 by Theodor B. Rais, MD, MS, and Alexandra Rais, BA
by Theodor B. Rais, MD, MS, and Alexandra Rais, BA
Dr. Rais is the Associate Professor of Psychiatry at the University of Toledo Medical Center, Toledo, Ohio; Ms. Rais is from the Public Health Masters Program at the University of Toledo, Health Science Campus, Toledo, Ohio.
Innov Clin Neurosci. 2014;11(5–6):18–22
Funding: There was no funding for the development and writing of this article.
Financial Disclosures: None of the authors have a conflict of interest in the conduct and reporting of this analysis.
Key words: Antidepressants, autistic spectrum disorders, pregnancy, neurodevelopmental delays, meta-analysis, r stats software
Abstract
Objective: Antidepressants have been reported in several studies in the literature to be associated with the development of autistic disorder symptoms in children exposed to them during the time of their mothers’ pregnancies. There have also been reports of neurodevelopment delays associated with exposure to antidepressants in the same conditions.
Design: We searched the PUBMED, MEDLINE, PsycARTICLES, and ERIC for original articles published between January 1983 and May 2013 to identify studies on the association between autistic spectrum disorders (ASD), neurodevelopment delays in children, and exposure to antidepressants during pregnancy.
Conclusion: At the end of our preliminary work, we retained only three articles that were pertinent to the purpose of our study. We extracted the available data in Excel files and then did a meta-analysis. The final results showed a positive association between the exposure to antidepressants in utero and autistic spectrum disorders.
Introduction
It is reported that between 9 and 14 percent of pregnant women show occasional symptoms of depression.[1] Bennett[2] reports that the prevalence rates of depression during pregnancy are as follows: 7.4 percent in the first trimester, 12.8 percent in the second, and 12 percent in the third trimester. Throughout the years, antidepressants have been used to treat depressive symptoms in pregnant women. Another situation sometimes met in clinical practice is women diagnosed with affective disorders becoming pregnant while on antidepressant medication. Many studies have looked into the question of neurodevelopment defects related to the use of antidepressant medication during pregnancy. In general, children who were exposed to antidepressants in utero were followed up, assessed, and compared to unexposed children as a control group. Many studies showed no statistically significant difference between the two groups.[15,16,17] However, Casper[3] and Mortensen[4] reported lower developmental scores in the antidepressant-exposed children.
We reviewed articles where autism spectrum disorders were described according to the Diagnostic Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria as communications and social interactions impairment manifested from the time of early childhood and associated with odd and stereotyped behaviors.
The term autism was first introduced by Leo Kanner in 1943 as the clinical expression of a major break in affective communication.[5] Today autism is considered a major developmental disorder, manifested by the individual’s inability to interact and communicate with others adequately and the presence of stereotyped, rigid, and restrictive behaviors. There is a 556-percent increase in the prevalence of autism in children as measured between 1991 and 1997. This increase is probably related to our enhanced awareness of the disease and to the change in diagnostic criteria.[6] For many years, it has been clear that autism is not a disorder by itself, but a syndrome with multiple causes that could be of genetic nature or not. For our meta-analysis, we are going to use the term autistic spectrum disorders (ASD). By this term, we mean a large variety of disorders related to early development and characterized by major impairments in the following domains:
1. Social interaction and affective expression
2. Language development and communication skills
3. Adequate play
4. Reduced imagination and interests
5. Narrowed sphere of activities.
A review of the literature published between 1961 and 2007 gives solid evidence for a variable but significant genetic component in ASD. However, there is clear evidence that other etiological factors may be responsible for the appearance of ASD, such as in-utero toxic exposure and infections (e.g., rubella, cytomegalovirus).
Antidepressants are the first line of treatment for depression and anxiety for pregnant women, and they are able to cross the placenta.[7] Not many studies have evaluated the effects of exposure to antidepressants during pregnancy in regard to the association with ASD, and no quantitative review has been performed so far. The purpose of this meta-analysis was to review the literature over the last 30 years to look for an association between the mother’s exposure to antidepressants during pregnancy and the appearance of ASD symptoms or neurodevelopmental delays in their children.
Method
We searched PUBMED, MEDLINE, PsycARTICLES, and ERIC for original articles published between January 1983 and May 2013 to identify studies on the association between ASD, neurodevelopment delays in children, and exposure to antidepressants during mother’s pregnancy.
We performed literature searches using the following combination of terms:
1. Antidepressants and pregnancy and autistic spectrum disorders
2. Antidepressants and pregnancy
3. Pregnancy and autism and antidepressants
4. Antidepressants and autism
5. Antidepressants and pregnancy and autism
6. Antidepressants and neurodevelopment delays
7. Antidepressants and pregnancy and neurodevelopment delays.
We found around 2,900 articles based on their titles, selected 307 articles based on their abstracts, and selected 15 articles that met the meta-analysis subject demands. After reading those articles, we selected three articles.
The studies were carefully reviewed, and estimators of relative risk with the 95-percent confidence intervals (CIs) were obtained. Relative risk estimates included the odds ratios (ORs) in the selected studies. In the last stage, we could select only three articles due to the scarcity of data in subject-related literature.
For the meta-analysis we used the R package rmeta. We decided to fit the fixed-effects model using the function meta.MH to compute the individual odds ratios, the Mantel-Haenszel weighted mean estimate, and Woolf’s test for heterogeneity. Then we implemented the random effects model with the same set of data using R function meta.DSL to compute the individual odds ratios, the Mantel-Haenszel weighted mean estimate, and the Woolf’s test for heterogeneity along with the estimate of the random effects variance. We then compared the results for both methods.
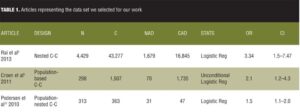
Table 1 lists the set of data we selected for our work.
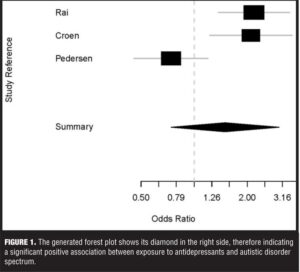
The articles were all case control studies: One article, Rai et al,[8] was a nested case control study, the second article was a population-based case control study,[9] and the third was a population-nested case control study.[10] The first article had a big sample, and the OR for association of ASD with exposure to antidepressants was 3.34. The second article had a midsized sample, with an OR of 2.1. And the third article had a smallest sample, with an OR of 1.5. The first two articles checked for the association between exposure to antidepressants and ASD, but the third article checked for the association between antidepressants and normal milestones development that is lacking in the context of ASD. From this standpoint, the sample of the meta-analysis for our data was not homogenous but we decided to include the article due to the fact that delay in milestones development is a major characteristic of ASD.
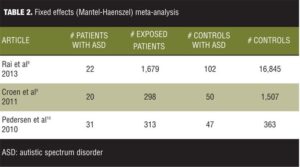
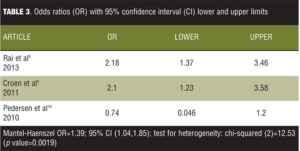
Tables 2 and 3 list the results for fitting the fixed effects mode for our set of data.
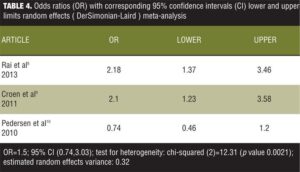
It is observed from the model fit that the overall OR is 1.39 with 95-percent CI from 1.04 to 1.85. The CI does not include the number 1, therefore indicating a significant weak overall positive association. However, if the meta-analysis would have encompassed only the first two studies of the data set, one can see that an effect size of 2.18 for the first and 2.10 for the second showing that excluding the third study from the meta-analysis would have produced more convincing results. The problem is that the chi-squared value for heterogeneity has a value of 12.53 with a p value of 0.0019 statistically significant heterogeneity. We then analyzed the data using the random effects (DerSimonian-Laird) meta-analysis (Table 4).
It can be seen that the estimated between-study variance is 0.32, and the global OR is 1.5 with a 95-percent CI of 0.74 to 3.03, which includes the number 1, rendering the results insignificant. However, the variance is 0.32 and the CIs are wider than those for the fixed mode, which are consistent with the theory that the random effect mode could be seen as an extension of the fixed effect model. We then used appropriate code to generate a forest plot (Figure 1).
Looking at the forest plot one can see that the diamond shows that altogether there is a positive association between in utero exposure to antidepressants and the development of ASD.
Discussion
The finding of this meta-analysis shows that there is a possible association between the exposure to antidepressants during pregnancy and the development of ASD symptoms in child. When analyzed separately, each article shows a positive association between antidepressants exposure and ASD8,[9] or antidepressants exposure and some neurodevelopment milestones.[10] The sample was heterogeneous, which is a major limitation of this work. It is likely that a stratified meta-analysis working only with the first two articles that have similar sets of data would have produced more convincing results. The forest plot diamond showed that altogether the positive association between antidepressants and ASD symptoms is very likely.
Conclusion
Altogether the results of this work have demonstrated an association between the exposure to antidepressants and ASD and this could certainly open an avenue for further work and awareness in the future.
References
1. Evans J, Heron J, Francomb H, et al. Cohort study of depressed mood during pregnancy and after childbirth.” BMJ. 2001;323(7307):257–260.
2. Bennett HA, Einarson A, Taddio A, et al. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103(4):698–709.
3. Casper RC, Fleisher BE, Lee-Ancajas JC. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402–408.
4. Mortensen JT1, Olsen J, Larsen H, et al. Psychomotor development in children exposed in utero to benzodiazepines, antidepressants, neuroleptics, and anti-epileptics. Eur J Epidemiol. 2003;18(8):769–771.
5. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50.
6. Muhle R1, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–486.
7. De las Cuevas C, Sanz EJ. Safety of selective serotonin reuptake inhibitors in pregnancy. Curr Drug Saf. 2006;1(1):17–24.
8. Rai D1, Lee BK, Dalman C, et al. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013 Apr 19;346:f2059. doi: 10.1136/bmj.f2059.
9. Croen LA, Grether JK, Yoshida CK, et al. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011 Nov;68(11): 1104-12. doi: 10.1001/ archgenpsychiatry.2011.73.
Epub 2011 Jul 4.
10. Pedersen LH. Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125;e600.
11. Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336(4):258–262.
12. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159(11):1889–1895.
13. Nulman I, Koren G, Rovet J, et al. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry. 2012;169(11): 1165–1174.
14. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402–408.
15. Laugesen K, Olsen MS, Telén Andersen AB, et al. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ. 2013 Sep 20;3(9):e003507. doi: 10.1136/bmjopen-2013-003507.
16. Sorensen MJ, Gronborg TK et al, Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459
17. Oberlander TF, Papsdorf M, Brain UM, et al. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164(5):444–451.
18. Chen DG, Peace KE. Applied meta-analysis with R. Boca Raton, FL:?CRC Press; 2013.