
by Allison Zuckerberg, MD; Nitin Pothen, MD; and Adriana Fitzsimmons, MD
Dr. Zuckerberg is with Department of Psychiatry, Temple University Hospital in Philadelphia, Pennsylvania. Dr. Pothen is with Department of Psychiatry, Ocean University Medical Center in Brick, New Jersey. Dr. Fitzsimmons is with Department of Psychiatry, Rutgers Robert Wood Johnson Medical School in Piscataway, New Jersey and Department of Psychiatry, Jersey Shore University Medical Center in Neptune, New Jersey.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2024;21(1–3):63–65.
Abstract
In our case report, a 29-year-old male patient with a known history of schizophrenia presented with altered mental status and catatonia and was found to have an enlarged (21mm) cavum septum pellucidum (CSP) on magnetic resonance imaging (MRI). He was subsequently treated with escitalopram, olanzapine, methylphenidate, lorazepam, and eight electroconvulsive therapy (ECT) treatments during his hospital course, after which his catatonia improved. We compared this to other cases in which a large CSP was identified and discussed the possibility of increased susceptibility to psychosis, specifically catatonia, which might be associated with this developmental anomaly.
Keywords: Cavum septum pellucidum, CSP, electroconvulsive therapy, ECT, psychosis, schizophrenia, catatonia, movement disorders, brain maturation and development
Schizophrenia is a psychiatric disorder characterized by positive and negative psychotic symptoms, mood symptoms, cognitive impairment, and, in a minority of patients, catatonia. Catatonia is a general term for abnormal motor behavior that is associated with both medical and psychiatric disease. Regardless of the cause, the first-line therapy for catatonia is benzodiazepines, namely in the form of a lorazepam challenge. However, electroconvulsive therapy (ECT) is another common treatment modality that is often considered for those who do not respond to benzodiazepines.
The exact pathophysiology of schizophrenia—and any associated catatonia—is unknown. However, there are known risk factors that may help in understanding its genesis. The goal of our case report is to highlight a neuroanatomical variant, a cavum septum pellucidum (CSP), which had already been identified as being associated with certain psychiatric disorders. In our case report, a patient who had an enlarged CSP, as identified on magnetic resonance imaging (MRI), had a particularly severe presentation of schizophrenia with catatonia, which eventually responded to ECT.
Case Presentation
A 29-year-old man with a past psychiatric diagnosis of schizophrenia presented to the emergency room after being found sitting unresponsive. He was given three doses of naloxone in the field with minimal improvement and was brought to the emergency department. A noncontrast computed topography (CT) head scan (Figure 1A) revealed no acute intracranial pathology. Urine drug screen was negative. Physical exam revealed that the patient had several features consistent with catatonia, including resisting eye opening and rolling his eyes upward when manual attempts were made to open his eyelids (stupor), not responding to painful stimuli (negativism), and lack of verbal response (mutism). He was subsequently admitted to the intensive care unit (ICU). An electroencephalogram (EEG) demonstrated no seizure activity, and a repeat CT was done which showed no interval changes. Further workup with MRI (Figure 1B) revealed normal brain structures and no chronic changes; however, it revealed a CSP et vergae, with a presumed associated caval cyst, which was 21mm in size.
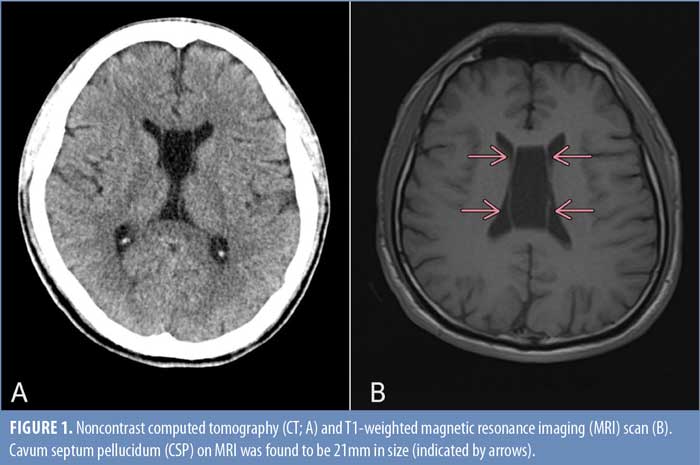
After three days in the ICU, the patient became verbal with more appropriate and safer behavior. After this improvement, he was downgraded to the medical floor for continued observation with telemetry. However, after a few days, he was involuntarily committed to the inpatient psychiatric unit. He was treated with escitalopram and olanzapine for anxiety and psychosis. However, within one week, the patient relapsed into the catatonic state, becoming mute and minimally responsive and refusing to eat; he was transferred back to the medical floor for management.
The patient was initiated on a lorazepam challenge and a subsequent trial of methylphenidate, but there was minimal improvement, so ECT was recommended. The patient was treated with intramuscular lorazepam 2mg four times per day and methylphenidate 10mg twice daily until he could be transferred to a hospital that provided ECT. Of note, there was a six-month delay in starting ECT due to the prolonged process of obtaining permanent guardianship in the absence of available family members.
After the patient was transferred to the second hospital, he had an episode of severe psychomotor agitation, characterized by combativeness, in which he tried to remove his feeding tube, thus requiring four-point restraints for safety. The patient demonstrated other symptoms of catatonia, such as grimacing, mutism, and negativism, including closing his eyes tightly and not responding to staff, although he did respond briefly to his family. He had no rigidity or tremors. A cardiology consult was completed for ECT clearance, and the patient had normal electrocardiogram and echocardiogram results. A complete metabolic panel demonstrated borderline low sodium (134mEq/L) and elevated alanine transaminase (88U/L). Complete blood count, vitamin B12, folate, and ceruloplasmin levels were found to be within normal limits. Thyroid stimulating hormone was borderline high at 5.233mU/L, but free T4 was within normal limits. The patient’s sodium and liver function tests returned to normal levels in a matter of days, but repeat lab work showed low total protein and albumin levels, suggesting poor nutritional status. Neurology was also consulted and completed a lumbar puncture to evaluate the cerebrospinal fluid (CSF). Of note, CSF was negative for multiple sclerosis, determined by the patient having normal levels of immunoglobulin G and being negative for oligoclonal bands. Central nervous system (CNS) infections were also ruled out by a negative venereal disease research laboratory test (VDRL) and negative cryptococcus levels. Furthermore, a culture of the CSF showed no white blood cells or organisms, and there was no growth up to five days after lumbar puncture was performed.
In preparation for ECT, the patient’s lorazepam was tapered to a low dose over the course of one week. ECT was administered a total of 12 times over the course of four weeks with the following parameters: a pulse width of 0.3ms, a duration of 6 to 8 seconds, a current of 800mA, and a frequency of 120Hz for the first three sessions and 80Hz for the rest of the sessions. After the patient received four ECT treatments, he was observed to blink his eyes, and he briefly spoke with his family members. After six sessions, the patient reported that he had been “confused” and did not recall what had occurred. However, he did report a memory of being “poked and prodded.” He had improved affect, denied paranoia against staff, and was able to be encouraged to eat and ambulate. He was then admitted to the psychiatric inpatient unit, where he was observed to be internally preoccupied and reported derogatory auditory hallucinations. At that point, the lorazepam was completely discontinued, and risperidone was initiated. With continued ECT and risperidone, the patient reported less frequent and softer auditory hallucinations, and he was administered the long-acting injection (LAI) paliperidone palmitate. He was discharged with sertraline for depression, low-dose lorazepam for catatonia prophylaxis, and plans to complete outpatient follow-up for a monthly LAI of paliperidone.
Discussion
A CSP is a congenital neuroanatomical variant in which there is a cavity between the membranes (also called leaflets) of the septum pellucidum, which is a midbrain structure. Although a CSP is present in all fetuses, the septum pellucidum fuses in 85 percent of individuals at about 3 to 6 months after birth1 and in more than 99 percent of individuals by adulthood.2 It is considered a CSP when there is a cavity of at least 1mm between the membranes.3 Structurally, a CSP is bordered by the following: anteriorly by the genu of the corpus callosum, superiorly by the body of the corpus callosum, posteriorly by the anterior limb and pillars of the fornix, inferiorly by the anterior commissure and the rostrum of the corpus callosum, and laterally by leaflets of the septum pellucidum.2 Functionally, a CSP is a part of the limbic system, carrying connections to both the medial and basolateral limbic circuits.3 It does not communicate with the ventricular system.
Although a CSP is generally considered benign, there is evidence that enlargement of any size is associated with certain psychiatric disorders. Landin-Romero et al4 examined a large cross-disorder sample and found that 639 patients with affective and psychotic disorders showed a significantly increased prevalence of CSP, compared to 233 healthy controls. A smaller study by Degreef et al5 showed an increased prevalence of CSP in individuals with schizophrenia (23%), compared to healthy controls (2%). While some studies have attempted to demonstrate an association between CSP and symptoms of schizophrenia, they have produced inconsistent results.6 To our knowledge, our case study is the first to identify an enlarged CSP associated with schizophrenia and catatonia. Furthermore, this patient’s catatonia belonged to a subtype that was refractory to medications, but eventually responded to ECT.
Conclusion
This case report reviewed increasing evidence of an association between CSP and psychiatric conditions, specifically psychotic disorders. Hypothetically, CSP enlargement of any size has the potential to cause a mass effect to any of its surrounding structures and cause alterations to limbic system function. Currently, this has unknown implications regarding prognosis and appropriate treatment for patients who have a CSP identified on MRI. Future directions include investigation into the mechanism by which CSP might predispose to catatonia and the development of targeted interventions that may be either medical or surgical in nature.
References
- Shaw CM, Alvord EC. Cava septi pellucidi et vergae: their normal and pathological states. Brain. 1969;92(1):213–223.
- Tubbs RS, Krishnamurthy S, Verma K, et al. Cavum velum interpositum, cavum septum pellucidum, and cavum vergae: a review. Childs Nerv Syst. 2011;27(11):1927–1930.
- Das JM, Dossani RH. Cavum septum pellucidum. In: StatPearls. StatPearls Publishing; 2023. Updated Apr 2023.
- Landin-Romero R, Sarró S, Fernández-Corcuera P, et al. Prevalence of cavum vergae in psychosis and mood spectrum disorders. J Affect Disord. 2015;186:53–57.
- Degreef G, Lantos G, Bogerts B, et al. Abnormalities of the septum pellucidum on MR scans in first-episode schizophrenic patients. AJNR Am J Neuroradiol. 1992;13(3):835–840.
- Wang LX, Li P, He H, et al. The prevalence of cavum septum pellucidum in mental disorders revealed by MRI: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2020;32(2):175–184.




