
by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
Dr. Nakamura is with the Department of Psychiatric Internal Medicine at Kosekai-Kusatsu Hospital in Hiroshima, Japan. Dr. Nagamine is with the Department of Psychiatric Internal Medicine at Sunlight Brain Research Center in Yamaguchi, Japan.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Psychotropic-induced hyponatremia is one of the most common electrolyte abnormalities observed in routine psychiatric practice. However, many features of hyponatremia mimic those seen in depression, which can lead to the condition being overlooked. Takotsubo cardiomyopathy, or apical ballooning syndrome (ABS), is a reversible cardiomyopathy that mimics acute myocardial infarction. Limited evidence has indicated that hyponatremia might have a role in the development of ABS. We present a case of female patient with bipolar disorder who developed acute hyponatremia and ABS while taking lamotrigine. After cessation of lamotrigine, a time-sequential improvement of hyponatremia and ABS was observed via repeated electrocardiogram, suggesting a relationship between hyponatremia and ABS. Due to the association between severe hyponatremia and Takotsubo cardiomyopathy, this case highlights the importance of carefully monitoring serum sodium levels and ECG changes in patients on psychotropic medications during their entire course of treatment to prevent serious or fatal complications.
Keywords: Apical ballooning syndrome, hyponatremia, lamotrigine, syndrome of inappropriate secretion of antidiuretic hormone, takotsubo cardiomyopathy
Innov Clin Neurosci. 2019;16(7–8):32–34
Hyponatremia is one of the most common electrolyte abnormalities encountered in the psychiatric clinical setting.1 A majority of patients with hyponatremia experience mild symptoms or are asymptomatic and do not require immediate treatment; however, acute hyponatremia can cause serious complications and should be treated immediately.2,3 Hyponatremia has been linked to various psychotropic medications (e.g., antidepressants, antipsychotics, mood stabilizers, sedative/hypnotics).4
Takotsubo cardiomyopathy, also known as apical ballooning syndrome (ABS), is a condition in which new ST-segment elevation and/or T-wave inversion on the electrocardigram and elevation in cardiac markers are observed, mimicing acute myocardial infarction. However, ABS differs from myocardial infarction by its absence of obstructive coronary disease and reversible dyskinesis of the left ventricle (LV) mid-segment with or without apical involvement.5,6 The precise mechanism of ABS remains unclear, but its onset can be triggered by acute physical trauma (e.g., surgery, injury) or acute emotional or psychiatric conditions (e.g., anxiety, panic attacks, depression).7 Although the long-term prognosis is favorable, ABS has been associated with fetal complications in pregnant women, including as cardiogenic shock, arrhythmias, and LV rupture.8
Several investigators have reported severe hyponatremia as a potential precipitating factor for ABS.9,10 Here, we present a case of lamotrigine-induced ABS with severe hyponatremia, likely due to syndrome of inappropriate secretion of antidiuretic hormone (SIADH).
Case Presentation
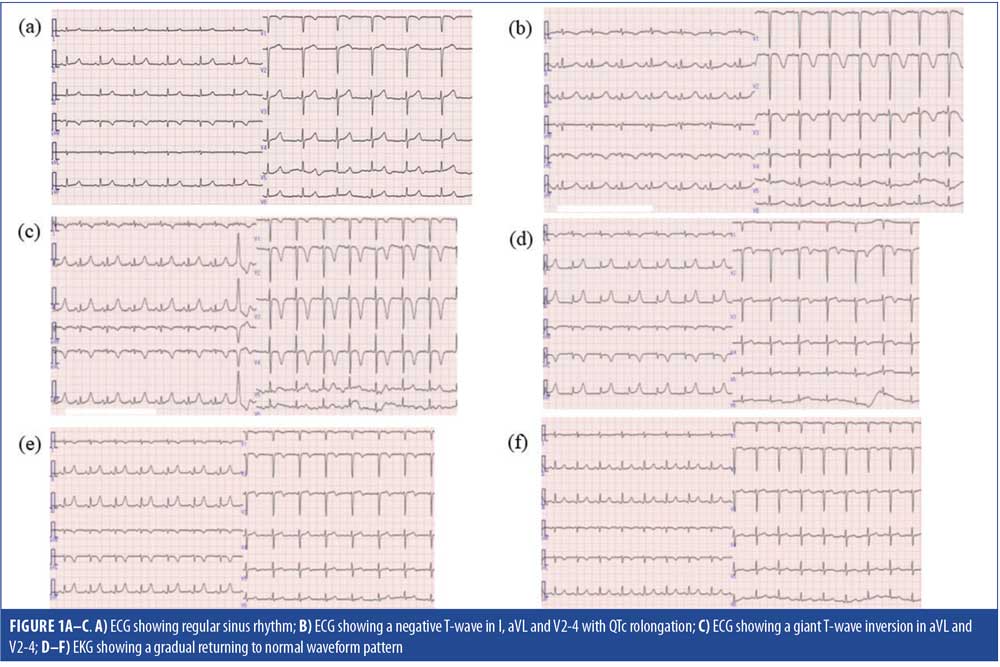
A 50-year-old Japanese woman with a five-year history of bipolar disorder presented to our hospital in an exacerbated catatonic state. She previously had been treated with several antidepressants, hypnotics, and thyroid hormone preparations in the psychiatry and internal medicine clinics, receiving alprazolam, escitalopram, etizolam, trazodone, triazolam, estazolam and levothyroxine sodium hydrate. Her vital signs, physical examination, chest X-ray, computed tomography (CT) scanning of the head, and 12-lead electrocardiogram (ECG) (Figure 1A) were unremarkable. Laboratory work-up, including hematological, liver, renal, and thyroid function tests, and serum electrolytes, were within normal limit except for low hemoglobin count (Hb=9.8g/dL) and mild elevation of N-terminal fragment pro-brain type natriuretic peptide (NT-proBNP=309pg/mL). On admission, she was placed in an isolation ward and treated with lorazepam (4mg/day) for symptoms of mania, followed by lamotrigine (12.5mg/day) as a mood stabilizer. Following pharmacological treatment, her psychiatric symptoms improved within four days but physical symptoms, such as nausea, imbalance, speech disturbance, and vomiting with loss of appetite began appearing.
On Day 13 after admission, she fell to the floor, experiencing urinary incontinence. Laboratory tests revealed low levels of serum sodium (98mEq/L), potassium (2.8mEq/L), chloride (67mEq/L) and osmolality (206mOsm/kgH2O [normal is 275–290]), as well as high levels of serum creatine phosphokinase (1,227IU/L [normal is 30–180]), without an elevation in serum cardiac troponin level. An ECG showed a negative T wave in I, aVL, and V2-4 ,with QTc prolongation (0.566msec) (Figure 1B), and echocardiography showed moderate hypokinesis of anteroseptal LV. Based on these data, an emergency cardiac catheterization was recommended to rule out significant coronary stenosis; however, her husband did not consent to invasive examinations; thus, the team decided to treat her for severe hyponatremia and ABS with lamotrigine-related SIADH, leading to discontinuation of lamotrigine. No other medications were altered. Following immediate intravenous sodium replacement therapy, the patient’s hyponatremia gradually improved; however, delirium with acute confusion developed.
On Day 20, the patient fell into a catatonic state with coldness of extremities. After approximately one hour, there were no problems in her consciousness level and vital signs, and she had recovered from catatonia, but subsequent blood analysis revealed high levels of NT-proBNP (2,661pg/mL) and the lower limit of normal range of sodium (132mEq/L). An ECG revealed a giant T-wave inversion in aVL and V2-4 (Figure 1C), and echocardiography revealed mild hypokinesis of anterior LV. We concluded that the patient’s ABS with hyponatremia were improving. Quetiapine (12.5–25mg/day) was added to lorazepam (1–2mg/day) for management of her bipolar disorder, and a nasogastric tube was inserted to aid her in taking in nutrition.
The patient’s mental and physical symptoms resolved one month after hospital admission, and quetiapine was switched to quetiapine extended release (XR) successfully. The follow-up ECG examination was performed on Days 29, 36, and 48 (Figure 1D, 1E and 1F, respectively) of hospitalization, which revealed a normal waveform pattern. On Day 54, the patient was discharged from the hospital with a serum sodium level of 137mEq/L and no cardiac complications.
Discussion
The temporal correlation between lamotrigine treatment and the development of significantly low levels of serum sodium and concerning T wave changes on ECG led us to the conclusion that lamotrigine, which acts as an antidiuretic hormone, contributed to the reversible cardiomyopathy in our patient.
There are some mechanistic links between hyponatremia and ABS. Catecholamine-mediated stunning of the myocardium is thought to be the primary mechanism underlying ABS.7 Significant adrenergic stimulation might shift the beta receptor, a stimulatory G protein-mediated pathway, to an inhibitory pathway, thereby decreasing cyclic adenosine monophosphate levels, calcium load, and myocardial contractility.11 Though catecholamine effects might have contributed to our patient’s symptoms, the development of severe hyponatremia upon initiation of lamotrigine and its resolution upon the drug’s discontinuation supports our conclusion that the ABS was drug-induced. Goldberg et al12 postulated that intercellular fluid shift causing cellular edema might be the mechanism behind lamotrigine’s sodium-lowering effect, and this is supported by cardiac resonance imaging evidence that 81 percent of patients with ABS develop myocardial edema.13 AbouEzzeddine et al9 reported two cases of Takotsubo cardiomyopathy and suggested that low sodium concentration can induce, through changing activity of sodium-calcium transporters, persistent calcium overload of myocardial cells, leading to dysfunction.14 Ex-vivo experiments in rat hearts showed that a change in sodium concentration had profound effects on cardiac contractility and relaxation.15
Mood stabilizers such as carbamazepine/oxcarbazepine, valproate, and lamotrigine have been shown in several studies to cause hyponatremia;16,17 however, reports are limited for lamotrigine-induced hyponatremia and SIADH.18–20 ADH, which is produced by the hypothalamus, plays a major role in controlling fluid balance in our body by promoting thirst. managing water retention, and upregulating reabsorption of water and sodium ions in the distal tubule of the nephron. Inappropriate or continuous secretion or action of ADH, despite the normal or higher plasma volume, results in hyposmolality and hyponatremia. The proposed mechanisms by which various psychotropics cause hyponatremia are different across different classes of drugs; however, lamotrigine is thought induce hyponatremia by potentiating the renal tubule effects of ADH.4 Mewasingh et al18 reported two patients with central diabetes insipidus receiving LTG for epileptic seizures whose need for desmopressin decreased after LTG treatment. Kiliç et al19 reported a child with central diabetes insipidus who developed symptomatic hyponatremia; an increase in urine density, serum sodium and osmolality, and hypouricemia, and a decrease in desmopressin requirement after administration of LTG, supporting a diagnosis of SIADH.
Although several examinations (e.g., urine sodium and osmolality, plasma ADH concentrations) were lacking and multiple factors were overlapping (e.g., decrease in salt and water intake) in the present case, we believe this is the first published report suggesting that lamotrigine-induced severe hyponatremia due to SAIDH led to the development of Takotsubo cardiomyopathy in a psychiatric patient. The symptoms of hyponatremia can easily be misinterpreted as a worsening of psychiatric symptoms; thus, when patients are placed on psychotropic medications, clinicians should consider the importance of severe hyponatremia as a stressor for Takotsubo cardiomyopathy and carefully monitor serum sodium levels and ECG changes during the entire course of treatment to prevent serious or fatal complications.
References
- Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol. 2009;29:227–238.
- Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM. 2005;98:529–540.
- Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653–658.
- Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3:108–122.
- Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001;38:11–18.
- Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417.
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548.
- Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865.
- AbouEzzeddine O, Prasad A. Apical ballooning syndrome precipitated by hyponatremia. Int J Cardiol. 2010;145:e26–29.
- Patnaik S, Punjabi C, Nathan R, et al. Bland and broken hearted: a case of hyponatremia induced Tako-tsubo cardiomyopathy. Int J Cardiol. 2015;187:267–271.
- Opie LH. Mechanisms of cardiac contraction and relaxation. In: Libby P, ed. Braunwald’s Heart Disease: Textbook of Cardiovascular Medicine. 8th ed. Philadelphia, PA: Saunders Elsevier; 2008:523–524.
- Goldenberg I, Jonas M, Thaler M, Grossman E. Elevated levels of serum creatine kinase induced by hyponatraemia. Postgrad Med J. 1997;73:511–512.
- Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286.
- Santos M, Dias V, Meireles A, et al. Hyponatremia—an unusual trigger of Takotsubo cardiomyopathy. Rev Port Cardiol. 2011;30:845–848.
- Wier WG, Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984;83:395–415.
- Van Amelsvoort T, Bakshi R, Devaux CB, Schwabe S. Hyponatremia associated with carbamazepine and oxcarbazepine therapy: a review. Epilepsia. 1994;35:181–188.
- Gupta E, Kunjal R, Cury JD. Severe hyponatremia due to valproic acid toxicity. J Clin Med Res. 2015;7:717–719.
- Mewasingh L, Aylett S, Kirkham F, Stanhope R. Hyponatraemia associated with lamotrigine in cranial diabetes insipidus. Lancet. 2000;356:656.
- Kiliç H, Ekici B, Ergul Y, et al. Lamotrigine-induced SIADH in a child with central diabetes insipidus. J Pediatr Neurosci. 2011;6:89–90.
- Tsuru T, Akiyama R, Kohashi K, Okumura K. Case of a 13-year-old boy with hyponatremia due to lamotrigine-induced syndrome of inappropriate secretion of antidiuretic hormone. No To Hattatsu. 2012; 44:73–74.





