by Michael Shuman, PharmD; Tammie Lee Demler, PharmD, MBA, BCPP; Eileen Trigoboff, RN, DNS; and Lewis A. Opler, MD, PhD
Dr. Shuman is from the Department of Pharmacy, Buffalo Psychiatric Center, Buffalo, New York; Drs. Demler and Trigoboff are from Buffalo Psychiatric Center in Buffalo, New York, and with the State University of New York at Buffalo; and Dr. Opler is Professor of Clinical Psychiatry, Columbia University Medical Center, New York, New York.
Innov Clin Neurosci. 2012;9(11–12):18–30
Funding: There was no funding for the development and writing of this article.
Financial Disclosures: None of the authors have a conflict of interest in the conduct and reporting of this study.
Key words: schizophrenia, antibiotic, side effect, clozapine, psychiatry, hematology
Abstract: Objective: The purpose of this study was to determine if the drop in white blood cell/absolute neutrophil count for clozapine patients on antibiotics is a normal response to the resolution of infection or if the concurrent administration resulted in an abnormal drop in blood counts and further reduction of white blood cell/absolute neutrophil below baseline prior to infection. Design: This was a retrospective record review of all patients who received clozapine and antibiotics concurrently between June 30, 2010, and June 30, 2011. Setting: Subjects included inpatients on clozapine therapy at a state psychiatric facility. Participants: This protocol was approved by the Institutional Review Board of record. A total of 42 patients prescribed 93 antibiotic regimens were found to meet all of the above requirements. Measurements and methods: Medications were placed into distinct groups based on approved use and mechanism of action. Pearson Correlation Coefficients were utilized and were found to be 0.409 (p<0.01), indicating that a statistically significant relationship existed between the use of systemic antibiotics and alterations in hematologic parameters. Results: Each regimen was classified by specific agent as well as whether the final white blood cell/absolute neutrophil was above or below the baseline established for each patient. Conclusion: Antibiotics have been identified as one category of medications that may cause decreased white blood cell/absolute neutrophil counts when combined with clozapine. Our study supports the use of either ciprofloxacin or moxifloxacin as agents that may have less risk of reductions in white blood cell/absolute neutrophil counts than are seen with penicillins, cephalosporins, and other antibiotics that may ultimately require interruption or discontinuation of clozapine therapy.
Introduction
As the first atypical antipsychotic to be introduced into the United States, clozapine is known for its superior efficacy compared to other antipsychotic agents, including other atypical agents (i.e., second generation antipsychotics).[1] Since its introduction in 1990, however, cases of agranulocytosis and neutropenia have been reported and associated with clozapine therapy. An increased risk has been noted during the first 6 to 18 weeks of treatment.[2] This rare but severe adverse event is idiosyncratic, and its mechanism of action remains unknown.[1,2] Studies have demonstrated that clozapine-induced agranulocytosis and neutropenia are not related to the dose of the antipsychotic.[1,2] Because of the significant risk of agranulocytosis, a potentially life-threatening adverse event, clozapine is reserved for specific populations. It may be used in the treatment of severely ill patients with schizophrenia who fail to show an acceptable response to adequate courses of standard antipsychotic drug treatment as well as reducing the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffectve disorder who are judged to be at risk of re-experiencing suicidal behavior.[3] Clozapine should not be used simultaneously with other agents having a well known potential to cause agranulocytosis or otherwise suppress bone marrow function due to the potential synergistic effects of these combinations.[3] Other medications thought to induce agranulocytosis when used as monotherapy include carbamazepine, sulfonamides, ticlodipine, and beta-lactams.[4] However, additional agents, including new drugs to market, may play a confounding role have not yet defined, and more research is needed to ensure safe and effective long-term clozapine use.
The human immune system is made up of leukocytes (white blood cells [WBC]), which include neutrophils, eosinophils, basophils, lymphocytes, monocytes, macrophages, and dendritic cells. These cells function through two distinct methods of cellular response. The first bodily response to a foreign entity (bacterial, fungal, viral, or parasitic) is through the innate immune system. This system involves a nonspecific response to an invading pathogen that is predominately carried out by phagocytes, including macrophages, natural killer cells (NK cells), and neutrophils.[5] Along with the innate immune system, the body also has adaptive immunity. This system mainly comprises lymphocytes, including T cells and B cells, both of which are produced in response to a specific antigen. Such a response produces immune memory. When the antigen is seen again in the body, the T and B cells will target that antigen and produce a more efficient immune response.[5]
Normal levels of leukocytes can differ with ethnic background.[6,7] One study has shown that the WBC and absolute neutrophil count (ANC) can range drastically between African American, Caucasian, and Mexican American populations. A normal WBC range for an African American man (>18 years of age) can be between 3.1 and 9.9 (x103/µL) with an ANC between 1.3 and 6.6. Caucasian and Mexican American men show a WBC range between 4.1 and 11.4 with an ANC between 2.1 and 8.0. Though these levels are different, African American men do not appear to have an increased risk of infection.[6]
Neutrophils are the most abundant WBCs. They are produced in the bone marrow at a rate of 0.85 to 1.63 x 109 cells/kg/day (~1011 cells/day) and remain for 4 to 6 days. At this point, the production rate overcomes the high turnover, and concentration reaches 5.59 x 109 cells/kg. The cells then travel to the blood at a rate of 109 cells/kg/day. An individual cell takes 6.6 days to reach the bloodstream, where the turnover rate is 0.87 x 109 cells/kg/day. Each neutrophil has a half-life in the blood of 6 to 8 hours. Under noninflammatory conditions, both neutrophil production and migration out of the bone marrow are controlled by granulocyte colony-stimulating factor (G-CSF).[8]
This neutrophil timeline may be altered by both neutropenia and infection. When an infection is initially identified by the immune system, cytokine release signals for neutrophils and macrophages circulating in the plasma to migrate to the area of infection.[9] In experimental models, this resulted in an initial decrease in plasma concentrations of neutrophils and macrophages that reached their nadir on Day 1 Postinoculation.[9,10] The same cytokines that cause circulating neutrophils to migrate to the area of infection then cause the bone marrow to release neutrophils, which migrate to the affected site. This increase in circulating neutrophils offsets the initial decrease seen in the first stage of infection. Concentrations rise in response to increasing bacteria counts, peaking at four days postinfection. Reduction in neutrophil release from bone marrow occurs as the bacterial load from the initial infection begins to decrease. This generally occurs seven days after the onset of infection.[9]
Transit time is expected to be reduced in patients with infection. This was illustrated by the canine model provided by Marsh et al.[11] Here, the granulocyte pool in the marrow becomes elevated and the transit time to the blood is reduced by two days. It appears that this reduced transit time is temporary and is followed by a period of normal to elevated transit time. It is understood that the process begins with release of the cells from the marrow into the circulating granulocyte pool (CGP) in the bloodstream. Neutrophils leave the bloodstream and migrate to the tissue at the site of infection. In order to do so, the marginal granulocyte pool (MGP) must increase. The CGP cannot be restored until bone marrow stores are replenished and released from the bone marrow. In both subacute and chronic infection, CGP is more frequently elevated than is MGP.[11]
Commonly the definition of neutropenia can be divided into mild (1000–1500 cells/µL), moderate (500–1000 cells/µL) and severe ( As aforementioned, the transit time is also expected to be reduced by two days in those with neutropenia. This has been shown in some models to be a function of bone marrow depletion.[11] The size of the mitotic compartment was used to determine the degree of neutrophil proliferation in neutropenic patients. As the bone marrow stores are depleted, regulatory processes should result in increased production of granulocytes. This held true in four patients (4/16; 25%), as the mitotic component was two standard deviations or more greater in size than those in normal individuals. In the remaining 12 (75%), low blood neutrophil counts did not result in increased cell proliferation, as evidenced by mitotic compartments at or below basal levels. This would be expected in the neutropenic patient, as these regulatory mechanisms are altered. Possible mechanisms for neutropenia include failure to repopulate the bone marrow with new cells, failure for the bone marrow to produce or release mature granulocytes, increased cell turnover in the tissue, and the increased cells adherence to endothelial surfaces known as margination.[12]
Certain hematological parameters may predict the incidence of infection secondary to neutropenia, including the absolute neutrophil count and duration of neutopenia.[7,13] For example, if peripheral consumption of neutrophils increases while bone marrow production remains constant, risk of infection is lessened as compared to reduced proliferation of myeloid precursors or depletion of bone marrow reserves.
Antibiotic treatment has the potential to affect the normal physiological actions of the innate immune system. Depending on the drug this effect can be beneficial or detrimental to the immune system and health of the infected host. Macrolide antibiotics decrease cytokine release and in turn decrease the migration of neutrophils to sites of infection. They have an immune-modulating effect by inhibiting the synthesis of pro-inflammatory cytokines and chemokines while simultaneously facilitating the release of anti-inflammatory cytokines. Macrolides may also exert a direct effect on neutrophils through alterations in rates of oxidative burst and apoptosis.[13] By facilitating the onset of apoptosis, cell death is produced without a subsequent release in inflammatory mediators. This prevents the death of surrounding neutrophils.[14,15] Beta-lactam antibiotics have shown an effect on decreasing total neutrophil concentration in the plasma leading to decreased WBC count and neutropenia. This is thought to be caused by a combination of decreased granulopoiesis and induction of antibodies against the formation of haptens on neutrophils.[16–18] Vancomycin has also been shown to cause neutropenia in patients due to the formation of hapten molecules on neutrophils.[19,20] Further studies are necessary to evaluate how great of a risk is actually associated with these antibiotics.
Few studies have been conducted and published on the combination of clozapine and antibiotics. The available data suggest that medication, including antibiotics, with increased risk of neutropenia poses an even greater risk when combined with clozapine.[1,16] When taking a closer look at concurrent therapy of clozapine and antibiotics, a defined drop in WBC count is therefore expected.[1] However, it is proposed that this may be due to various confounders, such as the actual resolution of the infection, rather than the antibiotic itself or its possible association with agranulocytosis.[1] Thus, it is inconclusive whether concurrent therapy of clozapine and antibiotics can be associated with an increased risk of agranulocytosis. To further examine this association between clozapine and antibiotics, we will take a closer look at the hematological trends in antibiotics as monotherapy as well as when used with concomitant clozapine therapy. More specifically, however, the hematological trends discussed will focus on the adverse events of agranulocytosis and neutropenia, as well as whether antibiotics have an increased risk of these events.
Overview of the Literature
Macrolides. A study that was completed by Culic et al[21] assessed the degree of anti-inflammatory action that azithromycin has on leukocyte function. In the trial, 12 healthy volunteers underwent a daily dosing of 500mg of azithromycin for three days. Rates of neutrophil degranulation and oxidative burst were obtained. Degranulation is thought to increase the antibacterial activity of the drug and decrease the inflammation potential of neutrophils later during the infection, while oxidative burst is responsible for releasing reactive oxygen molecules in the area of infection. These reactive oxygen molecules help with antibacterial actions but also lead to host tissue damage and further inflammation. The inhibition of the oxidative burst is another driving force of the anti-inflammatory effects of azithromycin.
The addition of azithromycin acutely induced neutrophil degranulation as well as oxidative burst. Three days after being treated with azithromycin, however, rates of neutrophil oxidative burst were found to be decreased.[21] In addition to the reduction of the oxidative burst, it was found that azithromycin also decreased the action of recruiting chemokines. An initial decrease in leukocyte count was seen 2.5 hours after dosing was completed, though levels returned to normal 24 hours after dosing was completed.
Several authors attributed these effects to attenuation of interleukin-8 (IL-8) and IL-6.[15,21–23] Both IL-6 and IL-8 are involved in the immune response. Inhibition of these two cytokines prevents chemotaxis, or the migration of neutrophils to the side of infection. IL-8 also has an additional effect on regulation of apoptosis. By inhibiting IL-8, azithromycin also works to induce apoptosis.[15,21,22] This yields a protective effect by reducing tissue damage and subsequent destruction of healthy neutrophils in the surrounding area.[24]
A follow-up study in patients with chronic obstructive pulmonary disease (COPD) failed to entirely replicate these results. While the anti-inflammatory effects were retained, patients on azithromycin displayed statistically significant reductions in relative neutrophil count (p=0.0422).[10] The failure of this and other studies to replicate the original findings was attributed to the presence of additional comorbidities. While the original study enrolled only healthy volunteers, other study populations may feature a compensatory increase in inflammation produced by COPD or bacterial infection.[10,21,24]
Aoshiba et al[25] evaluated the role of erythromycin in neutrophil apoptosis. Blood was drawn from healthy volunteers and neutrophils were isolated from the whole blood. Erythromycin was added to the samples of neutrophils along with other drugs and placebo for comparison. After 24 hours of incubation with erythromycin, a dose dependent increase in apoptosis was seen. In a similar fashion, the addition of the macrolides clarithromycin, roxithromycin, and midecamycin showed an increase in neutrophil apoptosis. Other antibiotic agents, including ampicillin, cefazolin, and gentamicin, showed no increase.
The summative effect that the macrolides have on immunity in vivo may serve to attenuate the negative effects of the inflammatory response by reducing the migration of neutrophils to the site of action. Furthermore, this may serve to prevent subsequent neutropenia.[21]
Fluoroquinolones. Fluoroquinolone antibiotics appear to have an immunomodulatory effect, manifested through increased production of IL-2, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF). The benefits have been found to be dependent on both the individual fluoroquinolone agent as well as the specific dose.[26] Fluoroquinolone antibiotics, which produce increases in WBC counts, appear to share a similar molecular structure.[27,28] While moxifloxacin and ciprofloxacin each contain a cyclopropyl moiety at position N1 of the quinolone group, levofloxacin does not. In an animal model administered saline, moxifloxacin, or the cephalosporin antibiotic ceftazidime, cyclophosphamide-induced neutropenia was reversed exclusively by moxifloxacin. The results were observed independently of whether the pathogen was susceptible to moxifloxacin. A follow-up study found significant increases with ciprofloxacin as well.[29]
Beta-lactams. Penicillins and other beta-lactam antibiotics have been previously associated with reductions in ANC/WBC resulting in neutropenia. A review study by Scheetz[18] included six case reports and three cohort studies of piperacillin-induced neutropenia.[18] Of the six case reports, three included patients who became neutropenic while receiving piperacillin and three who developed neutropenia secondary to therapy with a combination of piperacillin and tazobactam. Each of the six cases of neutropenia occurred after at least 15 days of therapy, though the average dose was not specified. Review of the cohort studies determined that rates of neutropenia were dependent upon both dose and duration of therapy.[18] Additionally, Neftel[17] examined the relationship between beta-lactam antibiotics and neutropenia. In the cases evaluated, the majority of treatments that resulted in neutropenia were regimens that lasted more than 10 days. The mean duration of therapy was 23.2 days; however, results differed between the different antibiotics.[17] Within the beta-lactam class, cephalosporin drugs were as much as 25 times more potent than penicillins in decreasing the amount of marrow growth.[17]
Data were also gathered regarding the effects beta-lactam antibiotics had on normal bone marrow cultures. Initial bone marrow colony formation decreased 90 to 100 percent when cultures were grown in the environment of penicillins and cephalosporins as compared to growth in the presence of placebo. The drugs were then washed from the bone marrow cultures and the cells recovered with as much viability as marrow exposed to placebo.
The formation of hapten molecules on the cell surface is also responsible for neutropenia.[7,16] A hapten is formed when a molecule, in this case a drug, binds to a protein on the surface of a cell.[30] The immune system reads the hapten as a foreign protein and produces an antibody against the hapten. The antibody binds in a similar way as it would to a bacterial or viral structure, and induces an immune response.[30–32] Unlike granulopoiesis, the formation of hapten molecules on cell surfaces is not time-related and can occur within hours to days from the initiation of the drug.[7] The incidence of neutropenia is reversible upon discontinuation of the offending agent.
Sulfamethoxazole/trimethoprim. Sulfa drugs are cited among those antibiotic agents with a propensity toward hematologic changes.[16,33] A small study was performed on 12 healthy adults given a standard regimen of sulfamethoxazole 800mg and trimethoprim 160mg twice daily for five days.[34] At Day 5, cell counts were analyzed and changes, though present, were not found to be statistically significant. The changes were found to be related to folate level. In colonies deprived of folate, granulocyte production decreased in correlation with increased concentrations of trimethoprim. When folinic acid was added to a patient’s regimen, the rates of colony formation increased.[34]
Doxycycline. Doxycycline may also have a role in facilitation of hematologic changes through the inhibition of matrix metalloproteinases (MMPs), which are important mediators of cytokine release and subsequent cellular development. Upon suppression of bone marrow, MMP-9 expression is up-regulated. Mice deficient in MMP-9 had, on average, an eight-day delay in the recovery of WBCs from dyscrasias induced by the cytotoxic agent 5-fluorouracil (5-FU).[35] Doxycycline has been shown to inhibit MMP-9 and prolong neutropenic episodes induced by 5-FU therapy.[36] Our findings indicate a delay in recovery of ANC and WBC, with reductions in both ANC and WBC noted at 47 days after the antibiotic regimen was completed. While the number of individuals prescribed doxycycline regimens (n=16) was larger than with other types of antibiotics, there remained a degree of variance between baseline and final hematologic parameters. While the average reduction in ANC was 20.3 percent (range: -57 to +2%), the corresponding decline in WBC was only 13.8 percent (range: -46 to +15%).
Vancomycin. Vancomycin has also demonstrated the potential to cause neutropenia. Pai et al[20] studied the incidence of neutropenia due to vancomycin between 1998 and 2004. Of the 114 patients receiving vancomycin, 14 (12%) developed neutropenia. Once neutropenia was evident, vancomycin was discontinued. Mean time to WBC resolution was 10 days. Researchers attributed the cause of neutropenia to an immune response against neutrophils. Incidence was not related to vancomycin dose, duration of therapy, or trough concentrations.20
In a case presented by Hsiao et al,[19] a 57-year-old patient presented to the hospital with drainage from a previous surgical incision, which occurred 15 days earlier. The blood tests from the recent surgical admission were used as baseline and showed a WBC of 8,000/µL and an ANC of 6,000µL. Upon admission, blood tests revealed a WBC of 12,000/µL and an ANC of 10,000/µL. Five days after admission, vancomycin was prescribed at a dose of 1 gram every 12 hours. WBCs and ANC returned to normal around Day 7 and remained consistent through Day 18. On the 19th day of vancomycin therapy, the WBC and ANC began to drop. By Day 24, counts were reduced to a WBC of 2,800/µL and an ANC of 280/µL. At this time, vancomycin was discontinued and teicoplanin was started. WBC and neutrophil levels began to increase, but after 11 days of teicoplanin therapy, cell levels dropped back down to previous levels. Linezolid was later initiated after failure on teicoplanin, and WBCs returned to baseline.[19]
Clindamycin. Reports of neutropenia or agranulocytosis secondary to clindamycin therapy are limited.[37–40] A Thai study evaluated 38 cases of agranulocytosis. Two were determined to be caused by clindamycin therapy, though a precise mechanism was not established.[37]
Metronidazole. As with clindamycin, reports of dyscrasias secondary to metronidazole therapy have been documented, albeit infrequently.[16,38,41] We identified one case report of chronic neutropenia that resolved upon discontinuation of metronidazole.[41]
Nitrofurantoin. There is also evidence in the literature for neutropenia or agranulocytosis secondary to nitrofurantoin therapy.[33,38] Cases of neutropenia or agranulocytosis occurring in the Netherlands between 1974 and 1994 were examined.[38] Of the 275 reported, five were found to be related to therapy with nitrofurantoin.
Not every study has shown such conclusive results. Healy et al[42] looked at the action of multiple antibiotics on neutrophils and found conflicting data. Ceftazidime, gentamicin, ciprofloxacin, trovafloxacin, azithromycin, erythromycin, tetracycline, and doxycycline were added to cultures containing neutrophils and K. pneumonia. The organism was sensitive to ceftazidime, gentamicin, ciprofloxacin, and trovaloxacin and was resistant to azithromycin, erythromycin, tetracycline, and doxycycline. Reductions in rates of apoptosis were identified regardless of whether the pathogen was susceptible to the specific agent. The authors proposed different theories for these results. The first theory was that bactericidal effects decreased the levels of K. pneumoniae in the samples leading to a longer neutrophil life, but that did not account for the drug against which the bacteria was resistant. A second theory was that the anti-inflammatory properties of the resistant drugs were the cause of the decreased apoptosis. The conclusion was made that macrolide and tetracycline antibiotics decreased the inflammatory actions of the neutrophils which increased the life span. The third explanation was that neutrophil samples were kept in whole blood. In most other in-vitro studies the neutrophils were separated from the whole blood and tested. It is thought that cytokines, present in the whole blood, in combination with the antibacterial and anti-inflammatory effects the antibiotics, have increased the lifespan of the neutrophils.[42]
Methods
This study was built on previous research that collected information regarding the use of concomitant medications in clozapine recipients at a state psychiatric facility at any point from January 1, 2004, until June 30, 2011.[43] Medications were placed into distinct groups based on approved use and mechanism of action. Pearson correlation coefficients were utilized in order to determine whether or not increased use of medications within a given class were associated with the presence of an adverse drug reaction (ADR) involving blood dyscrasias in clozapine recipients. The correlation coefficient was found to be 0.409 (p<0.01), indicating that a statistically significant relationship existed between the use of systemic antibiotics and alterations in hematologic parameters.
In order to evaluate the relationship further, we performed a one-year pilot study to assess hematologic trends related to the prescribing of antibiotics in all clozapine patients, not just the patients who had a reported ADR. A retrospective chart review of open and closed medical records was performed for all eligible research inpatient recipients of both clozapine and systemic antibiotic therapy at the state psychiatric center between June 30, 2010, and June 30, 2011. The following information was obtained:
• Baseline ANC/WBC prior to the initiation of the antibiotic regimen
• Category of antibiotic prescribed
• All subsequent ANC/WBC values collected up to 50 days post-discontinuation of the antibiotic, including the nadir. If no blood counts were obtained within 50 days of therapy, then the most recent values were evaluated.
Antibiotic regimens were excluded from analysis if one or more of the subsequent criteria were met:
• Total duration of therapy less than three days
• Most recent ANC/WBC values were obtained more than six months after the antibiotic regimen was completed
• Missing ANC/WBC data
While the normalization of neutrophil release after infection is expected to occur beginning at approximately seven days after infection, this frame of reference has not been established in a clozapine population.[10] In situations where drug-induced blood dyscrasias have occurred, reestablishment of granulopoiesis and resolution of neutropenia may not occur until at least 25 days after onset.[2] We therefore determined that an analysis of ANC/WBC 50 days after the end of antibiotic therapy would be an appropriate endpoint for analysis.
Results
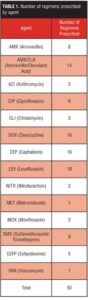
This protocol was approved by the Institutional Review Board of record. A total of 42 patients prescribed 93 antibiotic regimens were found to meet all of the above requirements (Table 1). Each regimen was then classified by specific agent as well as whether the final ANC/WBC was above or below the baseline established for each patient (Tables 2–8).
Discussion
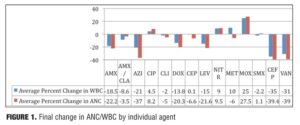
This study reports the preliminary findings of a one-year pilot study of clozapine patients concomitantly prescribed various antibiotic regimens. Of the individual antibiotic regimens, 28/93 (30%) of the final WBC values were within + 10 percent of the baseline, as compared to 21/93 (33%) of ANC values (Figure 1). A total of 14 discrete antibiotics were utilized within the time period identified. When evaluated against the patient’s pre-antibiotic baseline, 11/14 (79%) of the prescribed antibiotics were associated with reductions in ANC and/or WBC that remained 50 days after the antibiotic regimen was completed. Differences observed between classes may be a function of the heterogeneity in the pharmacodynamic properties of the various antimicrobials. Azithromycin (n=2) was the only macrolide antibiotic used by the facility and thus included in our data. On average, individuals prescribed azithromycin were noted to have a 37-percent decrease in ANC (range: -28 to -46%) and a 21-percent decrease in WBC (range: -25 to-17%) when compared to baseline. The reductions in hematologic parameters found in our study differ from results observed in previous studies. Therefore, while our preliminary data indicates that individuals prescribed clozapine may not retain the positive benefits of azithromycin on hematologic parameters which have been seen elsewhere, the relevance of our findings may be limited by the small sample size.
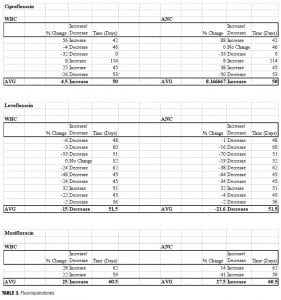
While the fluoroquinolone antibiotics ciprofloxacin and moxifloxacin have been studied in neutropenic patients, no previous studies have evaluated the use of these agents to increase cell counts in clozapine recipients. Three fluoroquinolones were included in our study, and distinct differences in ANC/WBC values were noted within this class. Both patients prescribed moxifloxacin displayed increases in ANC and WBC values (ANC: +14%, +41%; WBC: +22%, +28%), which were sustained at 60 days postcompletion of the antibiotic regimen. Similarly, individuals prescribed ciprofloxacin (n=6) experienced an overall increase in both values. There was an 8.2-percent increase in ANC (range:
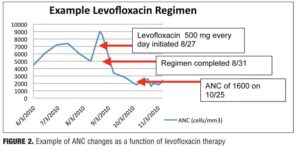
-50 to +88%) and a 4.5-percent increase in WBC (range: -32 to +56%). Levofloxacin, however, produced net decreases in ANC and WBC, which persisted upon discontinuation (Figure 2). The average ANC was found to be 15 percent decreased from baseline (range: -70 to +32%) while the average reduction in WBC was 22 percent (range: -53 to +32%).
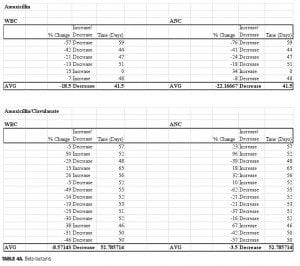
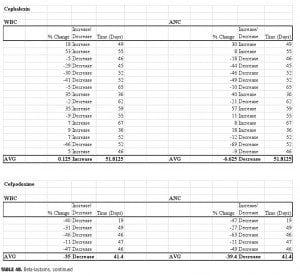
Four beta-lactam antibiotics were included in this study: two penicillins and two cephalosporins. As expected, individuals prescribed the cephalosporin agent cefpodoxime (n=5) displayed the greatest reductions of any beta-lactam agent. There was an average 39-percent decrease in ANC (range: -63 to -11%) and a 35-percent decrease in WBC (range: -47 to -11%) when compared to baseline. Amoxicillin (n=6) was also associated with profound changes; average ANC was found to be reduced by 22 percent (range: -76 to +34%) while WBC decreased by 18.5 percent (range: -57 to +7%) amoxicillin/clavulanate (n=14), however, did not result in major changes 50 days-post discontinuation. The ANC was found to be reduced by 3.5 percent on average (range: -62 to +96%), while the WBC decreased 8.6 percent (range: -49 to +50%). It is possible that the beta-lactamase inhibitor clavulanate may have a role in suppressing some of the negative effects on ANC/WBC produced by amoxicillin. If the process is dependent on the potency of the antibiotic used, then differences may also be explained by the varying doses within the specific agent used. Amoxicillin was given three times daily at either 250mg or 500mg per dose, while the strength of amoxicillin in the combination with clavulanate was 500mg or 875mg dosed twice daily. Our results were not separated by dose of the amoxicillin component. Therefore, it is possible that individuals prescribed amoxicillin received a higher total daily dose as compared to those prescribed amoxicillin/clavulanate.
Surprisingly, our study found that cephalexin (n=16) did not result in the substantial ANC or WBC reductions seen with cefpodoxime. On average, ANC remained 6.6 percent below baseline at 52 days after the antibiotic regimen was completed (range -69 to +57%), while WBC essentially returned to baseline (average 0.1% increase, range: -46 to +53%).
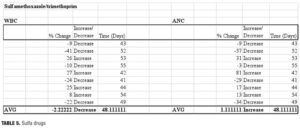
Our data showed that patients prescribed sulfamethoxazole/ trimethoprim (n=9) experienced minimal change from baseline. At 48 days postantibiotic, ANC values had returned to previous levels (average 1.1% decrease, range -57 to +81%). Changes in WBC counts were also similar to baseline (average 2.2% increase, range -41 to +27%). As with other classes of antibiotics, the changes in ANC/WBC varied to a great extent.
We did not differentiate between strengths of the trimethoprim component, which may account for some of the variance. In colonies deprived of folic acid or its derivatives, granulocyte production decreased in correlation with increased concentrations of trimethoprim. When folinic acid was added to a patient’s regimen, the rates of colony formation increased. Therefore, some of the variance may also be related to differences in folate levels and dietary requirements among our patient population.
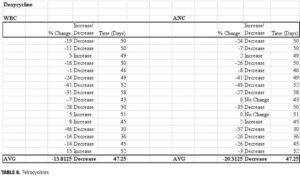
Our findings indicate a delay in recovery of ANC and WBC, with reductions in both ANC and WBC noted at 47 days after the antibiotic regimen was completed. While the number of individuals prescribed doxycycline regimens (n=16) was larger than with other types of antibiotics, there remained a degree of variance between baseline and final hematologic parameters. While the average reduction in ANC was 20.3 percent (range: -57 to +2%), the corresponding decline in WBC was only 13.8 percent (range: -46 to +15%).
The authors enrolled one patient who was prescribed vancomycin within the past year. Reductions in ANC and WBC at 50 days postantibiotic discontinuation were 39 percent and 31 percent, respectively. Our study found that patients prescribed clindamycin, metronidazole, or nitrofurantoin were found to have ANC/WBC values that returned within 10 percent of baseline after discontinuation of the antibiotic agent. The relevance of our data is limited, however, by the limited use of these agents within our facility.
Conclusions
While it is not unexpected that antibiotics may reduce WBC/ANC values during the infectious process, the tendency for these alterations in ANC/WBC to persist months after the completion of the antimicrobial regimen has not been revealed elsewhere in the literature. Therefore, we present evidence that antibiotic regimens may potentiate the blood dyscrasias associated with clozapine. The combination of the two also appears to either slow the return to baseline or facilitate the establishment of a new baseline. This may be validated though an extension of the endpoint beyond 50 days.
Our pilot study indicates a high degree of variability within classes of medication, thus picking an agent with less impact on the WBC baseline would be beneficial to patients who are on clozapine therapy. The use of either ciprofloxacin or moxifloxacin agent may be beneficial in avoiding the reductions in ANC/WBC seen with penicillins, cephalosporins, and other antibiotics and may prevent interruption or discontinuation of clozapine therapy.
If sustained hematologic changes are the result of antibiotic regimens prescribed to an individual who has improved with clozapine therapy and not solely to the clozapine therapy, then the discontinuation of clozapine may not provide any benefit in resolving these changes. Rather, it may worsen psychiatric outcomes.
While these data are certainly promising, larger studies will be required in order to obtain more definitive results. Our study lacked a control population, as we did not have access to patients prescribed antibiotics but not clozapine. Instead, the literature was searched for comparison. We also included data from combination therapies of multiple antibiotics over a similar time course. This may magnify changes in ANC/WBC beyond what would be present if the agents were assessed individually. Other factors such as gender, race, and the presence of comorbid inflammatory diseases may also have a role in reducing white cell counts. Additionally, our findings are limited by their retrospective nature as well as the small sample size.
In order to evaluate a greater population, we will expand upon the current research by examining antibiotic therapies prescribed over a greater period of time, as well as through direct comparison to the hematologic profiles of individuals prescribed systemic antibiotic therapy in the absence of concomitant clozapine therapy.
Acknowledgment
The authors wish to thank Phil Basko and Josephine Cheuk for their kind editorial assistance with this article.
References
1. Demler TL, Trigoboff E. Are clozapine blood dyscrasias associated with concomitant medications? Innov Clin Neurosci. 2011;8:35–41.
2. Raja M, Azzoni A, Maisto G. Late onset neutropenia associated with clozapine. J Clin Psychopharmacol. 2011;31:780–781.
3. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2011.
4. Andersohn F, Konzen C, Garbe E. Systemic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657–665.
5. Paul W. Fundamental Immunology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
6. Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. Int J Lab Hematol. 2010;32(6 Pt 2):590–597.
7. Schwartzberg LS. Neutropenia: etiology and pathogenesis. Clinical Cornerstone 2006;8(Suppl 5):S5–S11.
8. Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58(3):705–715.
9. Takumi K, Garssen J, de Jonge R, et al. Release kinetics and cell trafficking in relation to bacterial growth explain the time course of blood neutrophils and monocytes during primary Salmonella infection. Int Immunol. 2005;17(1):85–93.
10. Parham P. The Immune System, Third Edition. New York, NY: Garland Science; 2009:506.
11. Marsh JC, Boggs D, Cartwright E, Wintrobe M. Neutrophil kinetics in acute infection. J Clin Invest. 1967;46:1943–1953.
12. Price TH, Lee M, Dale D, Finch C. Neutrophil kinetics in chronic neutropenia. Blood.1979;54:581–594.
13. Altenburg J, de Graaf C, van der Werf T, Boersma W. Immunomodulatory effects of macrolide antibiotics. part 1: biological mechanisms. Respiration. 2011;81:67–74.
14. Koch CC, Esteban D, Chin A, et al. Apoptosis, oxidative metabolism and interleukin-8 production in human human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J Antimicrob Chemother. 2000;46:19–26.
15. Anderson R, Tintinger G, Cockeran R, et al. Beneficial and harmful interactions of antibiotics with microbial pathogens and the host innate immune system. Pharmaceuticals. 2010;3(5):1694–1710.
16. Bhatt V, Saleem A., Drug-induced neutropenia: pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34(2):131–137.
17. Neftel KA, Simon PH, Müller MR. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J Infec Dis. 1985;152(1):90–98.
18. Scheetz MH, McKoy J, Parada J, et al. Systematic review of piperacillin-induced neutropenia. Drug Safety. 2007;30(4):295–306.
19. Hsiao S-H, Chang C, Tsai J, et al. Glycopeptide-induced neutropenia: cross-reactivity between vancomycin and teicoplanin. Ann Pharmacother. 2007;41(5):891–894.
20. Pai MP, Mercier RC, Koster SA. Epidemiology of vancomycin-induced neutropenia in patients receiving home intravenous infusion therapy. Ann Pharmacother. 2006;40(2):224–228.
21. Culic O, Erakovic V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;50(3):277–289.
22. Sharma S, Jaffe A, Dixon G. Immunomodulatory effects of macrolide antibiotics in respiratory disease: therapeutic implications for asthma and cystic fibrosis. Paediatr Drugs. 2007;9(2):107–118.
23. Tauber SC. Immunomodulatory properties of antibiotics. Curr Molecul Pharmacol. 2008;1(1):68–79.
24. Koch CC, Estaban D, Chin A, et al . Apoptosis, oxidative metabolism and interleukin-8 production in human human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J Antimicrob Chemother. 2000;46:19–26.
25. Aoshiba K, Nagai A, Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob Agents Chemother. 1995;39(4):872–877.
26. Shalit I, et al. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob Agents Chemother. .2002;46:2442–2449.
27. Shalit I, Kletter Y, Weiss K, et al. Enhanced hematopoiesis in sublethally irradiated mice treated with various quinolones. Eur J Haematol. 1997;58:92–98.
28. Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–371.
29. Shalit I, et al. Immunomodulatory effects of moxifloxacin in comparison to ciprofloxacin and G-CSF in a murine model of cyclophosphamide-induced leukopenia. Eur J Haematol. 2001:;66: 287–296.
30. Ariza A, Montañez MI, Pérez-Sala D. Proteomics in immunological reactions to drugs. Curr Opin Aller Clinic Immunol. 2011;11(4):305–312.
31. Martin SF. T lymphocyte-mediated immune responses to chemical haptens and metal ions: implications for allergic and autoimmune disease. Int Arch Aller Immunol. 2004;134(3):186–198.
32. Weitzman S, Stossel T. Drug-induced immunological neutropenia. Lancet. 1978;311(8073):1068–1072.
33. Carey PJ. Drug-induced myelosuppression diagnosis and management. Drug Safety. 2003;26:691–706.
34. Bjornson BH, McIntyre A, Harvey J, Tauber A. et al. Studies of the effects of trimethoprim and sulfamethoxazole on human granulopoiesis. Am J Hematol. 1986;23:1–7.
35. Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637.
36. Tjwa M, et al. Antibiotics may impair hematopoietic recovery after cytotoxic myeloablation. Blood.2009;113:1608-1609.
37. Apinantriyo, B., A. Lekhakula, and P. Rujirojindakul. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011; 16:50-53.
38. Van der Klauw, M.M., J.H.P. Wilson, and B.H.Ch. Stricker. Drug-induced agranulocytosis: 20 years of reporting in the Netherlands (1974-1994). American Journal of Hematology. 1998; 57:206-211.
39. Montserrat M, et al. Venlafaxin and/or clindamycin induced agranulocytosis: a case report. International Journal of Clinical Pharmacy. 2011; 33:290.
40. Pisciotta AV. Agranulocytosis during antibiotic therapy: drug sensitivity or sepsis? American Journal of Hematology. 1993; 42:132-137.
41. Khan, A.Y., M.H. Golewale, D.A. Kahn. A case of chronic drug-induced neutropenia. Journal of Psychiatric Practice. 2008; 14: 246-250.
42. Healy, D.P., et al., Effect of Antibiotics on Polymorphonuclear Neutrophil Apoptosis. Pharmacotherapy, 2002. 22(5): p. 578-585.
43. Shuman MD, Trigoboff E, Demler TL. Adverse Drug Reactions Involving Clozapine Recipients. Presented at ASHP Midyear Clinical Meeting; December 7, 2011; New Orleans, LA.