 by Katie T.B. Touma, PharmD, BCPP, BCPS; Allysa M. Zoucha, PharmD, BCPP; and Jonathan R. Scarff, MD
by Katie T.B. Touma, PharmD, BCPP, BCPS; Allysa M. Zoucha, PharmD, BCPP; and Jonathan R. Scarff, MD
Drs. Blissit and Zoucha are with William Jennings Bryan Dorn VA Medical Center, Anderson Community Based Outpatient Clinic, Anderson, South Carolina, and Dr. Scarff is with William Jennings Bryan Dorn VA Medical Center, Spartanburg Community Based Outpatient Clinic, Spartanburg, South Carolina.
Innov Clin Neurosci. 2017;14(3–4):24–29.
Funding: No funding was received for the preparation of this article.
Financial disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: Triiodothyronine, liothyronine, T3, treatment-resistant depression, augmentation, enhancement
Abstract: Objective: The objective of this review is to discuss triiodothyronine’s (T3, liothyronine) mechanism of action, efficacy in enhancement and augmentation trials, and dosing and safety considerations for the treatment of depression.
Method: A literature search of PubMed was performed using search terms depression, augmentation, antidepressant, and liothyronine. Only English-language studies of subjects with unipolar depression were included from the past 50 years.
Results: Most studies have shown that liothyronine is an efficacious enhancement and augmentation strategy for depression in combination with antidepressants, primarily tricyclic antidepressants and selective serotonin reuptake inhibitors.
Conclusion: With appropriate baseline and follow-up safety monitoring, liothyronine augmentation can be a safe and effective treatment for unipolar depression. Larger studies of longer duration assessing liothyronine efficacy with serotonin norepinephrine reuptake inhibitors and multimodal antidepressants are needed.
Introduction
Major depressive disorder (MDD) has a 12-month prevalence of 6.7 percent and a lifetime prevalence of 16.9 percent.[1,2] Treatment-resistant depression is defined as lack of response after trials of two or more antidepressants with different mechanisms of action for sufficient duration.[3] Such antidepressants include tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), monoamine oxidase inhibitors (MAOI), bupropion, mirtazapine, and trazodone. Current guidelines suggest augmentation of antidepressants for individuals with treatment-resistant unipolar depression.[4,5] There are several augmenting agents, one of which is the thyroid hormone triiodothyronine (T3 or liothyronine). This review discusses T3’s mechanism of action, efficacy in enhancement and augmentation trials, and dosing and safety considerations.
Mechanism of Action
Thyroid hormone is postulated to exert antidepressant efficacy through multiple mechanisms. Depression is associated with neuronal death, so it follows that decreasing neuronal stress, atrophy, and death would be associated with an antidepressant effect. T3 has been shown to increase gene expression by increasing levels of thyrotropin-releasing hormone, corticotrophin releasing factor, and brain derived neurotrophic factor.[6–8] There are changes in sensitivity and transcription of serotonin (5-HT) receptors and possibly a net increase in serotonin signaling after T3 administration.[9,10] Administration of T3 has also led to increased basal 5-HT levels in the frontal cortex.[11] There is a possible but unclear association between T3 and adrenergic transmission and activity of second-messenger systems.[10] It has not been demonstrated to increase concentrations of monoamine metabolites, even in patients whose depression improved after treatment with T3.[12]
Methods
Ovid Medline and PubMed databases were used to search for original studies, reviews, and meta-analyses in English published in journals or books from 1966 to June 2016 using search terms depression, augmentation, antidepressant, and liothyronine. We included open-label studies, randomized, placebo-controlled trials, meta-analyses, case reports, and case series; however, we focused on randomized, placebo-controlled or open-label trials given their higher evidence base compared to case reports or case series. Our searches yielded a total of 26 articles: six open-label trials with TCAs, nine randomized trials with TCAs, three open-label reports with SSRIs, three randomized studies with SSRIs, four reports utilizing other antidepressants, and one meta-analysis.
TCAs and T3
The use of T3 in combination with TCAs for the treatment of MDD has been evaluated as a method to enhance or accelerate a treatment response (both agents initiated simultaneously) or as augmentation after an adequate trial of TCA monotherapy. Controlled trials rated depression severity using the Hamilton Rating Scale for Depression (HRSD)[13] and defined response to treatment as a 50 percent or greater reduction in HRSD score. A meta-analysis of six placebo-controlled, double-blind studies evaluating the effects of T3 enhancement found T3 to be significantly more effective compared to placebo, and that the effects of T3 enhancement were greater with the larger percentage of women subjects.[14] In the individual studies, doses of T3 ranged from 25mcg daily to 62.5mcg daily; however, some T3 toxicity was noted after a few days of 62.5mcg daily dose. Four of these six trials found a significantly quicker time to response and/or greater response rate with T3 enhancement.[15–20] Additional details of the individual enhancement studies are noted in Table 1.
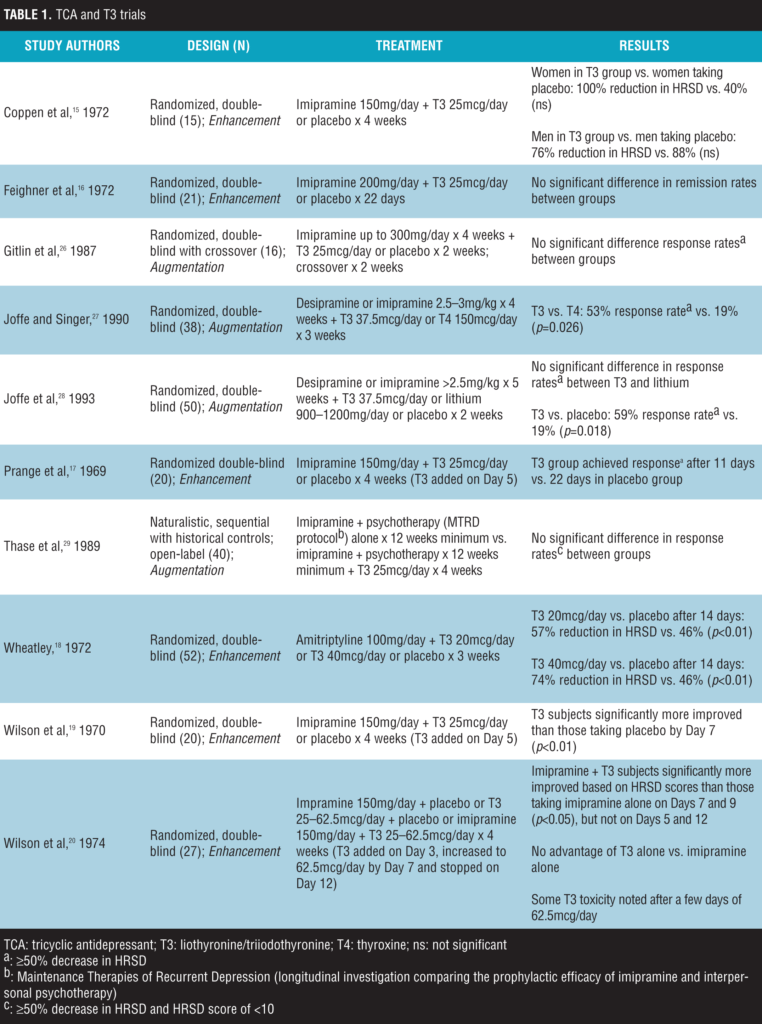
In regard to augmentation studies, uncontrolled, open-label trials in outpatients have suggested that T3 is an efficacious augmentation strategy as well with response rates of greater than 50 percent.[21–25] A small number of controlled trials in subjects without a full response to adequate trials of TCAs for the treatment of unipolar depression followed these previous findings (Table 1).[26–29] Doses of T3 ranged from 25mcg daily to 37.5mcg daily; however, only the 37.5mcg daily dose showed greater response rates compared to controls. Two of these four trials found a significantly greater response rate with T3 augmentation. Additional details of controlled augmentation studies are noted in Table 1.
SSRIs and T3
The combination of SSRIs and T3 has also been studied in both open-label and randomized, placebo-controlled trials either as enhancement or as augmentation (Table 2). An enhancement study demonstrated a response rate of 70 percent when T3 was initiated with sertraline in patients with non-treatment-resistant MDD compared with a 50-percent response in participants that received sertraline alone; improvement in HRSD by 50 percent or greater was considered a response.[30] This study utilized a maximum dose of 100mg/day for the sertraline group, and thus possible underdosing of this SSRI may have occurred. Two additional enhancement studies did not find a significant difference in response rates when T3 was added to paroxetine or sertraline compared to paroxetine or sertraline monotherapy.[31,32]
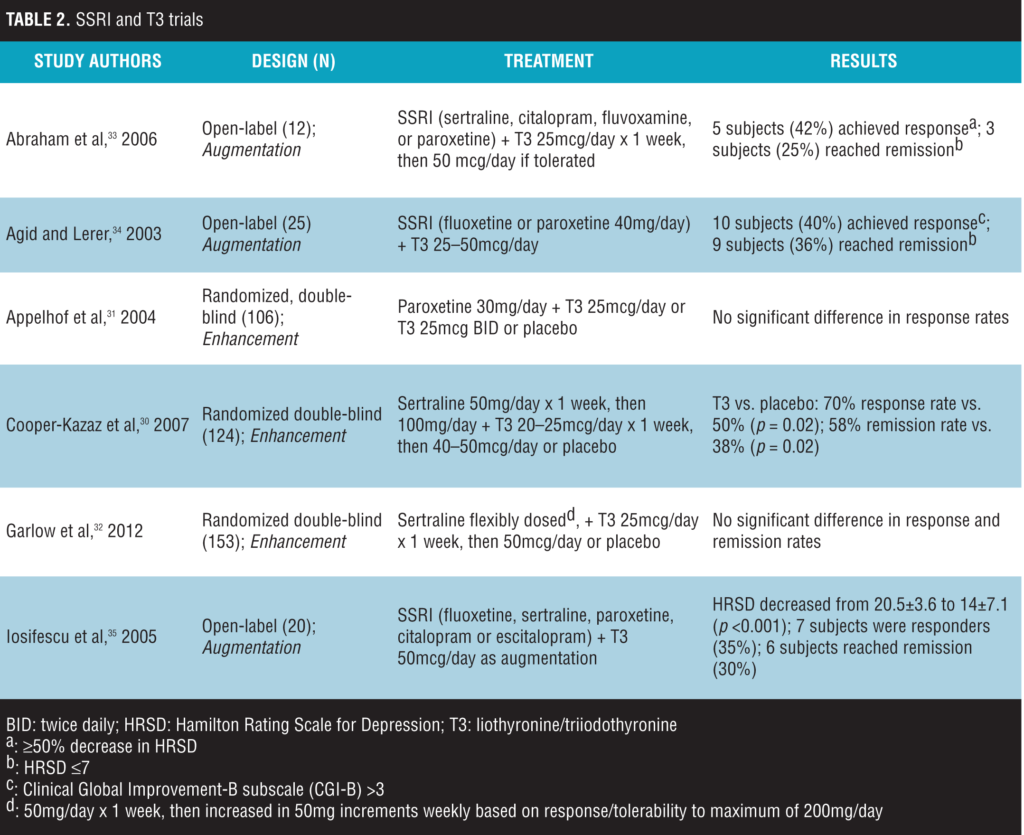
In three augmentation studies, T3 doses of 25- to 50mcg/day led to response and remission in patients that had an inadequate response to SSRI treatment.[33–35] However, the studies utilized an uncontrolled, open-label design, which does not adequately evaluate the true efficacy of T3 augmentation. This highlights the need for randomized, placebo-controlled trials.
Other Antidepressants and T3
The Sequenced Alternatives to Relieve Depression (STAR*D) study[36] compared T3 to lithium for augmentation in patients receiving sustained-release bupropion or extended-release venlafaxine. Subjects were randomly assigned to T3 25mcg/day for one week then increased to 50mcg/day (mean exit dose 45.2mcg/day), compared to lithium up to 900mg/day for 14 weeks. Approximately 23 percent of subjects who received T3 augmentation achieved a response, and close to 25 percent of the subjects received remission. Overall, T3 was better tolerated than lithium as an augmentation strategy.
The same year the STAR*D trial was published, Joffe et al[27] also reported data comparing T3 and lithium augmentation in a small randomized, double-blind, placebo-controlled study. All subjects (N=36) had received a trial of an antidepressant for at least five weeks and received augmentation with T3 37.5mcg/day, lithium up to 900mg/day, T3 plus lithium, or placebo for two weeks. Antidepressants other than SSRIs and TCAs included moclobamide 600- to 750mg/day, nefazodone 150- to 300mg/day, or venlafaxine 187.5- to 375mg/day. No significant difference was found in change in HDRS scores between groups. However, it is difficult to determine the efficacy of T3 with these antidepressants, as a majority of the subjects were receiving SSRIs, not other antidepressants. All subjects that received T3 alone as augmentation were taking SSRIs, and more than 60 percent of the subjects that received T3 plus lithium or placebo were taking SSRIs.
The addition of T3 to the MAO-I phenelzine has been described in three case reports. The first case report noted significant improvement in depressive symptoms after augmenting with T3 titrated up to 30mcg/day in a subject who had received phenelzine for at least four weeks prior.[38] The two latter cases described the efficacy of enhancement of the MAO-I effect with T3. These two subjects had trials of TCA monotherapy and TCA in combination with lithium or TCA in combination with T3 prior to receiving phenelzine in combination
with T3.[39]
Treatment and Monitoring
Laboratory studies and frequency of monitoring are detailed in Table 3. We recommend deferring the administration of T3 to a patient with abnormally high or low thyroid-stimulating hormone (TSH) level for further evaluation. However, low or high free T3 or free T4 levels in the context of a normal TSH does not preclude treatment with T3, as these elevations or decreases may be physiological or due to concurrent medications. Prior to prescribing T3, the patient and clinician should discuss and document the risk-benefit profile of T3, including risk for arrhythmia and osteoporosis. Throughout treatment the risks and benefits of T3 should be re-evaluated, focusing on depressive symptoms or cardiovascular status. If efficacy has been demonstrated and there are no symptoms of hyperthyroidism and no known cardiac disease, consider maintenance T3 supplementation even if the TSH level is below the normal reference range.[40]
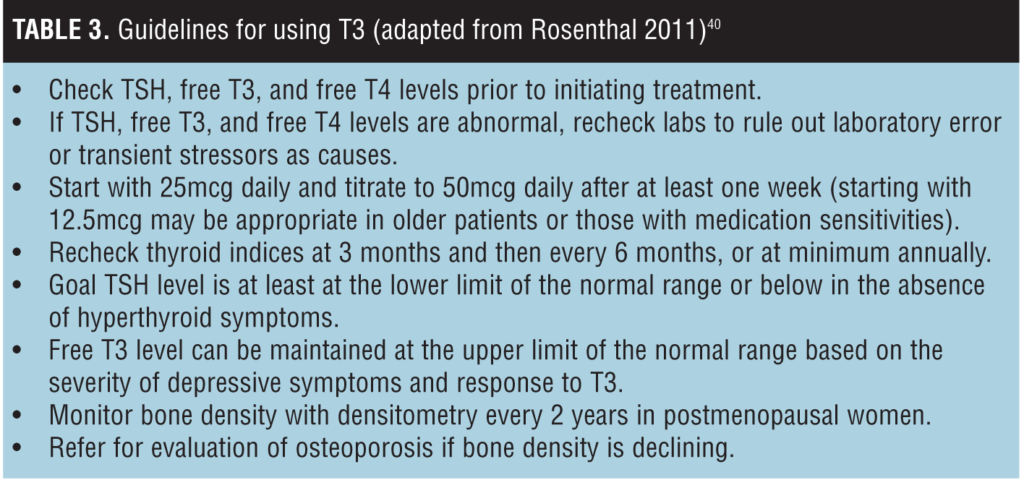
Doses above 50mcg daily and long-term therapy with T3 may be reasonable if the patient has a history of multiple depressive episodes or significant treatment resistance, despite limited data.[40] In an observational study of 14 patients, no subjects developed any cardiac or skeletal disease after receiving doses from 25- to 150mcg over a two-year period.[41] In pre- and postmenopausal women receiving T4 (levothyroxine), there was no clinically significant difference in bone mineral density between groups after one year, but bone density did decrease in post-menopausal women compared to the population standard.[42]
conclusion
Most trials that evaluated T3 enhancement or augmentation were published earlier than 10 years ago using small samples of patients, and there are limited data available for liothyronine’s efficacy with newer antidepressants. The majority of reports have focused on the use of T3 in combination with TCAs and have yielded mixed results. Larger studies of longer duration are needed to evaluate T3’s efficacy with newer and other classes of antidepressants. Despite these limitations, T3 augmentation appears to be a safe and effective alternative treatment for euthyroid patients with unipolar depression who receive appropriate baseline and follow-up safety monitoring.
References
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627.
- Harvard Medical School site. National Comorbidity Survey-Replication (NCS-R). http://www.hcp.med.harvard.edu/ ncs/. Accessed 31 March 2016.
- McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7.
- American Psychiatric Association site. Practice Guideline for Treatment of Patients with Major Depressive Disorder, Third Edition. http://psychiatryonline.org/guidelines November 2010. Accessed 1 June 2016.
- Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III: pharmacotherapy. J Affect Disord. 2009;117(Suppl 1):S26–S43.
- Segerson TP, Kauer J, Wolfe HC, et al. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science. 1987 2;238(4823):78–80.
- Ceccatelli S, Giardino L, Calzá L. Response of hypothalamic peptide mRNAs to thyroidectomy. Neuroendocrinology. 1992;56(5):694–703.
- Giardino L, Ceccatelli S, Zanni M, et al. Regulation of VIP mRNA expression by thyroid hormone in different brain areas of adult rat. Brain Res Mol Brain Res. 1994;27(1):87–94.
- Lifschytz T, Segman R, Shalom G, et al. Basic mechanisms of augmentation of antidepressant effects with thyroid hormone. Curr Drug Targets. 2006;7(2):203–210.
- Newman ME, Agid O, Gur E, Lerer B. Pharmacological mechanisms of T3 augmentation of antidepressant action. Int J Neuropsychopharmacol. 2000;3(2):187–191.
- Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther. 1999;288(1):81–87.
- Banki CM. Cerebrospinal fluid amine metabolites after combined amitriptyline-triiodothyronine treatment of depressed women. Eur J Clin Pharmacol. 1977;11:311–315.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
- Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? a review and metaanalysis of the literature. Am J Psychiatry. 2001;158:1617–1622.
- Coppen A, Whybrow P, Noguera R, et al. The comparative antidepressant value of L-tryptophan and imipramine with and without attempted potentiation by liothyronine. Arch Gen Psychiatry. 1972;26:234–241.
- Feighner JP, King LJ, Schuckit MA, et al. Hormonal potentiation of imipramine and ECT in primary depression. Am J Psychiatry. 1972;128:1230–1238.
- Prange AJ Jr,Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry. 1969;126(4):457–469.
- Wheatley D. Potentiation of amitriptyline by thyroid hormone. Arch Gen Psychiatry. 1972;26(3):229–233.
- Wilson IC, Prange AJ, McClane TK, et al. Thyroid-hormone enhancement of imipramine in nonretarded depressions. N Engl J Med. 1970;282:1063–1067.
- Wilson IC, Prange AJ Jr, Lara PP. L-triiodothyronine alone and with imipramine in the treatment of depressed women. In: Prange AJ Jr (ed). The Thyroid Axis, Drugs, and Behavior. New York, Raven Press; 1974:49–62.
- Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry. 1970;126:1667–1669.
- Ogura C, Okuma T, Uchida Y, et al. Combined thyroid (triiodothyronine)-tricyclic antidepressive treatment in depressive states. Folia Psychiatr Neurol Jpn. 1974;28:179–186.
- Tsutsui S, Yamazaki Y, Namba T, Tsushima M. Combined therapy of T3 and antidepressants in depression. J Int Med Res. 1979;7:138–146.
- Schwarcz G, Halaris A, Baxter L, et al. Normal thyroid function in desipramine nonresponders converted to responders by the addition of L-triiodothyronine. Am J Psychiatry. 1984;141:1614–1616.
- Nakamura T, Nomura J. Comparison of thyroid function between responders and nonresponders to thyroid hormone supplementation in depression. Jpn J Psychiatry Neurol. 1992;46:905–909.
- Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord. 1987;13:267–272.
- Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res. 1990;32:241–251.
- Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in uni-polar refractory depression. Arch Gen Psychiatry. 1993;50:387–393.
- Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression, I: an open clinical trial of adjunctive L-triiodothyronine. J Clin Psychiatry. 1989;50:385–388.
- Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007; 64:679–688.
- Appelhof BC, Brouwer JP, van DR, et al. Triiodothyronine addition to paroxetine in the treatment of major depressive disorder. J Clin Endocrinol Metab. 2004;89:6271–6276.
- Garlow SJ, Dunlop BW, Ninan PT, Nemeroff CB. The combination of triiodothyronine (T3) and sertraline is not superior to sertraline monotherapy in the treatment of major depressive disorder. J Psychiatr Res. 2012;46(11):1406–1413.
- Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord. 2006;91:211–215.
- Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol. 2003;6(1):41–49.
- Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry. 2005;66:1038–1042.
- Nierenberg AA, Fava M, Trivedi MH, et al, STAR*D Study Team. A comparison of lithium and T3 augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–1530.
- Joffe RT, Sokolov ST, Levitt AJ. Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry. 2006;51(12):791–793.
- Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318–320.
- Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409–410.
- Rosenthal LJ, Goldner WS, O’Reardon JP. T3 augmentation in major depressive disorder: safety considerations. Am J Psychiatry. 2011;168(10):1035–1040.
- Kelly TF, Lieberman DZ. Long term augmentation with T3 in refractory major depression. J Affect Disord. 2009;115:230–233.
- Gyulai L, Bauer M, Garcia-Espana F, et al. Bone mineral density in pre-and post-menopausal women with affective disorder treated with long-term l-thyroxine augmentation. J Affect Disord. 2001;66:185–191.





