 by Steven D. Targum, MD; Christopher Murphy, PhD; Jibran Khan, BA; Laura Zumpano, BA; Mark Whitlock, PhD; Arthur A. Simen, MD, PhD; and Brendon Binneman, MD†
by Steven D. Targum, MD; Christopher Murphy, PhD; Jibran Khan, BA; Laura Zumpano, BA; Mark Whitlock, PhD; Arthur A. Simen, MD, PhD; and Brendon Binneman, MD†
Drs. Targum and Murphy are with Bracket Global LLC in Boston, Massachusetts. Dr. Khan was with Bracket Global LLC in Boston, Massachusetts, at the time of this study. Ms. Zumpano is with Pfizer Inc. in New York, United States, and Dr. Whitlock is with Pfizer Inc. in Cambridge, United Kingdom. Dr. Simen was with Pfizer Inc. at the time of the study but is now with Takeda Pharmaceuticals in Cambridge, Massachusetts. Dr. Binneman was with Pfizer Inc. in Cambridge, Massachusetts, at the time of this study (†deceased).
Innov Clin Neurosci. 2018;15(3–4):37–42
Funding: Pfizer sponsored this study. Support for this study came from the sponsor Pfizer (Cambridge, Massachusetts) to conduct quality assurance in a clinical trial with additional support for the data analysis from Bracket Global LLC and Clintara LLC (Boston, Massachusetts).
Disclosures: Dr. Targum is an employee of Bracket LLC and has received vendor grants, retainers, or honoraria from Acadia Pharmaceuticals, Alkermes Inc., Functional Neuromodulation Inc., Intracellular Therapeutics, Janssen Research & Development, LLC, Karuna, Methylation Sciences Inc., Navitor Pharmaceuticals, Neurim Pharmaceuticals, Pfizer Inc., Prana Biotechnology Ltd., Resilience Therapeutics, and Sunovion. Dr. Murphy is an employee and Mr. Khan was an employee of Bracket Global LLC at the time of this study and have no other disclosures. Drs. Binneman and Simen, Mr. Whitlock, and Ms. Zumpano are employees of Pfizer Inc. and have no other disclosures relevant to the content of this article.
Abstract: Objective. The assessment of patients with generalized anxiety disorder (GAD) to deteremine whether a medication intervention is necessary is not always clear and might benefit from a second opinion. However, second opinions are time consuming, expensive, and not practical in most settings. We obtained independent, second opinion reviews of the primary clinician’s assessment via audio-digital recording.
Design. An audio-digital recording of key site-based assessments was used to generate site-independent “dual” reviews of the clinical presentation, symptom severity, and medication requirements of patients with GAD as part of the screening procedures for a clinical trial (ClinicalTrials.gov: NCT02310568).
Results. Site-independent reviewers affirmed the diagnosis, symptom severity metrics, and treatment requirements of 90 moderately ill patients with GAD. The patients endorsed excessive worry that was hard to control and essentially all six of the associated DSM-IV-TR anxiety symptoms. The Hamilton Rating Scale for Anxiety scores revealed moderately severe anxiety with a high Pearson’s correlation (r=0.852) between site-based and independent raters and minimal scoring discordance on each scale item. Based upon their independent reviews, these “second” opinions confirmed that these GAD patients warranted a new medication intervention. Thirty patients (33.3%) reported a previous history of a major depressive episode (MDE) and had significantly more depressive symptoms than patients without a history of MDE.
Conclusion. The audio-digital recording method provides a useful second opinion that can affirm the need for a different treatment intervention in these anxious patients. A second live assessment would have required additional clinic time and added patient burden. The audio-digital recording method is less burdensome than live second opinion assessments and might have utility in both research and clinical practice settings.
Keywords: Generalized anxiety disorder (GAD), anxiolytic medications, anxious symptoms, audio-digital recording, dual review, second opinions
Anxiety symptoms are prevalent in most populations where people experience the stress of modern life. A cluster of anxiety symptoms becomes an anxiety disorder when the symptoms are difficult to control, cause significant distress for the person, and impact the ability to function.1,2 It has been reported that patients who meet diagnostic criteria for generalized anxiety disorder (GAD) might also have substantial economic and social burden as a consequence of the disorder.3–7
There are several effective treatments for GAD, including psychotropic medications, complementary and alternative medicines, psychotherapy, cognitive-behavioral therapy, and/or other nonpharmacological strategies that can be given to address troubling and/or disruptive anxiety symptoms.6–10 The use of psychotropic medications for treating anxiety symptoms, including approved antidepressants, is sometimes challenged because other treatment alternatives might be effective and do not convey the same potential adverse effects. Furthermore, the clinical decision about whether to prescribe a medication intervention is not always clear in some patients and might rely more on personal bias than the clinical evidence. As a result, some clinicians might withhold anxiolytic medications from some patients who really need them, whereas others might prescribe medications indiscriminately to patients who do not.
A confirmatory assessment of treatment needs (a “second” opinion) is always a useful clinical tool, but the reality is that a second independent clinical assessment is rarely feasible in most busy clinical settings. A second opinion would be particularly helpful to assess the need for medications and/or to change treatments after an intervention has been initiated.
The digital revolution has abetted the introduction of innovative tools to facilitate site-independent second opinions. In the present study, we used a time-efficient audio-digital recording method to obtain clinical interviews and independently confirm the GAD diagnosis and that these patients actually warranted a change in anxiolytic medication. As part of a clinical trial site, independent reviewers listened to and scored key site-based recorded interviews that were conducted at the first (screening) visit of the patients to confirm the specific clinical characteristics and metrics that might delineate anxious patients who warranted a medication intervention. The audio review method added minimal burden to the assessment during the clinical trial and might have broader utility for clinical practice as well.
Methods
The audio-digital recording and “dual” review analysis of anxious patients was conducted as part of a clinical trial on GAD (ClinicalTrials.gov Identifier: NCT02310568) titled, “An 8-week, randomized, Phase 2, double-blind, sequential parallel-group comparison study of two dose levels of PF-06372865 compared to placebo as an adjunctive treatment in outpatients with inadequate response to standard of care for generalized anxiety.” The objective of the trial was to evaluate the anxiolytic efficacy of PF-06372865 based upon changes in the total score of the Hamilton Rating Scale for Anxiety (HAM-A) (1959) using the Structured Interview Guide for the Hamilton Anxiety Rating Scale (SIGH-A).11 The study was conducted at 47 clinical trial sites in the United States, of which 34 sites ultimately enrolled patients. The study was carried out in accordance to the code of ethics of the World Medical Association (Declaration of Helsinki). All potential patients considering the trial consented to participate and signed an IRB-approved consent form that described their participation in the trial and the possibility of receiving active medication or placebo prior to the conduct of any study procedures.
In this study, eligible patients needed to meet the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for GAD, as confirmed by the Mini International Neuropsychiatric Interview (MINI) at the screen interview as follows:1,12
- Excessive anxiety and worry (apprehensive expectation) regarding a number of events or activities (e.g., work or school performance) that occurs more days than not for at least six months
- Difficulty controlling the worry
- Anxiety and worry that are associated with three (or more) of the following six symptoms (with at least some symptoms present for more days than not for the past six months):
- Restlessness or feeling keyed up or on edge
- Being easily fatigued
- Difficulty concentrating or mind going blank
- Irritability
- Muscle tension
- Sleep disturbance (difficulty falling or staying asleep or restless, unsatisfying sleep)
- Focus of anxiety and worry that is not confined to features of another Axis I disorder or having a serious illness (as in hypochondriasis) and anxiety and worry that do not occur exclusively during posttraumatic stress disorder (PTSD)
- Anxiety, worry, or physical symptoms that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning
- Disturbance that is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition (e.g., hyperthyroidism) and does not occur exclusively during a mood or a psychotic disorder
The study excluded patients with current comorbid conditions that might obscure assessment of the experimental treatment intervention. Thus, patients with a diagnosis within the past six months of panic disorder with or without agoraphobia, PTSD, dissociative disorder, obsessive-compulsive disorder, attention deficit disorder, DSM-IV-TR defined substance abuse (within 3 months), or substance dependence (within 12 months) were not eligible. Furthermore, patients with a history of bipolar disorder or any psychotic or cognitive disorder, or who had comorbid personality disorders (Axis II) were not eligible for the study. A mid-study amendment allowed patients with a recent history of major depressive disorder (MDD) to participate, provided GAD was the primary disorder and their anxiety symptoms predominated over their depressive symptoms as measured by the Covi Anxiety Scale (CAS) and the Raskin Depression Scale (RDS).13,14 The amendment also permitted enrollment of patients with social phobia or specific phobias, provided the anxiety symptoms generated by these disorders were clinically less significant than those of the primary GAD disorder.
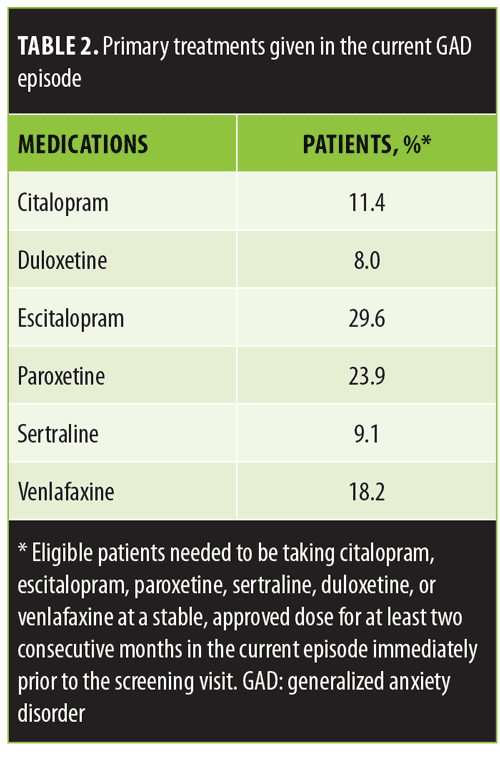
The study was designed for patients with GAD who required a change in medication because their current regimen was not effective. Hence, eligible patients needed to have had an inadequate response (<50% effective) to an adequate dose of an acceptable, approved anxiolytic medication at a stable, approved dose for at least two consecutive months in the current episode immediately prior to the screening visit. Acceptable medications initially included escitalopram (10–20mg total daily dose per day), paroxetine (20–50mg per day), duloxetine (60–120mg per day), and venlafaxine (75–225mg per day). The mid-study amendment added citalopram (20–40mg per day) and sertraline (50–100mg per day) to the acceptable list of medications.
Additional study eligibility criteria included being 18 to 65 years of age (inclusive) with a total SIGH-A score of 22 or greater, a CAS score of 9 or greater, and a RAS score of 7 or less at the screening visit to ensure predominance of anxiety symptoms over depression symptoms.11,13–15 The screening assessment also included a clinical and anxiolytic treatment history review, the Structured interview guide for the Clinical Global Impression of Severity (CGI-S), called the SIGGI, a patient self-rated generalized anxiety disorder 7-item scale (GAD-7), and completion of the Clinical-Validation Inventory for Study Admission (C-VISATM) for site-independent eligibility affirmation.16–19
There were 57 site-based raters and six site-independent reviewers/raters who successfully completed a rater training and qualification program that included an inter-rater reliability assessment and certification for the SIGH-A interview.
Site-independent “dual” reviews were employed at the screening visit as a quality assurance (surveillance) strategy to assure appropriate subject selection. The “dual” review included the C-VISATM to assure diagnostic accuracy, ratings precision, pharmacologic history, and documentation that the patient met the inclusion/exclusion criteria of the protocol. Furthermore, the MINI, SIGH-A, and CGI-S assessments were audio-digitally recorded at the screening visit using a commercially available pen-recording device and specially manufactured booklets that captured digital notes. All patients consented to the use of the audio-digital recordings as part of the screening visit. The recordings permitted a site-independent review and scoring of the key screen data by an independent quality assurance group (Clintara LLC, Boston, Massachusetts) that included diagnostic verification of GAD, assessment of current treatment response, and “dual” scoring of the site-based CGI-S and SIGH-A interviews.19,20 Dual scores that yielded greater than three-points difference (discordance) between the site-based raters and independent reviewers were re-scored by a second independent reviewer for confirmation as part of the quality assurance program. Rater remediation by telephone was provided whenever issues related to scoring discordance or insufficient (incomplete) interviews were identified,
Statistical analysis included demographic and clinical tabulation, Pearson’s product moment correlation, and Student’s t tests when appropriate.
Results
Data for this analysis was derived from 101 patients who were screened for study eligibility and completed audio-digitally recorded screen interviews for the MINI, CGI-S, and SIGH-A that were received between December 2014 and June 2015. Ninety reviewed patients were deemed eligible to enroll in the study.
Table 1 depicts the demographics of the enrolled population (n=90), presenting clinical metrics, and the DSM-IV-TR anxiety symptoms endorsed by the study-eligible patients with GAD. The patients were mostly female (74.4%), ranged in age from 20 to 64 years (mean age=41.2±13.7 standard deviation [SD]), and were generally obese (mean body mass index [BMI]=31.5±7.1 SD). Forty-seven of the 90 patients (52.2%) met World Health Organization (WHO) standards for obesity (BMI >30)22 and 10 subjects (12.2%) had a BMI greater than 40 (morbid obesity). The women had higher BMIs than the men (32.1±7.3 and 29.8±6.1 respectively), but this difference was not statistically significant.
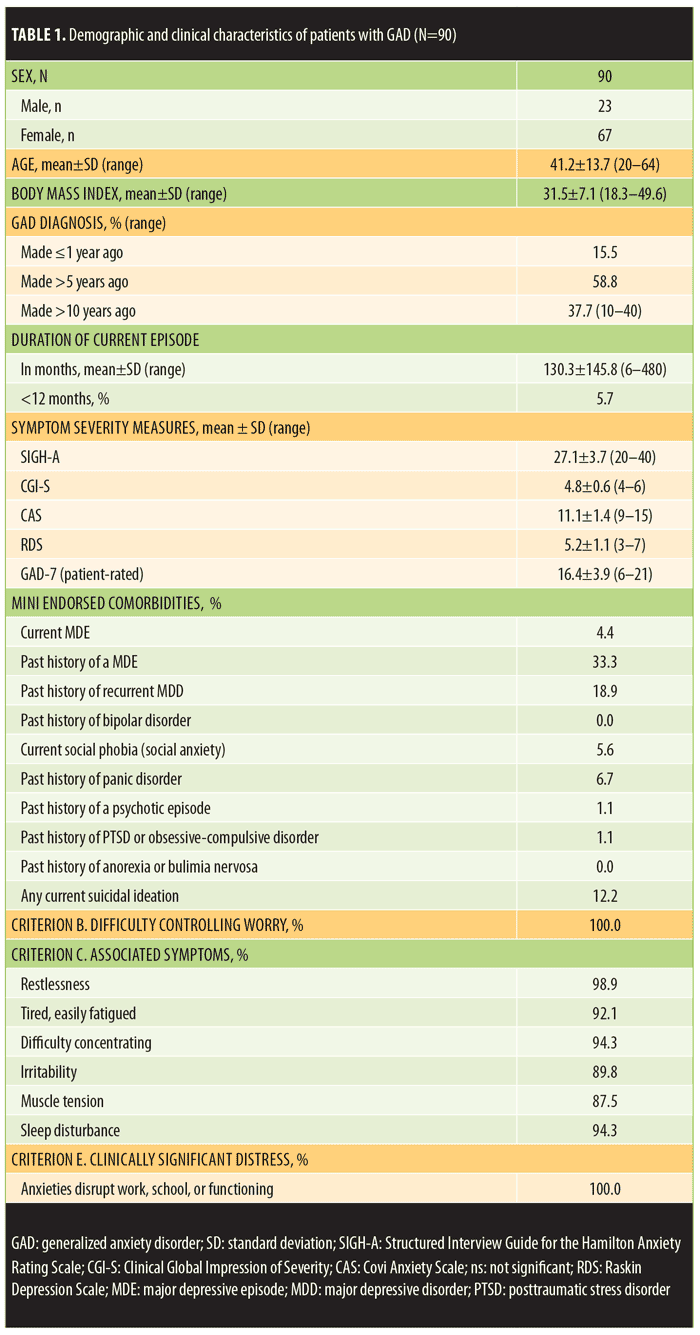
All 90 eligible patients reported an inadequate response (<50%) to their current anxiolytic medication despite taking an adequate dose for an adequate duration of time. Sixty-five patients (72.2%) reported achieving less than a 25-percent response to their ongoing anxiolytic medication. The most commonly prescribed medications were escitalopram, paroxetine, and venlafaxine (Table 2). Additionally, 10.6 percent of patients were taking a second medication (including a second antidepressant, alprazolam, clonazepam, buspirone, or gabapentin) at the time of the screening visit.
Diagnostic confirmation of GAD. Among the study participants, the original diagnosis of GAD had been made 1 to 40 years prior to the screening visit, and 37.7 percent of the enrolled patients had been ill for at least 10 years.
Patients with GAD entering this study endorsed virtually all of the six GAD symptoms listed on the DSM-IV-TR-associated symptom checklist in addition to 100-percent endorsement of persistent anxiety or worry that was hard to control and 100-percent significant clinical distress associated with these symptoms. The mean CGI-S score was 4.8±0.6 (SD), and 68.9 percent of the patients were scored a 5 or higher (moderately severe illness) at the screening visit. The site-independent reviewers affirmed these endorsements based on the MINI documentation and the level of clinical distress endorsed on the narrative description provided in the structured format of the CGI-S.
Eleven patients (10.9%) failed screening as a result of the site-independent review process based upon the submitted screening materials. Two patients had subthreshold SIGH-A scores, and one patient gave inconsistent responses that suggested poor reliability as a study candidate. Two patients were excluded because of a comorbid current diagnosis of panic disorder, and one because she had a binge eating disorder. Three patients had social phobia, and one patient had a current major depressive episode. These last four patients were excluded before the mid-study amendment that allowed these disorders.
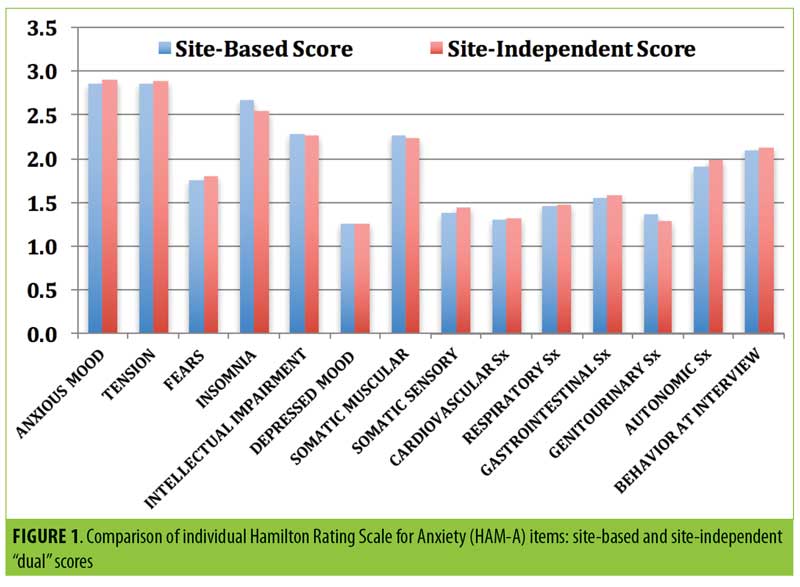
“Dual” scoring comparisons of SIGH-A scores. The mean site-based total SIGH-A score was 27.1±3.7 (SD). Fourteen patients scored the SIGH-A Item 1 (anxious mood) as a 2 (15.6%), and 76 patients scored it as a 3 (84.4%), reflecting moderate anxious symptoms within the past week. The mean site-independent total SIGH-A score (derived by listening to the audio-digital recordings of the site-based SIGH-A interviews) was 27.2±4.3 and was not significantly different than the site-based total mean SIGH-A score (t=0.42, p=0.67, 95% confidence interval [CI] for mean difference 0.1 [-0.37, 0.57]).
The mean absolute discordance (“dual” total SIGH-A scoring deviations between site-based and site-independent raters in either direction) was only 1.50±1.66, and the correlation coefficient was 0.852 (p<0.0001). Only eight of the paired SIGH-A scores (8.9%) were greater than three points deviant from each other, whereas 70 scores (77.8%) were within two points of each other. Rater remediation was provided for the eight discordant scores. Furthermore, there were no significant mean “dual” scoring differences on any of the 14 individual SIGH-A items between the site-based and site-independent scores (Figure 1).
Depressive symptoms in the GAD population. By protocol criteria, depressive symptoms, if present, needed to be clinically less significant than anxious symptoms. Hence, the RDS score was always less than the CAS score in all study-eligible patients. Both scales range in scores from 3 to 15. In this study, the highest individual patient’s RDS score was 7, whereas the CAS scores ranged from 9 to 15. As noted above, a mid-study amendment permitted enrollment of patients with a current major depressive episode (MDE) but still specified that the depressive symptoms needed to be clinically less significant than the anxious symptoms. As noted in Table 1, four patients were enrolled with a current MDE, 33 percent of enrolled patients reported a past history of MDE, and 18.9 percent reported a history of recurrent MDD.
Table 3 compares the clinical metrics obtained at the screen visit between the enrolled patients with GAD, with or without a previous history of MDE. There were no significant differences noted on the CAS score, GAD-7, total SIGH-A, or the CGI-S. However, the patients with a previous history of MDE had significantly more depressive symptoms than the patients with no history of MDE. The mean RDS score was 5.73±1.05 in patients with GAD and a history of MDE and 4.95±1.08 in patients without a history of MDE (t=3.27; p=0.0015, 95% CI for mean difference 0.78 [0.31, 1.26]).
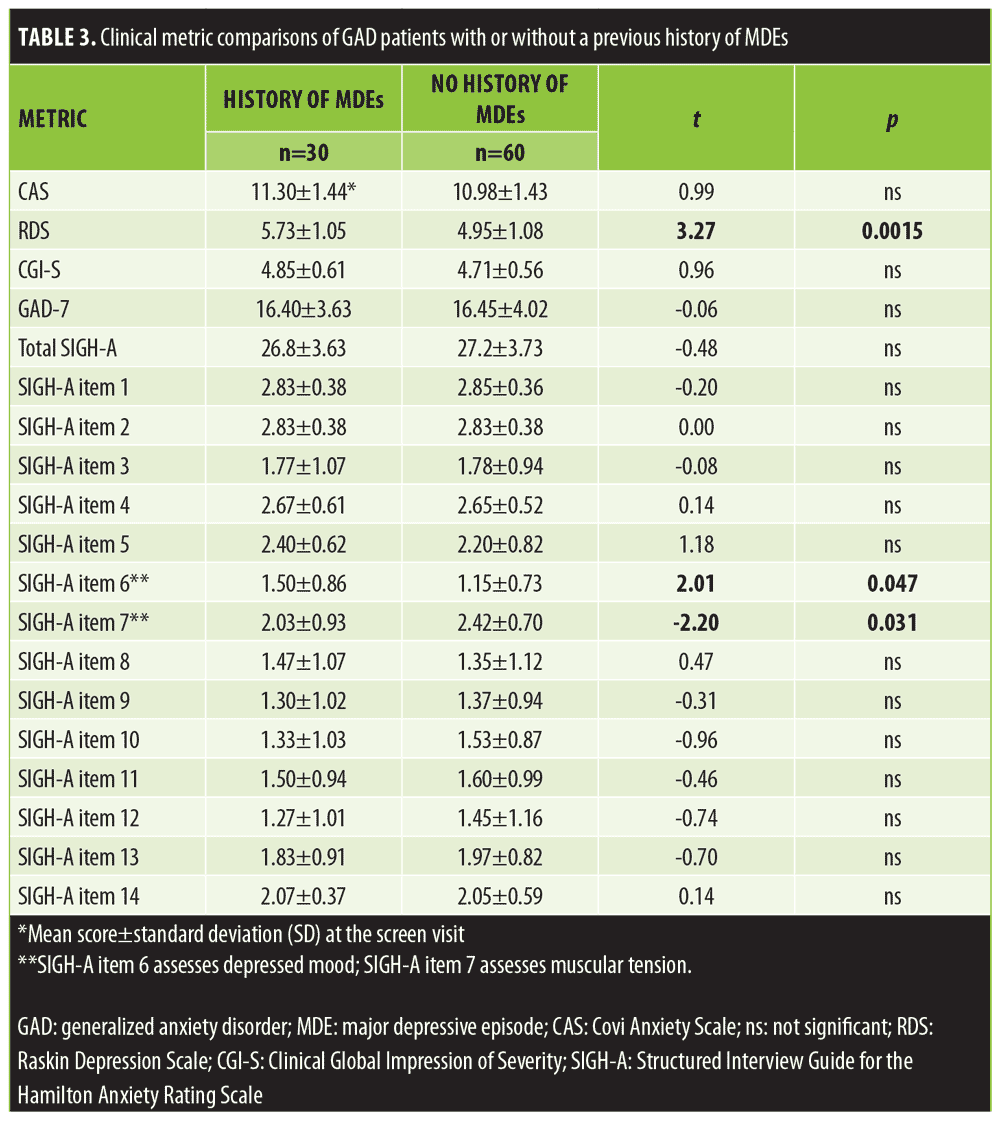
Two of the 14 individual SIGH-A items revealed statistically significant differences between these two GAD cohorts. The mean SIGH-A Item 6 depression score was significantly greater in the patients with versus those without a history of a previous MDE (t=2.01; p=0.047, 95% CI for mean difference 0.35 [0.01, 0.70]), whereas their mean muscular tension score (SIGH-A item 7) was significantly less (t= -2.2; p=0.030, 95% CI for mean difference -0.38 [0.04, 0.73]) than the patients without a history of MDE.
Discussion
In this clinical trial, a site-independent “dual” review method using audio-digital recordings of site-based interviews at the screening visit was employed to independently assess and confirm the symptomatic presentation and treatment history of patients with GAD who were seeking new treatment. The site-independent reviewers examined the impact of the presenting anxious symptoms on behavior and function to determine whether the symptoms caused sufficient distress to warrant a change in treatment. The “dual” review confirmed that these patients were suffering from excessive uncontrollable worry and had a cluster of anxiety symptoms that caused distressing and/or disruptive behavioral and/or functional consequences in their lives despite receiving adequate anxiolytic treatment. In fact, 65 patients (72.2%) reported achieving less than a 25-percent response on their current regimen of antidepressant/anxiolytic medication(s). In this study population, every eligible patient endorsed excessive worry, and most endorsed all six of the associated anxiety symptoms listed in the DSM-IV-TR checklist for Criterion C. The mean total site-based HAM-A score of 27.1 and the mean CGI-S score of 4.8 reflect a moderately ill population of patients with anxiety. Hence, these patients with GAD warranted a different treatment intervention to address their disruptive symptom profile.
The clinical characteristics and consequential distress reported in this GAD study population are consistent with the GAD treatment guidelines issued by the National Institute for Health Care Excellence (NICE) in 2011, which recommends drug treatment if the persistent anxious symptoms cause marked functional impairment as reflected by difficulty in functioning in the domains of work or school, social or leisure, and/or family life.22
Limitations. This analysis has limitations that require cautious interpretation of the findings. We only enrolled patients with GAD who needed a change in their current antidepressant medication, and thus we did not assess patients with GAD who were either untreated or successfully treated. We limited our study to patients with GAD and did not explore the characterization of patients with other anxiety disorders or patients with GAD and comorbid acute depression. This was a drug trial to assess a different medication, and we did not consider alternative treatment regimens, such as complementary or alternative medicines. Further, we did not examine the treatment outcome or the placebo at the end of this trial.
The diagnosis of GAD is often comingled with depressive disorders, other anxiety disorders (e.g., social anxiety or panic disorders), or substance abuse disorder or dependency problems.6,9,23,24 In fact, anxiety symptoms can be masked by coexisting depressive symptoms, obfuscating the diagnosis and making it difficult to clarify the need for psychotropic medication.6,10,23–25 A sizable number of patients with GAD in this study (33%) reported experiencing a previous MDE. Patients with a history of MDE had significantly more depressive symptoms at the screening visit than patients without a history of MDE, although their anxiety symptom scores on the CAS, GAD-7, and total SIGH-A were not significantly different (Table 3). One study identified different risk factors for GAD and MDE and argued that these two disorders are not merely different manifestations of a single underlying condition or that GAD is merely a prodrome or severity marker of MDE.23 Our screening data do not allow us to speculate whether patients with GAD, with or without concomitant depressive symptoms, are different from each other or whether a history of MDE alternating with GAD actually matters in the course of the illness.
The widely used DSM classification system has evolved since the third edition of the DSM and now describes GAD as a disorder characterized by excessive worry that is hard to control. It includes a relatively short checklist of six associated anxious/arousal symptoms, of which only three are required to qualify the diagnosis as GAD.1,2,24 Many “normally” anxious but reasonably well-functioning people could potentially have worries that are hard to control and might also endorse many, but not all, of the associated symptoms. Clearly, prescribing medication to manage anxiety in patients who don’t have disruptive symptoms would not be justified. To address this issue, the DSM-IV-TR Criterion E differentiated “true” GAD from nonpathological anxiety and required that the persistent anxiety or worry must cause clinically significant distress.1 The recently adapted DSM-5 criteria retained this requirement as Criterion D.2 Clearly, all of the enrolled patients in this study met the DSM distress criterion so that there was no doubt that they needed a new treatment intervention. Our data do not address milder illness and cannot inform whether patients with milder GAD can be differentiated from normally anxious people with nonpathological anxiety and would benefit from earlier treatment intervention.
The digital revolution has made it possible to conduct remote independent assessments of patients. In this study, we used audio-digital recordings as a site-independent “dual” review method to assess patients with GAD as part of the eligibility procedures for a clinical trial. The second opinions were obtained from site-independent clinical experts who received the audio-recorded data in lieu of conducting a second, live interview. A possible limitation of this method is that the precision of the second independent opinion relies on the quality of the primary site-based clinician interview. Based on our experience with surveillance strategies, the use of audio recording actually improves the quality of site-based interviews.19,20
Conclusion
The audio-recording method is a time-efficient, pragmatic approach that minimizes patient burden because it does not require longer clinic times and extra scheduling to conduct a second live clinical assessment. This method might have broader utility in clinical practice where second opinions might be desirable but not always feasible.
Contributors
Dr. Targum participated in the design, implementation, and analysis of the original study and conceived, analyzed, and wrote the current analysis reported in this manuscript. Dr. Binneman, Dr. Simen, Dr. Whitlock, and Ms. Zumpano participated in the design, implementation, monitoring, and analysis of the study and review of the manuscript on behalf of the sponsor, Pfizer, and communicated with Dr. Targum about the data analysis for this current manuscript. Dr. Murphy and Mr. Khan assisted with the collection, collation, and analysis of the data and assisted with the preparation of the final manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Press, Inc; Arlington, VA; 1994.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Arlington, VA: American Psychiatric Press Inc; 2013.
- Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60(7):427–435.
- Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety. 2002;16(4):162–171.
- Barrera T, Norton P. Quality of life impairment in generalized anxiety disorder, social phobia, and panic disorder. J Anxiety Disord. 2009;23(8):1086–1090.
- Hoge EA, Ivkovic A, Fricchione Gl. Generalized anxiety disorder: diagnosis and treatment. BMJ. 2012;345:e7500.
- Bandelow B, Boerner R, Kasper S, et al. The diagnosis and treatment of generalized anxiety disorder. Dtsch Arztebl Int. 2013;110(17):300–9.
- Struzik L, Vermani M, Coonerty-Femiano A, Katzman MA. 2004. Treatments for generalized anxiety disorder. Expert Rev Neurother. 2004;4(2):285–294.
- Kavan MG, Elsasser GN, Barone EJ. Generalized anxiety disorder: practical assessment and management. Am Fam Physician. 2009;79(9):785–91.
- Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199.
- Williams JBW. A Structured Interview Guide for the Hamilton Anxiety Rating Scale. New York, NY: New York State Psychiatric Institute; 1993.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22 33;quiz 34–57.
- Raskin A, Schulterbrandt J, Reatig N, McKeon JJ. Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis. 1969;148:87–98.
- Covi L, Rickels K, Lipman O. Effects if psychotropic agents on primary depression. Psychopharmacol Bull. 1981:100–101.
- Hamilton MA. The assessment of anxiety states by rating. Br J Med Psychol. 1959;23:50–55.
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare (DHEW) Publication ADM-76-338).1972;218–222.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Int Med. 2006;166(10):1092–7.
- Targum, SD, Houser C, Northcutt J, et al. A Structured Interview Guide for Global Impressions: reliability and validity for CNS trials. Ann Gen Psychiatry. 2013;12(1):2.
- Targum SD, Pendergrass JC. Site-independent confirmation of subject selection for CNS trials: “dual” review using audio-digital recordings. Ann Gen Psychiatry. 2014;13:21.
- Targum SD, Pendergrass JC, Toner C, et al. Audio-digital recordings used for independent confirmation of site-based MADRS interview scores. Eur Neuropsychopharmacol. 2014;24(11):1760–6.
- NICE Clinical Guideline CG113. 2011. Generalized anxiety disorder and panic disorder (with or without agoraphobia) in adults: management in primary, secondary and community care. NICE (CG113) guidance.nice.org.uk/cg113.
- World Health Organization (WHO) Obesity: preventing and managing the global epidemic. report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000.
- Kessler RC, Gruber M, Hettema JM, et al. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38(3):365–374.
- Andrews G, Hobbs MJ, Borkovec TD, et al. Generalised worry disorder: a review of DSM-IV generalised anxiety disorder and options for DSM-V. Depress Anxiety. 2010;27(2):134–47.
- Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47–57.





