 by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
Dr. Nakamura is with the Department of Psychiatric Internal Medicine, Kosekai-Kusatsu Hospital, in Hiroshima, Japan, and Dr. Nagamine is with the Department of Psychiatric Internal Medicine, Sunlight Brain Research Center, in Yamaguchi, Japan.
Innov Clin Neurosci. 2017;14(3–4):30–37.
Funding: No funding was received for the preparation of this article.
Financial disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: Autonomic nervous, insomnia, neuroendocrine, orexin antagonist, psychiatry, suvorexant
Abstract: Aim: The aim of this study was to investigate the neuroendocrine, autonomic, and metabolic system responses to suvorexant in psychiatric subjects with insomnia.
Design: This prospective study was conducted in Kusatsu Hospital in Hiroshima, Japan and included 40 psychiatric inpatients treated with suvorexant from December 2014 to April 2016.
Methods: Questionnaire of Pittsburgh Sleep Quality Index (PSQI), Generalized Anxiety Disorder-7 (GAD-7), and Patient Health Questionnaire-9 (PHQ-9) scores were checked at baseline, Week 2, and Week 4, and fasting serum levels of prolactin, insulin, cortisol, noradrenaline, white blood cell count, and average pulse rate were measured at baseline and Week 4 and Week 8 after suvorexant initiation. Sequential change of the values were compared against baseline respectively.
Results: Subjective sleep quality scores were significantly decreased at Weeks 2 and 4, and sleep duration, habitual sleep efficacy, and global scores were significantly decreased at Week 4 from baseline. Total scores on the Generalized Anxiety Disorder-7 and the Patient Health Questionnaire-9 significantly decreased at Week 4 from baseline. The levels of cortisol and white blood cell count were decreased, significantly at Week 8, and the levels of pulse rate were significantly decreased at Week 4 from baseline. The levels of noradrenaline decreased, although not significantly. The prolactin levels remained unchanged, and no trend was found in the insulin levels.
Conclusion: Suvorexant treatment resulted in overall improvement in the quality of sleep and the severity of anxiety and depression. This dual orexin antagonist may be related to autonomic functions and neuroendocrine systems, especially in the hypothalamus-pituitary-adrenal axis in psychiatric subjects.
Introduction
The hypothalamus plays a critical role in the integrated control of sleep/wakefulness, thermogenesis, and energy homeostasis, and the lateral hypothalamus area (LHA) in this region has been regarded as an important center for arousal and feeding.[1] The neuropeptide orexin A and orexin B (also known as hypocretin 1 and hypocretin 2, respectively) were initially identified as endogenous ligands for two orphan G-protein-coupled receptors exclusively expressed in the LHA neurons.[2,3] Orexin-producing neurons (orexin neurons) project their axons throughout the entire brain, excluding the cerebellum,[4,5] which suggests that their functions are varied. Particularly, dense projections of orexin neurons are observed in the serotonergic dorsal raphe nucleus (DR), noradrenergic locus coeruleus (LC), and histaminergic tuberomammillary nucleus (TMN), and all of these nuclei are involved in promoting arousal.[6] On the other hand, orexin neurons receive abundant input from the limbic system and also have reciprocal links with hypothalamic arcuate (Arc) nucleus,[4,7] which regulates feeding behavior. Moreover, the responsiveness of orexin neurons to peripheral metabolic cues, such as leptin, ghrelin, and glucose, suggest that these neurons have a role as a link between the energy homeostasis and vigilance states.[8]
Consistently, deficiencies of orexin function found in humans and animals produce characteristic symptoms of the sleep disorder narcolepsy[9,10] and cause abnormalities in energy homeostasis and reward systems.[11] In addition, the orexin system regulates autonomic functions, such as blood pressure and heart rate,[12,13] and neuroendocrine systems, including the hypothalamic-pituitary-adrenal (HPA) axis.[14,15] When orexins are administered centrally to rodents, they elevate sympathetic tone, metabolic rate, food intake, locomotor activity, and wakefulness, and their neuroendocrine effects include a lowering of plasma prolactin and growth hormone and an increase in the levels of corticotropin and cortisol, insulin, and luteinizing hormone.[16] These findings demonstrate that orexin neurons play a critical, adaptive role in the coordination of central and peripheral states according to the environmental changes, which is beneficial for survival in nature.
The physiological roles of orexin have encouraged pharmaceutical companies to develop drugs targeting orexin receptor agonists or antagonists as treatment for sleep-wake cycle disorders, such as narcolepsy and insomnia, and might also be useful in novel treatments for obesity, eating disorders, or other autonomic/metabolic disorders.[17] Suvorexant was approved in late 2014 by the United States Food and Drug Adminstration (FDA) and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) for the treatment of insomnia characterized by difficulty achieving and/or maintaining sleep. Suvorexant is a dual orexin receptor antagonist (DORA) and is first drug in its class to reach the market.[18,19] Suvorexant has been generally effective and well tolerated; however, its pharmacological properties remains unverified in a “real-world” clinical setting.
The aim of this study was to investigate the neuroendocrine, sympathetic, and metabolic responses to suvorexant associated with insomnia in psychiatric inpatients.
Methods
Subjects. Psychiatric inpatients treated with suvorexant, 15- or 20mg per night, for any type of sleep disorder were eligible to enter the study. Use of psychiatric medication, such as antipsychotics, mood stabilizers, antidepressants, and antianxiety/ hypnotics, was permitted. Diagnosis of mental and behavioral disorders were made according to the International Statistical Classification of Disease and Related Health Problems 10th Revision (ICD-10).[20] Throughout the observation period, the exclusion criteria included an alternation in psychiatric medication and/or any medical treatment that directly affected the patient’s sleeping or neuroendocrine and metabolic systems. Under these criteria, subjects were enrolled consecutively, and a total of 40 subjects were studied prospectively from December 2014 to April 2016 at Kusatsu Hospital in Hiroshima, Japan.
Outcome measures and statistical methods. Quality of sleeping using the Pittsburgh Sleep Quality Index (PSQI),[21] severity of anxiety using the Generalized Anxiety Disorder-7 (GAD-7),[22] and severity of depression using the Patient Health Questionnaire-9 (PHQ-9)[23] were evaluated at baseline and two and four weeks following suvorexant treatment.
Fasting plasma samples for analysis of prolactin, insulin, cortisol, and noradrenaline were drawn through routine practice and measured together with white blood cell count at baseline, Week 4, and Week 8 during suvorexant treatment. The average pulse rate was calculated by routine vital measurement at 9:00AM on three consecutive days—the day before, the day of, and the day after each of the following visits: baseline, Week 4, and Week 8. The collected average pulse rate data were assessed together with the other laboratory test data.
Multiple comparisons of the measured values at baseline, Week 2, and Week 4 or at baseline, Week 4, and Week 8 were individually performed with repeated-measured ANOVA, followed by post-hoc analysis using Boneferroni adjustment, and considered statistically significant at P error (SE).
Ethical approval. The ethics committee of Kusatsu Hospital approved this study, and written informed consent was obtained from all participants.
results
Sample characteristics and treatment disposition. Subjects consisted of 24 men (2 with mild cognitive impairment, 7 with alcoholic psychosis, 7 with schizophrenia/schizoaffective disorder, 7 with mood disorder, and 1 with neurotic/stress-related disorder) with a mean age of 55.4 years; and 16 women (5 with schizophrenia/schizoaffective disorder, 9 with mood disorder, and 2 with neurotic/stress-related disorder) with a mean age of 54.9 years. Of the 40 subjects, two men dropped out of the study for somnolence and fatigue, one man for patient refusal to continue the study at Week 4, and 10 men and one woman for patient discharge at Week 8. The clinical characteristics of subjects taking suvorexant along with concomitant use of psychiatric drugs from baseline to the end observation period are summarized in Table 1.
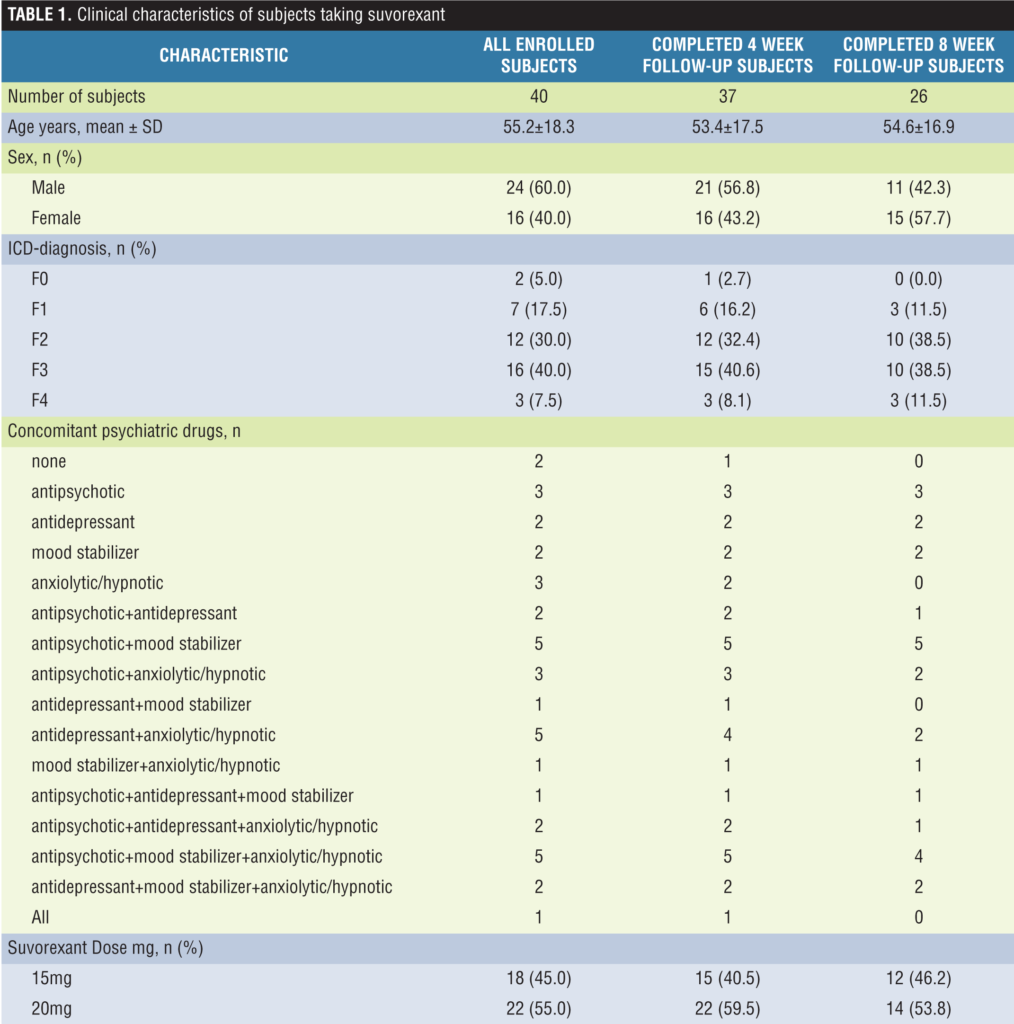
PSQI, GAD-7, and PHQ-9 scores with Week 4 follow-up data and evaluation. Subjective sleep quality scores (Component 1) decreased and use of sleep medication (Component 6) scores increased significantly at Weeks 2 and 4 from baseline; sleep duration (Component 3), habitual sleep efficacy (Component 4), and global scores (the total of all 7 component scores) significantly decreased at Week 4 from baseline; and no differences were found in sleep latency (Component 2), sleep disturbance (Component 5), and daytime dysfunction (Component 7) scores.
The total scores on the GAD-7 and the PHQ-9 scores significantly decreased at Week 4 from baseline.
The values of sleeping and psychiatric-related signs are presented in Table 2.
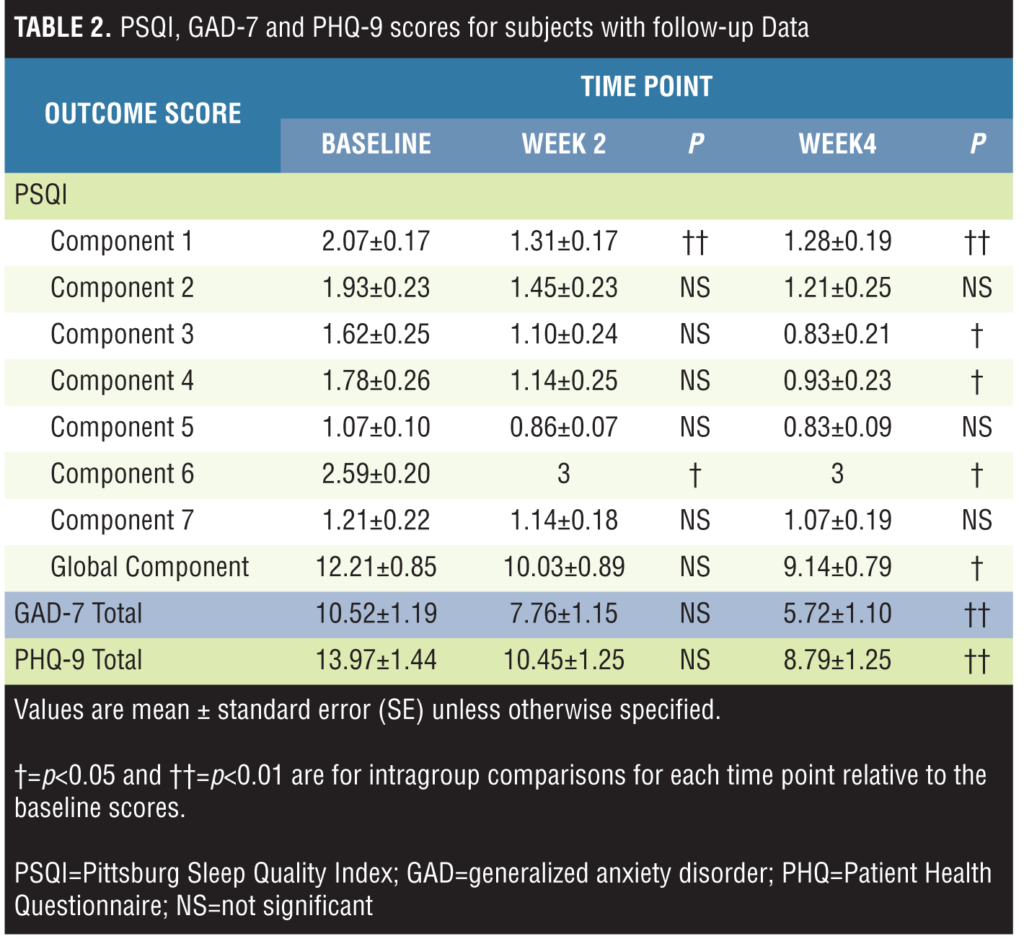
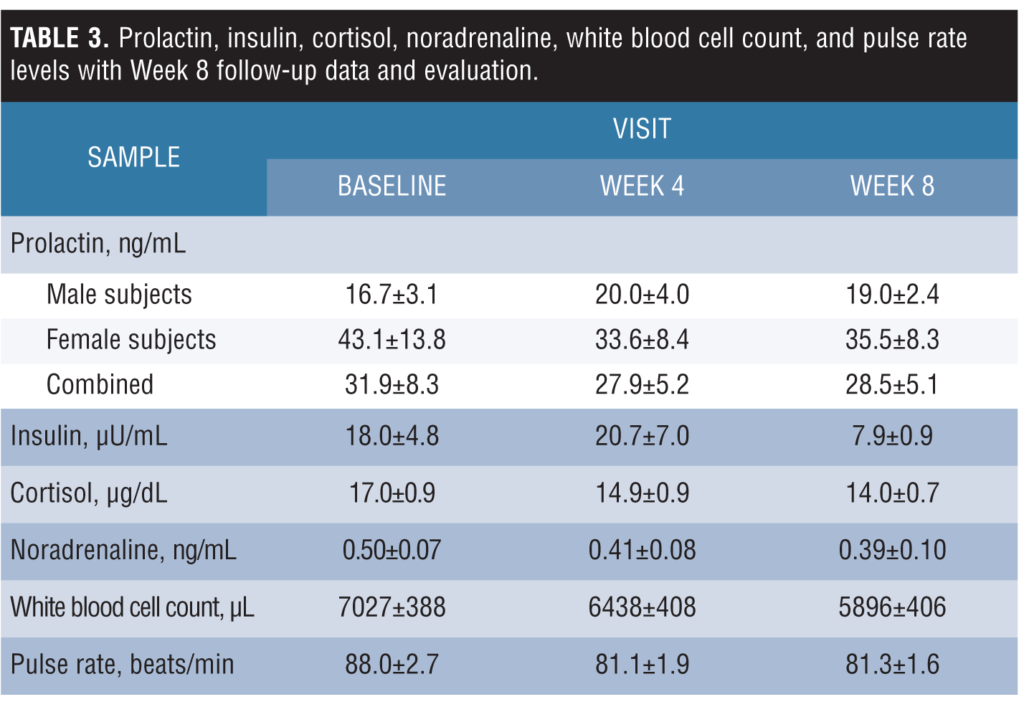
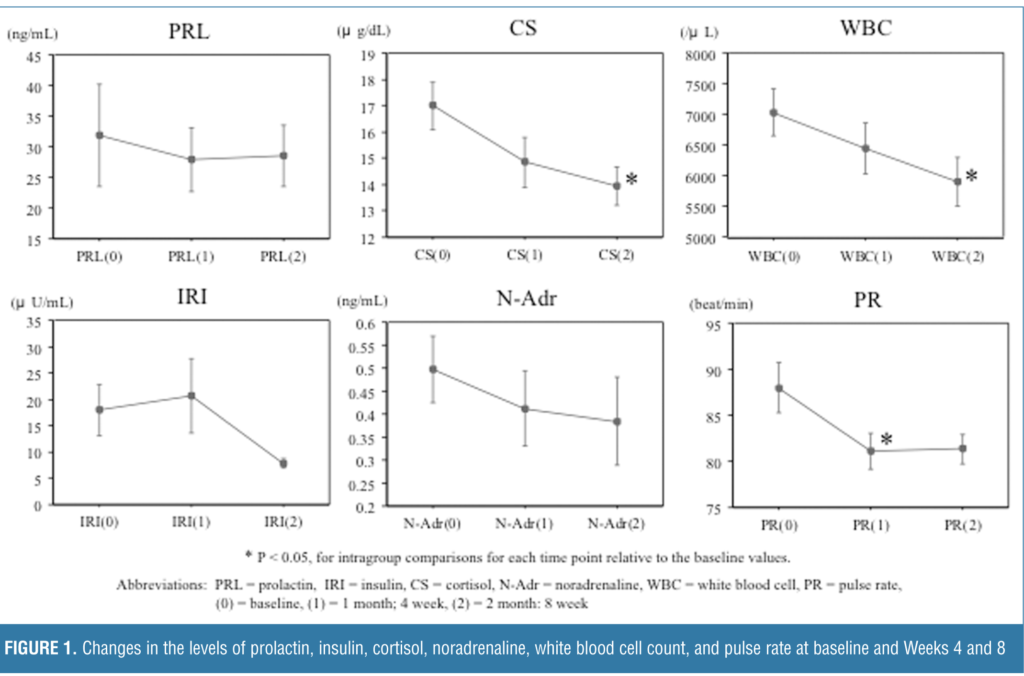
Prolactin, insulin, cortisol, noradrenaline, white blood cell count, and pulse rate levels with Week 8 follow-up data and evaluation. The levels of cortisol and white blood cell count were decreased significantly at Week 8 from baseline. Levels of pulse rate were significantly decreased at Week 4 from baseline. Levels of noradrenaline decreased, although not significantly from baseline. Prolactin levels in male subjects, and female subjects, and both sexes combined remained unchanged, and no trend was found in the insulin levels during the observed period. Laboratory test values are listed in Table 3, and changes of these values over time from baseline, Week 4, and Week 8 are shown as 0, 1 month, and 2 months, respectively, in Figure 1.
Discussion
Clinical trials. Suvorexant is the first DORA to be approved in the United States and has demonstrated efficacy at decreasing both time to sleep onset and increasing total sleep time in patients with insomnia compared with placebo in three Phase 3 clinical trials.[24,25]
Herring et al evaluated the efficacy of suvorexant at Month 3 of administration to participants in two separate trials,[24] whereas Michelson et al evaluated the safety and tolerability of suvorexant at one year of administration to participants.[25] Efficacy for the trials was assessed by a variety of primary and secondary endpoints that included sleep diaries, subjective total sleep time (sTST), subjective time to sleep onset (sTSO), subjective wake after sleep onset (sWASO), wake after sleep onset (WASO), subjective quality of sleep (sQUAL), subjective refreshed feeling on waking (sFRESH), subjective number of awakenings (sNAW), and latency to onset of persistent sleep (LPS). The former two trials demonstrated that suvorexant 30mg and 40mg was superior to placebo by improving sleep maintenance, measured by sTST and WASO, at Months 1 and 3 for both. The third trial showed that after one month, suvorexant improved sTST (38.7min) versus placebo (16min), and sTSO was also improved with suvorexant (-18min) versus placebo (-8.4min). Although these trials excluded subjects if they had other sleep disorders, confounding neurologic disorders, current major affective or psychotic psychiatric illness, substance abuse, or an unstable medical condition, we investigated the efficacy of suvorexant in psychiatric patients with concomitant use of psychotropic drugs and/or with medical complications and found improvement in quality of sleep using the PSQI self-report questionnaire. It is possible that this efficacy reduced severity of anxiety and depression caused by sleeping disorders in our participants.
Prolactin secretion. There has been controversy over the effect of orexin on the hypothalamic control of prolactin secretion. Russell et al[26] reported that intracerebroventicular (i.c.v.) administration of orexin profoundly suppressed plasma prolactin in male rats, and the inhibition of prolactin secretion by orexin is not entirely reversed by the administration of the dopamine D2 antagonist domperidone, suggesting that other pathways are involved. Pituitary prolactin release is under tonic inhibition from tuberoinfundibular (TIDA) dopamine.
Orexin may affect multiple conflicting pathways. For example, it densely innervates adrenergic, histaminergic, and serotogenic neurons that can increase prolactin secretion via the TIDA, while acting on neuropeptide Y (NPY) neurons, which may stimulate the TIDA and thus decrease prolactin secretion. Orexin may stimulate prolactin-inhibitory factors (PIFs) or inhibit prolactin-releasing factors (PRFs) that are independent of the TIDA. Samson et al[27] observed that a dose of the OX1R antagonist was capable of abolishing the ACTH response to immobilization stress was without effect on the prolactin response in their models.
It is well established that secretion of prolactin concentrations are highest during sleep and lowest during wakefulness,[28] which suggests sleep and circadian rhythmicity interact in determining the temporal organization of prolactin secretion during the day.
High prolactin level increase the duration and frequency of rapid eye movement (REM) sleep. The reduction in plasma prolactin with orexin administration may therefore be related to its effect on increasing arousal and reducing REM sleep. In their study, Chang et al[29] showed that rotating night shifts may cause high prolactin levels during the subsequent day, which suggests a DORA administration at night may reduce plasma prolactin levels in the early morning and lead to an improvement of arousal and sleep. We could not add further support to this hypothesis in our study because most of our subjects received antipsychotics.
Metabolic disorders. According to Schwartz et al,[30] glucose metabolism is regulated by both central and peripheral regulatory systems through their cooperative interactions. Funato[31] describes the daily rhythm of activated hypothalamic orexin as being controlled by the central biological clock located in suprachiasmatic nuclei, by blood glucose levels, and by brain signals that center around hunger/food-seeking behaviors. Acting through the sympathetic and parasympathetic nervous systems, the rhythmic activation of orexin helps to stabilize the sleep/wake cycle and modify hepatic glucose production and as well as enhance glucose consumption in skeletal muscles and thermogenesis in brown adipose tissue.[31–35] Orexin’s role in modifying/reducing blood glucose is believed to be critical in the prevention of insulin resistance caused by age, obesity, and depression.[36]
Currently, suvorexant is only approved for insomnia, and the effect this drug has on glucose metabolism remains to be proven. An early study reported that the orexin-1 receptor antagonist, SB-334867-A, exerted anti-obese and anti-diabetic effects when repetitively injected into genetically obese ob/ob mice at daytime for two weeks.[37] Through the enhancement of brown adipose tissue thermogenesis, SB-334867-A appears to reduce adiposity and increase energy expenditure. Moreover, the administration of SB-334867-A at the resting state may strengthen normal sleep/wake and feeding cycles, which can improve glucose metabolism in the liver and skeletal muscle.
In our present study, the fasting insulin levels at Week 8 were decreased from baseline, although not significantly, which may indicate that suvorexant at the clinical dosage for insomnia has the potential to have an advantageous effect on several metabolic disorders.
Mechanism of autonomic and neuroendocrine system. Orexin-A and orexin-B are endogenous neuropeptide agonist for orexin receptor-1 (OX1R) and orexin receptor-2 (OX2R). Orexin-A has equal affinity for OX1R and OX2R, whereas orexin-B displays higher affinity for OX2R.2
It was demonstrated that i.c.v. injection of orexin increased blood pressure, heart rate, and renal sympathetic nerve activity. These effects were abolished by administration of ?- or ?-adrenoceptor antagonists. In addition, a high dose of orexin increased plasma noradrenaline (NA) concentration,[38] which suggests that orexin increases sympathetic nerve tone. The autonomic response induced by orexin A is greater than that induced by orexin B. The novel OX1R antagonist SB-334867, which alone did not significantly change baseline hemodynamic variables and plasma catecholamine levels, markedly attenuated increase in mean arterial pressure, heart rate, and plasma noradrenaline concentration induced by orexin-A in rats.[12] Li A et al[39] measured orexin-A mRNA expression in the rostral ventrolateral medulla and anatomized both orexin receptors using an orally administered potent dual orexin receptor antagonist, almorexant, in spontaneously hypertensive rats. The researchers found there was a strong trend toward an increased orexin-A mRNA expression in the rostral ventrolateral medulla, and blocking orexin receptors markedly lowered blood pressure, heart rate, sympathetic vasomotor tone, and the noradrenaline levels in cerebrospinal fluid and plasma. These results suggest that orexin A positively regulates sympathetic nerve tone primarily through the OX1R. Therefore, suvorexant might attenuate some of the sympathetic nervous system markers.
There is much evidence showing that orexin might play a role in the regulation of the HPA axis via a central mechanism.[14,15] I.c.v injection of both orexin A and orexin B was shown to activate neurons in the paraventricular nucleus (PVN) and to increase plasma adrenocorticotrophin (ACTH) and corticosteroid levels in rats.[14,40] As orexin neurons innervate the PVN in which OX2R is abundantly expressed,[41,42] this response might be mediated by corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP) in the PVN. The effect of centrally injected orexins on HPA axis function was completely inhibited by a CRH receptor antagonist, demonstrating the ability of orexins to activate hypothalamic CRH neurons in the PVN, most likely via the OX2R, with subsequent activation of the HPA axis. However, other studies suggest that orexin might activate adrenocortical cells without involvement of the HPA axis. In one study, chronic administration of orexin for one week did not affect plasma ACTH levels, but increased plasma cortisol and aldosterone levels.[43] In another study, orexin increased cortisol secretion in a dose-dependent manner using freshly dispersed normal and adenomatous human adrenocortical cells.[44] In fact, it was reported that orexin-deficient, male patients with narcolepsy displayed blunted basal and total ACTH production without basal pulsatile cortisol secretion.[45] These studies suggest that orexin might directly activate adrenocortical cells in the adrenal gland where OX1R, OX2R are distributed, without an involvement of the HPA axis responsiveness to different type of stress via CRH and AVP neurons in the PVN. Whether these observations have any practical implications in narcolepsy or other sleep disorders remains to be studied.
The reduction of white blood cell count after suvorexant treatment may be linked with the significant decrease of cortisol levels and slight decrease of noradrenaline levels, because glucocorticoid and catecholamine are known to induce leukocytosis by several biological mechanism.[46,47]
Patel et al[48] reported that the low-dose pharmacology of dual orexin receptor antagonist, SB-649868, had no impact on neuroendocrine hormones (cortisol, ACTH, and prolactin) and most sympathetic nervous system markers (pulse rate, mean arterial blood pressure, plasma noradrenaline, and adrenaline) in insulin-induced hypoglycemic stress in 24 healthy male subjects. The investigators also indicated the exploring the utility of the insulin tolerance test model as a basis for evaluating orexinergic antagonism over a wider dose range, including doses known to have efficiency on other marker, such as measures of insomnia.
Limitations. There are several limitations to the present study. First, subjects were selected under rigorous criteria to investigate the effect of suvorexant treatment; nevertheless, most of them received some psychiatric and/or medical treatment. In particular, the use psychotropic medications is a confounder in this study. Second, it was an open-label study, not a double-blind study design, with a small sample size and a limited observation period. Third, we assumed that the closed-ward setting would minimize inter-individual differences in sleep habituation, energy intake, and expenditure; however, some patients were discharged before the study ended, and it would have been difficult to evaluate these subjects as outpatients. Fourth, serum markers were measured and compared at only one point in the day, so that circadian variation patterns of neuroendocrine hormones were not taken into account.
Conclusion
The results of the present study could be summarized as follows: In psychiatric inpatients being treated with suvorexant for sleep disorders, 1) suvorexant was effective in improving the quality of sleep in general, especially subjective sleep quality, sleep duration, and habitual sleep efficiency; 2) suvorexant ameliorated the severity of anxiety and depression; 3) suvorexant slightly attenuated the sympathetic nervous system markers (fasting plasma noradrenaline level and pulse rate) and had no certain effect on fasting plasma prolactin and insulin; and 4) suvorexant reduced the hypothalamus-pituitary-adrenal axis markers (fasting plasma cortisol level and white blood cell count). Further studies investigating the role that suvorexant plays in the coordination of central and peripheral states of the CNS?need to be performed in various “real-world” treatment settings to further support our conclusion.
Acknowledgment
The authors would like to thank our colleagues in all department of Kusatsu Hospital for their assistance with this report.
References
- Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287.
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585.
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327.
- Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015.
- Nambu T, Sakurai T, Mizukami K, et al. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260.
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;537:1257–1263.
- Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753.
- Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713.
- Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451.
- Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474.
- Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354.
- Hirota K, Kushikata T, Kudo M, et al. Effects of central hypocretin-1 administration on hemodynamic responses in young-adult and middle-aged rats. Brain Res. 2003;981:143–150.
- Kayaba Y, Nakamura A, Kasuya Y, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–593.
- Al-Barazanji KA, Wilson S, Baker J, et al. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol. 2001;13:421–424.
- Brunton PJ, Russell JA.?Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J Neuroendocrinol. 2003;15:633–637.
- Hagan JJ, Leslie RA, Patel S, et al.?Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916.
- Smart D, Haynes AC, Williams G, Arch JR. Orexins and the treatment of obesity. Eur J Pharmacol. 2002;440:199–212.
- United States Food and Drug Administration FDA News Release: FDA Approves New Type of Sleep Drug. Belsomra. 2014. [Accessed March 17, 2015]. Available from:http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm.
- Parker T. Merck enters the Insomnia market through the launch of Belsomra in Japan. 2014. [Accessed May 12, 2015]. GlobalData Website. Available from: http://healthcare.globaldata.com/resources/expert-insights/pharmaceuticals/merck-enters-the-insomnia-market-through-the-launch-of-belsomra-in-japan.
- World Health Organization site. International Statistical Classification of Diseases and Related Health Problems 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en. Accessed 30 April 2017.
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097.
- Spitzer RL, Kroenke K, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
- Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in Patients With Insomnia: Results From Two 3-Month Randomized Controlled Clinical Trials. Biol Psychiatry. 2016;79:136–148. doi: 10.1016/j.biopsych.2014.10.003.
- Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:461–471. doi: 10.1016/S1474-4422(14)70053-5.
- Russell SH, Kim MS, Small CJ, et al. Central administration of orexin A suppresses basal and domperidone stimulated plasma prolactin. J Neuroendocrinol. 2000;12:1213–1218.
- Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R382–387.
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631.
- Chang YS, Chen HL, Wu YH, et al. Rotating night shifts too quickly may cause anxiety and decreased attentional performance, and impact prolactin levels during the subsequent day: a case control study. BMC Psychiatry. 2014;14:218. doi: 10.1186/s12888-014-0218-7.
- Schwartz MW, Seeley RJ, Tschöp MH, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709.
- Funato H. Orexin and metabolism. In: Sakurai T, Pandi-Perumal SR, Monti JM (eds). Orexin and Sleep: Molecular, Functional and Clinical Aspects. New York:?Springer; 2015:363–390.
- Kalsbeek A, Yi CX, la Fleur SE, et al. Suprachiasmatic nucleus and autonomic nervous system influences on awakening from sleep. Int Rev Neurobiol. 2010;93:91–107.
- Tsuneki H, Wada T, Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocr J. 2012;59:365–374.
- Shiuchi T, Haque MS, Okamoto S, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013.
- Perez-Leighton CE, Billington CJ, Kotz CM. Orexin modulation of adipose tissue. Biochim Biophys Acta. 2014;1842:440–445.
- Tsuneki H, Tokai E, Nakamura Y, et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes. 2015;64:459–470. doi: 10.2337/db14-0695.
- Haynes AC, Chapman H, Taylor C, et al. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104:153–159.
- Shirasaka T, Nakazato M, Matsukura S, et al. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–1785.
- Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271.
- Kuru M, Ueta Y, Serino R, et al. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980.
- Lu J, Bjorkum AA, Xu M, et al. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576.
- Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25.
- Malendowicz LK, Hochol A, Ziolkowska A, et al. Prolonged orexin administration stimulates steroid-hormone secretion, acting directly on the rat adrenal gland. Int J Mol Med. 2001;7:401–404.
- Spinazzi R, Rucinski M, Neri G, et al. Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab. 2005;90:3544–3549.
- Kok SW, Roelfsema F, Overeem S, et al. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-keeping by the master pacemaker. J Clin Endocrinol Metab. 2002;87:5085–5091.
- Nakagawa M, Terashima T, D’yachkova Y, et al. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–2313.
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10:77–91.
- Patel AX, Miller SR, Nathan PJ, et al. Neuroendocrine and sympathetic responses to an orexin receptor antagonist, SB-649868, and alprazolam following insulin-induced hypoglycemia in humans. Psychopharmacology (Berl). 2014;231:3817–3828. doi: 10.1007/s00213-014-3520-7.





