 by Sandeep Grover, MD; Aditya Somani, MD; Neeru Sahni, MD; Sahil Mehta, DM; Swati Choudhary, MBBS; Rahul Kumar Chakravarty, MBBS; and Anju Moni Rabha, MBBS
by Sandeep Grover, MD; Aditya Somani, MD; Neeru Sahni, MD; Sahil Mehta, DM; Swati Choudhary, MBBS; Rahul Kumar Chakravarty, MBBS; and Anju Moni Rabha, MBBS
Drs. Grover, Somani, Sahni, Mehta, Choudhary, Chakravarty, and Rabha are with the Post Graduate Institute of Medical Education and Research, Chadigarh in Chandigarh, India.
Innov Clin Neurosci. 2017;15(1–2):23–27.
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: The authors report no conflicts of interest relevant to this article.
ABSTRACT: Depression is a common comorbidity in patients suffering from Parkinson’s disease (PD). Available evidence suggests that electroconvulsive therapy (ECT) is an effective treatment for depression and also improves symptoms of PD. However, literature on usefulness of ECT in parkinsonian symptoms is limited. A review of records of all patients receiving ECT from 2010 to April 2017 in the authors’ clinic yielded six cases (0.63% of all patients who received ECT at the authors’ center over last 7 years) of depression with PD who were treated with ECT. All six patients had improvement in both depression and symptoms of PD following ECT treatment. The improvement achieved with ECT was sustained in four patients. Worsening of PD symptoms 3 to 4 months post-treatment was seen in two patients. ECT appears to be an effective treatment option for management of motoric symptoms in patients with PD, especially those with comorbid psychiatric disorders.
KEYWORDS: Depression, Parkinson’s disease, electroconvulsive therapy
INTRODUCTION
Depression is a frequent comorbidity in patients with Parkinson’s disease (PD). Evidence suggests that depression has a bidirectional relationship with PD, with either of the conditions increasing the risk of developing the other.[1,2] Incidence of depression in patients with PD has been reported to range from 1.86 to 5.1 percent per year,[3] with a prevalence rate of 2.7 to 55.6 percent.[1] This wide range of reported prevalence might be due to variations in assessment methods, clinical samples, and identification thresholds of mood disorders. Presence of comorbid depression often leads to further deterioration in quality of life and cognitive profile in these patients with PD.[4] Treatment options for comorbid depression and PD include antidepressants, cognitive behavioral therapy, and electroconvulsive therapy (ECT).[1,2,4]
In addition to depression, ECT has also been shown to have a beneficial effect on the symptoms of PD, although the data are limited.[5,6] In 1947, Gallinek first reported the beneficial effect of ECT on symptoms of PD,[7] and, in 1975, Lebensohn et al[8] reported improvement in comorbid depression and PD symptoms following ECT.[8]
In the current retrospective case series, we identified, via a search of our facility’s ECT registry (2010–April 2017), six patients with comorbid depression and PD who underwent ECT. We describe their treatment and review the current literature on the use of ECT among patients with PD.
CASE SERIES
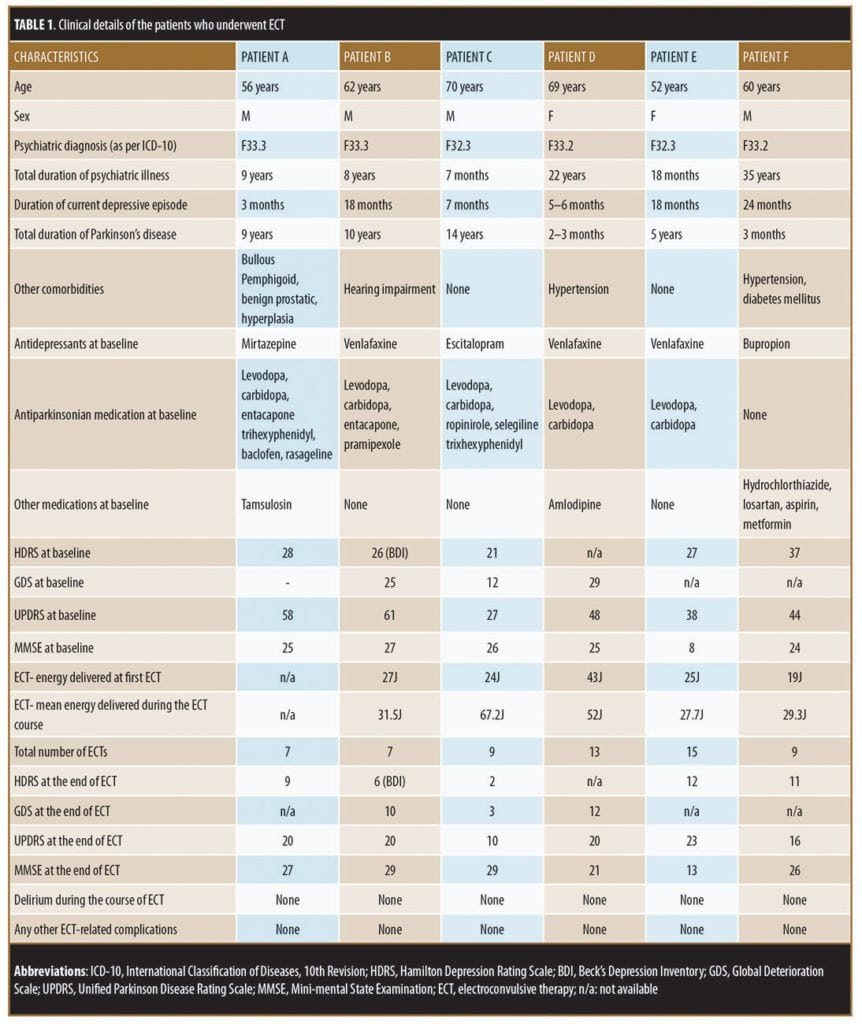
All six patients in our retrospective chart review received bitemporal modified brief pulse ECT. Prior to ECT, the patients underwent pre-ECT evaluation, which involved hemogram analysis, renal and liver function tests, fasting blood glucose analysis, chest X-ray (posteroanterior view), and serum electrolyte analysis, all of which were within the normal range for all six patients, and none of the patients showed evidence of raised intracranial pressure on fundoscopy examination. All patients provided written informed consent prior to starting ECT. Glycopyrrolate was used as a premedication, thiopentone was used for induction, and suxamethonium was used for muscle relaxation. Motor seizure of at least 15 seconds was considered effective. Details of different parameters are provided in Table 1.
Case 1. Mr. A, 56 years old, presented with a nine-year history of PD with recurrent depressive disorder (as per International Classification of Diseases, 10th Revision [ICD-10]). His medications included levodopa-carbidopa-entacapone, trihexyphenidyl, baclofen, rasageline, and mirtazapine. The patient reported that he had been experiencing a worsening of PD symptoms (e.g., increase in rigidity and tremors) and the need for assistance with activities of daily living (ADL)for the three months prior to presentation to our clinic, despite being on regular treatment. During the first month that he had begun to experience worsening PD symptoms, Mr. A reported that he had relapse of depressive symptoms in the form of feelings of sadness, crying spells, loss of interest in pleasurable activities, loss of self-confidence, feelings of guilt, poor sleep, and loss of appetite. Gradually, over the next two months, the symptoms of depression progressed, and the patient developed delusions of persecution, feelings of hopelessness and worthlessness, and suicidal ideations. He attempted to harm himself by consuming insecticide but was found by family members and brought to our emergency room. After initial stabilization, he was moved to the psychiatry inpatient unit.
On initial examination in the psychiatry ward, Mr. A presented with a mask-like face, tremors in the hands, and sad affect. The patient expressed feelings of hopelessness and worthlessness, plans of self-harm, and ideas of reference. His Hamilton Depression Rating Scale (HDRS) score was 28, and his Unified Parkinson Disease Rating Scale (UPDRS) score was 58. He was continued on levodopa-carbidopa-entacapone, and his antidepressant was changed to milnacipran. Due to the risk of self-harm, Mr. A underwent ECT. He was treated with seven ECT sessions over three weeks without any complications. During the course of ECT, doses of antiparkinsonian medications were not altered. Along with resolution of depression (reduction in HDRS score to 9), there was complete relief from tremors, rigidity, and other symptoms of PD. Mr. A was able to resume ADLs without the need for assistance, and he was discharged in an improved state. His UPDRS score had decreased to 20 by the end of the ECT sessions. However, three months after the completion of the ECT course, the patient reported mild worsening of symptoms of PD, which required an increase in the dose of antiparkinsonian medications. Mr. A remained stable on antidepressants and antiparkinsonian medications for the next eight years.
Case 2. Mr. B, 62 years old, had a 10-year history of PD and an eight year history of recurrent depression (as per ICD-10 criteria). His medications included levodopa-carbidopa and pramipexole for PD. He had not taken antidepressant medications for the last two years. A year and a half prior to presentation, Mr. B had a relapse of depression. Symptoms included feelings of sadness, decreased interest in pleasurable activities, lethargy, poor attention and concentration, ideas of hopelessness and worthlessness, decreased sleep, decreased appetite, and delusions of persecution and reference. With the emergence of depression, his adherence to medications for PD became poor, and PD symptoms worsened. At the time of presentation to our clinic, Mr. B was found to have tremors in the hands (more on right side), increased tone and cogwheel rigidity of both upper limbs, slow gait, and reduced arm swing. In liaison with the neurologist, our team prescribed Mr. B levodopa-carbidopa-entacapone and pramipexole. For management of depression, we initiated venlafaxine and quetiapine. Over the next four weeks, the patient did not show much improvement in depressive symptoms, and his PD symptoms improved only marginally. In view of his suicidal ideations and severity of depression, we began treatment with ECT. He received seven sessions of ECT over a period of three weeks without any complications. Prior to starting ECT, Mr. B scored 61 on UPDRS and 26 on Beck Depression Inventory (BDI). After the third session of ECT, the patient started to show improvement in both depressive and PD symptoms. After his final session of ECT, Mr. B scored 20 on UPDRS and 6 on BDI. Subjectively, the patient reported a return to baseline both in terms of mood and ability to perform ALDs without assistance. He stayed on antidepressants and PD medications for four months, after which PD symptoms worsened again, requiring an increase in the dose of PD medications.
Case 3. Mr. C, 70 years old, had a 14-year history of PD, and was being treated with levodopa-carbidopa, ropinirole, selegiline, and trihexyphenidyl. Seven months prior to presentation, he had worsening symptoms of PD, despite regular treatment. His dose of selegiline was increased, but Mr. C did not perceive any benefit. After a month of worsening PD symptoms, he developed symptoms of depression, characterized by feelings of pervasive sadness, crying, excessive worry about his worsening physical condition, reduced interaction with people, lethargy, anhedonia, reduced appetite, poor sleep, and poor self-care. With the onset of the depressive symptoms, Mr. C became nonadherent with the medications, causing his symptoms of PD to worsen. His speech became slurred and incomprehensible, and Mr. C developed marked rigidity and tremors. On examination, he had a mask-like face, increased muscle tone and cogwheel rigidity in all limbs, axial rigidity, coarse tremors in the right hand, and festinating gait. Mental status examination revealed sad affect, feelings of worthlessness, a bleak and pessimistic view of the future, a death wish, delusions of persecution, poor attention and concentration, impaired judgment, and poor insight. A diagnosis of severe depressive disorder with psychotic features was made (per the ICD-10 criteria). Initially, Mr. C was reluctant to take medication for depression, but he eventually agreed to start escitalopram, which failed to alleviate his depression after six weeks. He was then considered for ECT, of which he was administered nine sessions over three weeks without any complications. Prior to starting ECT, his HDRS score was 21 and UPDRS score was 27. After the first three ECTs, he showed partial improvement in depressive symptoms and a reduction in PD symptoms (UPDRS reducing to 16), and he agreed to start PD medications. By the end of the ECT course, Mr. C showed marked improvement in symptoms of depression and PD with an HDRS score of 2 and UPDRS score of 9. His speech improved and became comprehensible, he could walk with support, and he was free from symptoms of depression. He continued taking escitalopram after the completion of ECT. The patient remained in remission from depressive symptoms for the next four months, and symptoms of PD did not worsen over this period.
Case 4. Ms. D, 69 years old, had a 22-year history of depression and had been on antidepressant medications irregularly. Upon presentation, she reported having had six episodes of depression. Her most recent episode had been present for the last 5 to 6 months. She reported feelings of sadness, decreased interaction with others, poor sleep, crying spells, and one suicide attempt. She was admitted to the psychiatry ward. At the time of admission, she was also found to have coarse-resting tremors in both hands along with cogwheel rigidity, which was equal in severity in both upper limbs. After consultation with a neurologist, she was diagnosed with PD, and Ms. D was prescribed levodopa-carbidopa. Because of symptoms of severe depression and risk of self-harm, she was offered ECT. She underwent 13 ECT sessions over the course of one month. Her baseline score on the Geriatric Depression Scale (GDS) was 29, and her UPDRS score was 48. After ECT, her GDS score decreased to 12, and her UPDRS score decreased to 20. She also had significant improvement in symptoms of tremors and rigidity. At the time of discharge, Ms. D could perform ADLs independently. At the three-month followup, she was still symptom free from depression and PD.
Case 5. Ms. E, 52 years old, presented to our clinic with stiffness in her right arm and right leg, which she reported had been present for five years and for which she had not undergone any treatment. She also reported having symptoms of depression for the past 1.5 years, including sadness of mood, worries about future, decreased interest in activities, decreased sleep and appetite, and easy fatigability. Both her body stiffness and depressive symptoms worsened to the extent that she would remain in bed, had difficulty in speech, and would pass stool and urine in bed. She was admitted to the neurology ward, was diagnosed with PD, and was prescribed levodopa, carbidopa, and entacapone. She had mild improvement in that she could speak but reported feelings of fearfulness, suspiciousness, hopelessness, helplessness, and a wish to die. She was then admitted to the psychiatry ward. She was diagnosed with psychosis (as per ICD-10 criteria) and was offered ECT. Baseline assessment revealed an HDRS score of 27 and an UPDRS score of 38. Ms. E underwent 15 sessions of ECT over the course of one month. At the time of discharge, she had a HDRS score of 12 and UPDRS score of 23 and was fully mobile, could perform ALDs without assistance, and reported an absence of fearfulness and suspiciousness. She was prescribed venlafaxine, quetiapine, levodopa, carbidopa, and entacapone. She remained stable at the one-year followup.
Case 6. Mr. F, 60 years old, had a 35-year history of recurrent depressive disorder, and upon presentation reported that he had been experiencing a relapse for the past two years. He reported symptoms of sadness, crying spells, excessive worries, decreased confidence, pessimistic view of future, and poor sleep and appetite. His symptoms waxed and waned despite treatment with antidepressants. For the previous year, his depressive symptoms had worsened, and he reported having suicidal thoughts. Three months prior to the presentation, he also started experiencing tremors in both hands, slowness in initiating and maintaining movements, and slurred speech. A day prior to presentation, he attempted suicide and was subsequently admitted to the psychiatry inpatient unit. Mental status examination at the time of admission showed that he had depressed affect, feelings of hopelessness and helplessness, death wishes, and suicidal ideations. A physical examination revealed presence of rigidity and tremors in both upper limbs, but more so on left side. The patient was not taking medications for his PD. Because of significant risk for self-harm, ECT was initiated. He was administered nine sessions of ECT over one month. His HDRS score before starting ECT was 37 and at the end of ECT was 11. His UPDRS score decreased from 44 to 16. He reported relief from depressive symptoms as well as from symptoms of PD. There was no rigidity or tremors at the time of discharge. He was released on bupropion, along with medicine for hypertension and diabetes mellitus. No PD medicine was initiated at the time of discharge. He was advised to follow up in neurology outpatient department (OPD) if the tremors and rigidity returned. In the follow-up, he was prescribed a combination of levodopa and carbidopa. He remained stable without any relapse of symptoms of depression and PD for the next six months.
DISCUSSION
This case series demonstrates the beneficial effects that ECT can have in patients with comorbid depressionand PD. The beneficial effect of ECT on symptoms of PD were reported as early as 1947 by Gallinek.[5] In a review of literature published in 1991, the authors reported that ECT showed benefit in patients with movement disorders, irrespective of the presence of psychiatric comorbidity. There are 35 case reports on use of ECT in patients with PD. In six of the cases, patients with PD did not have comorbid psychiatric disorder.[9] ECT has also been shown to have positive effects on patients with drug-induced Parkinsonism, tardive dystonia, and tardive dyskinesia. Another review of literature from 1990 to 2000 reported 21 cases of ECT in patients with PD, as well as four additional reports on use of ECT in patients with parkinsonian symptoms.[10] The four reports included 135 patients, with motor scores being available for 76 patients to determine the efficacy of ECT. When combining information from the previous review,[9] the authors had information on 213 patients total.[10] Most of the reports (17 out of 25) published during the evaluation period were case reports and series, but there were two retrospective studies and four prospective open-label studies.[10] Eighty-eight percent (36 out of 41) of patients with PD who received ECT in the absence of a comorbid psychiatric disorder were reported to show improvement in motor symptoms. Among those with psychiatric disorders, 77 percent (58 out of 75) were reported to show improvement following ECT.[10] The six patients from our clinic showed improvement in both depressive and motoric symptoms followoing ECT. In terms of PD, although there is lot of inconsistency in reporting, there is evidence to suggest that ECT offers a beneficial effect on tremors, on/off time, rigidity, and cogwheeling.
Our six patients showed improvement in tremors and rigidity. In terms of predictors of treatment response, some of the reports we reviewed suggest that a lower level of pre-existing impairment is associated with better response to ECT, whereas others suggest that a higher severity of symptoms and older age are associated with better response in motor symptoms.[10] In terms of severity, our six patients had severe PD symptoms prior to starting ECT. In terms of complications with ECT in patients with PD, the literature shows that 44 percent have been reported to develop delirium,[10] and one study reported delirium in 85 percent of the patients.[11] This suggests that patients with PD have higher a risk of developing delirium while being treated with ECT. It is important to understand that underlying pathogenesis for delirium includes dopamine activation; hence, concomitant use of antiparkinsonian medications can also contribute to the development of delirium. However, the available studies do not discuss this issue in detail. None of our patients developed delirium.
A recent review has been published that evaluates data on ECT use in patients with depression and comorbid PD.[7] The authors of this review located 43 articles—27 were individual patient case reports, 13 were case series describing 2 to 11 patients, two were retrospective chart reviews, and one was a retrospective case control study that included 19 patients. All of these articles were published between 1975 and 2015. All except for one of these articles had data from 115 patients with PD and depression and one patient with PD and mania. The majority (93.1%) of the patients described in the review article were reported as having improvement in depression. The reviewed studies reported that 83 percent of the patients perceived improvement in motor symptoms and depression following ECT, 15 percent reported no improvement in motoric symptoms despite improvement in depression, and two percent reported worsened motoric symptoms despite improvement in depression.[7] Based on the available information, the authors of that review article proposed an algorithm for management of depression in patients with PD, suggesting ECT should be considered if the depression is severe or if there is a worsening of depression. They further suggested that cognitive testing should be done prior to starting an ECT course. If a patient experiences significant cognitive deficits during the course of ECT, increasing the time between sessions or reducing the amount of energy used during treatment should be considered. In terms of electrodes, the recommendations suggest that a right unilateral ECT should be started with an ultra-brief pulse width. Patients with pre-existing dementia should be prescribed cholinesterase inhibitors, which might act as protective agents for ECT-associated cognitive deficits.[7] One study compared the effect of ECT on patients with or without PD. Both study arms included 25 patients, and 56 percent of those with PD reported improvement in motoric symptoms, 40 percent reported no change, and four percent reported worsening symptoms. More than half (56%) of patients with PD experienced side effects, which was significantly higher than the 12 percent reported among patients without PD.[12]
A recent open-label study included six patients with PD and depression and used a right unilateral brief pulse ECT. All of the patients reported improvement in both depressive and motoric symptoms without worsening of cognitive symptoms immediately after completion of ECT and at the one-month follow-up. ECT has also recently been reported to benefit patients with PD with refractory psychosis,[13] refractory anxiety,[14] severe obsessive-compulsive disorder,[15] drug-induced psychosis,[16,17] residual axial symptoms partially unresponsive to L-dopa,[18] and drug-included Parkinsonian symptoms.[19]
Various mechanisms have been proposed for the effectiveness of ECT in the management of motoric symptoms in patients with PD. One of the proposed mechanisms suggests that improvement in symptoms of depression leads to improvement in motoric symptoms. In terms of neurotransmitter mechanisms, alteration of dopaminergic and gamma-aminobutyric acid systems have been proposed, but an exact mechanism is not yet well understood.[20]
In terms the of long-term effects of ECT on PD symptoms, it isn’t clear how long the improvement in PD symptoms last after discontinuing ECT.[7,8,21] Two of our patients showed worsening in motoric symptoms 3 to 4 months after discontinuing ECT. Some of the available data suggest that maintenance ECT can be used to sustain improvement in motoric symptoms.[22,23]
Despite the reported evidence, ECT has not found its due place in treatment protocols and guidelines for management of PD. Most protocols do not even mention ECT as a treatment option.[8] Possible stigma associated with ECT likely precludes its use in these patients, especially those without psychiatric comorbidity. With newer and more established treatments, like deep brain stimulation (DBS), further research into ECT use in PD does not seem urgent to researchers.[24]
To conclude, ECT appears to have a beneficial effect on motoric symptoms in patients with PD, especially those with comorbid psychiatric disorders. However, proper precautions must be taken to reduce the associated complications, and patients must be closely monitored while undergoing ECT.
REFERENCES
- Grover S, Somaiya M, Kumar S, Avasthi A. Psychiatric aspects of Parkinson’s disease. J Neurosci Rural Pract. 2015;6(1):65–76.
- Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry. 1992;149(4):443–54.
- Dooneief G, Mirabello E, Bell K, et al. An estimate of the incidence of depression in idiopathic Parkinson’s disease. Arch Neurol. 1992;49(3):305–7.
Allain H, Schuck S, Mauduit N. Depression in Parkinson’s disease. BMJ. 2000;320(7245):1287–8. - Borisovskaya A, Bryson WC, Buchholz J, et al. Electroconvulsive therapy for depression in Parkinson’s disease: systematic review of evidence and recommendations. Neurodegener Dis Manag. 2016;6(2):161–76.
- Popeo D, Kellner CH. ECT for Parkinson’s disease. Med Hypotheses. 2009;73(4):468–9.
Fromm GH. Observation on the effects of electroshock treatment in patients with Parkinsonism. Bull Tulane Univ. 1959;18:71–3. - Lebensohn ZM, Jenkins RB. Improvement of Parkinsonism in depressed patients treated with ECT. Am J Psychiatry. 1975;132(3):283–5.
- Faber R, Trimble MR. Electroconvulsive therapy in Parkinson’s disease and other movement disorders. Mov Disord. 1991;6(4):293–303.
- Kennedy R, Mittal D, O’Jile J. Electroconvulsive therapy in movement disorders: an update. J Neuropsychiatry Clin Neurosci. 2003;15(4):407–21.
- Figiel GS. ECT and delirium in Parkinson’s disease. Am J Psychiatry. 1992;149(12):1759; author reply -60.
- Moellentine C, Rummans T, Ahlskog JE, et al. Effectiveness of ECT in patients with parkinsonism. J Neuropsychiatry Clin Neurosci. 1998;10(2):187–93.
- Nishioka K, Tanaka R, Shimura H, et al. Quantitative evaluation of electroconvulsive therapy for Parkinson’s disease with refractory psychiatric symptoms. J Neural Transm (Vienna). 2014;121(11):1405–10.
- Marino L, Friedman JH. Letter to the editor: successful use of electroconvulsive therapy for refractory anxiety in Parkinson’s disease. Int J Neurosci. 2013;123(1):70–1.
- Gadit AM, Smigas T. Efficacy of ECT in severe obsessive-compulsive disorder with Parkinson’s disease. BMJ Case Rep. 2012;2012.
- Muralidharan K, Thimmaiah R, Chakraborty V, Jain S. Bifrontal ECT for drug-induced psychosis in Parkinson’s disease. Indian J Psychiatry. 2011;53(2):156–8.
- Ueda S, Koyama K, Okubo Y. Marked improvement of psychotic symptoms after electroconvulsive therapy in Parkinson disease. J ECT. 2010;26(2):111–5.
- Pintor LP, Valldeoriola F, Fernandez-Egea E, et al. Use of electroconvulsive therapy in Parkinson disease with residual axial symptoms partially unresponsive to L-dopa: a pilot study. J ECT. 2012;28(2):87–91.
- Sadananda SK, Holla B, Viswanath B, et al. Effectiveness of electroconvulsive therapy for drug-induced parkinsonism in the elderly. J ECT. 2013;29(1):e6–7.
- Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9–10):25–8.
- Lieberman A. Depression in Parkinson’s disease—a review. Acta Neurol Scand. 2006;113(1):1–8.
- Balke LD, Varma A. A case of long-term maintenance ECT in a 78-year-old with depression and possible Parkinson’s disease. CNS Spectr. 2007;12(5):325–6.
- Shulman RB. Maintenance ECT in the treatment of PD. Therapy improves psychotic symptoms, physical function. Geriatrics. 2003;58(11):43–5.
- Cunningham MG, Yadollahikhales G, Vitaliano G, van Horne C. Administration of electroconvulsive therapy for depression associated with deep brain stimulation in a patient with post-traumatic Parkinson’s disease: a case study. BMC Psychiatry. 2016;16(1):399.





