
by JP Lindenmayer, MD; Beverly J. Insel, PhD; Anzalee Khan, PhD; McKenzie Osborne, BA; Abraham Goldring, MA; and Mary Seddo, BA
Drs. Lindenmayer, Insel, and Khan, Ms. Osborne and Seddo, and Mr. Goldring are with Manhattan Psychiatric Center in New York City, New York. Drs. Lindenmayer and Khan and Mr. Goldring are with Nathan S. Kline Institute for Psychiatric Research in Orangeburg, New York. Dr. Lindenmayer is with the Department of Psychiatry at New York University in New York City, New York. Mr. Goldring is with Medgar Evers College, City University of New York in Brooklyn, New York.
FUNDING: No funding was provided for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2021;18(10–12):40–46.
ABSTRACT: Objective. While clozapine is recognized as the most effective antipsychotic for individuals with treatment-resistant schizophrenia, its effects on neurocognition remain unclear. This study aimed to compare the neurocognitive effects of clozapine treatment to those of non-clozapine antipsychotics in patients with schizophrenia and to examine the role of anticholinergic burden on cognitive impairments.
Design. This was a naturalistic study. Cross-sectional data were drawn from participants with chronic schizophrenia in two clinical trials assessing cognition. Cognition was evaluated using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB). Anticholinergic burden was calculated for each medication using the Anticholinergic Cognitive Burden (ACB) scoring system. We stratified the participants treated with non-clozapine antipsychotics into high ACB score versus low ACB score groups.
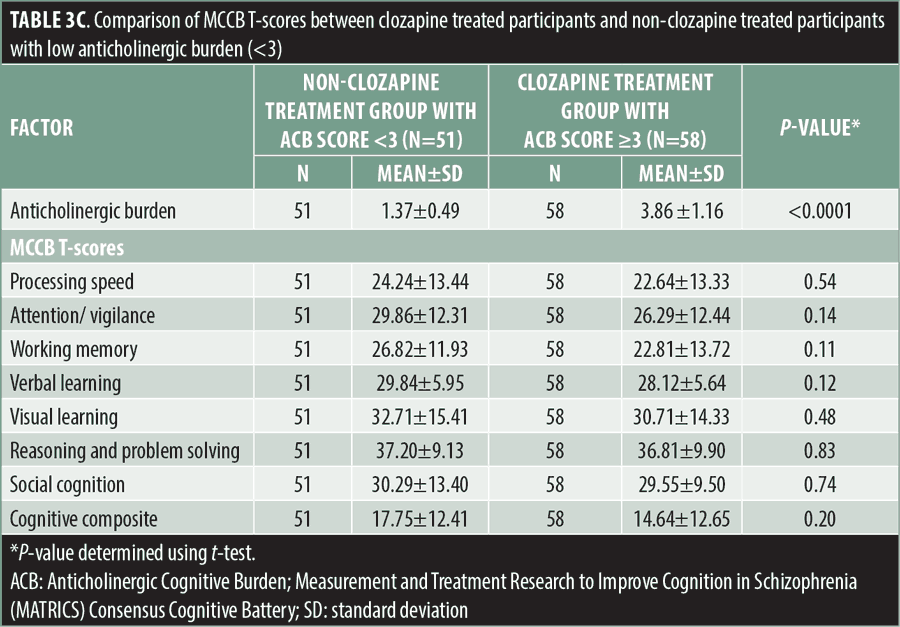
Results. One hundred and seventy participants were enrolled and treated with clozapine (n=58) or non-clozapine antipsychotics (n=112). We observed no significant differences in the MCCB T-scores between the clozapine and the total non-clozapine groups for the cognitive composite score and the seven domain scores. However, the non-clozapine high ACB group showed significant impairments in processing speed and attention/vigilance, in contrast to the non-clozapine low ACB group (p<0.05).
Conclusion. Our results show that cognitive effects of clozapine might be no different from other antipsychotics. Negative effects on neurocognition in participants treated with antipsychotics with a high ACB score were related to their total ACB score.
Keywords: Treatment-resistant schizophrenia, clozapine, non-clozapine antipsychotics, neurocognition, anticholinergic burden
Although clozapine is generally recognized as the most effective antipsychotic for individuals with treatment-resistant schizophrenia, its effects on neurocognition remain unclear.1–3 Clozapine’s antagonist activity on M1, M2, and M3 muscarinic receptors contributes to neurocognitive impairment in some individuals with schizophrenia, which has been reported in the context of clozapine’s high anticholinergic activity.4,5 One of the first studies assessing clozapine’s effect on neurocognition reported that it failed to improve any neurocognitive domains,6 while other studies found some improvement in neurocognition following clozapine treatment, particularly in executive functioning, verbal fluency, and delayed memory.1,7–9 Some of the divergent findings might have been due to small sample sizes, different clozapine dosages, concomitant medications, different cognitive measures, different durations of treatment, and lack of an adequate comparison group. Given these inconsistent results and the importance of the role of neurocognition in the daily functioning of individuals with schizophrenia, we examined the effects of stable clozapine treatment on neurocognition in a large, well-defined population of individuals with treatment-resistant schizophrenia compared to individuals with treatment-resistant schizophrenia treated with non-clozapine antipsychotics. This cross-sectional naturalistic study also examined the role of elevated anticholinergic burden on cognitive impairments in both groups. We hypothesized that neurocognition in participants with treatment-resistant schizophrenia who are treated with clozapine would be more impaired than participants treated with non-clozapine antipsychotic medications.
Materials and Methods
Study population. Data were drawn from baseline assessments of two clinical trials assessing cognitive remediation interventions among inpatients with treatment-resistant schizophrenia at a tertiary care facility.10,11 The first study (n=64; years 2012–2016) examined two methods of cognitive remediation.10 The second study (n=108; years 2015–2016) assessed social cognitive remediation in individuals with a diagnosis of chronic schizophrenia and impulsivity.11 In both studies, the same cognitive assessments were administered in a controlled environment by trained Masters-level psychologists using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB);12 MD-level raters conducted all other clinical assessments. Both datasets included the same demographic, cognitive, and clinical symptom measures. Both studies were approved by the Institutional Review Board (IRB) of the Nathan S. Kline Institute for Psychiatric Research (NKI) in Orangeburg, New York, and all participants signed an informed consent form. The second study was also approved by Weill Cornell Medical Center in Westchester, New York.
Participants were in the post-acute illness phase awaiting placement into a community residence. All participants were treated clinically by their hospital clinicians and had undergone at least two failed trials of antipsychotic medications. Those who agreed to the required blood draws were placed on clozapine.
Participant inclusion criteria for both parent studies were identical and consisted of the following: 18 years of age or older, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) diagnosis of schizophrenia or schizoaffective disorder, auditory and visual acuity adequate to complete cognitive tests, stable dose of antipsychotic medications for at least four weeks prior to enrollment, good physical health determined by physical examination and laboratory tests, capacity and willingness to give written informed consent, at least an eighth grade reading level (as evidenced from an educational assessment based on chart review or the Wide-Range Achievement Test–Third Edition [WRAT-3]),13 Positive and Negative Syndrome Scale (PANSS) score of 90 or lower, and Mini-Mental State Examination (MMSE) score of 24 or higher. For both studies, participants were excluded if they met at least one of the following criteria: inability to read or speak English, major neurological disorder, history of intellectual impairment predating onset of symptoms of psychosis, or pregnancy.
Assessments. All participants underwent assessments of cognition and psychopathology. Raters were trained and certified on the MCCB by a senior psychometrician (AK). Demographics and illness course variables, years of education, length of current hospitalization (LOS), and antipsychotic daily dose expressed in chlorpromazine (CPZ) equivalents, together with doses of any anticholinergic agents, were gathered from all participants’ medical charts. In case of antipsychotic polypharmacy, CPZ equivalent values of the different antipsychotics were added together.
Neurocognitive functions. A broad range of neuropsychological functions were assessed utilizing the MCCB, which is composed of 10 tests that target seven cognitive functioning domains, to measure cognition. The scores of these domains were combined to give an overall composite score for cognitive functioning by standardizing each of the cognitive measures (i.e., computing T-scores) and summing the T-scores.12
Psychiatric symptoms. Psychopathology symptoms were assessed using the PANSS within the week prior to baseline.
Assessment of anticholinergic burden. The anticholinergic burden of prescribed antipsychotics was assessed and rated according to the Anticholinergic Cognitive Burden (ACB) scoring system14 and the Anticholinergic Burden Calculator (http://www.anticholinergicscales.es/) for consistency. The ACB scale categorizes medications on a scale of 0 to 3, according to their anticholinergic activity, with a higher score denoting higher anticholinergic activity.15 An ACB score of 1 indicates possible anticholinergic effect based on in-vitro evidence that the medication has antagonist activity at muscarinic receptors. An ACB score of 2 means definite anticholinergic effect. An ACB score of 3 represents definite anticholinergic effect.
If participants were on a polypharmacy regimen, the ACB score of each antipsychotic was calculated and the results were summed. In addition to the antipsychotics ACB score, we added the ACB score for benztropine (ACB score=3) if a participant received concomitant anticholinergics; however, other psychotropics, such as mood stabilizers, were not included in the ACB calculation since information on their anticholinergic load is not well-established. Each participant could receive a total ACB score of 1 to 10.
Assessment of clozapine plasma levels. To assess the relationship of clozapine and norclozapine plasma levels with neurocognitive function, we calculated the clozapine/norclozapine plasma concentration ratio as an index of drug metabolism. The primary clozapine metabolite norclozapine is a partial-agonist at the M1, M3, and M5 muscarinic sites, in contrast to the parent compound clozapine, which is an antagonist on these sites.
Statistical analysis. We used the T-scores for the MCCB cognitive domains, corrected for age and sex. ACB scores were summed across all individual antipsychotic medications to create a total ACB score for each participant. Since a total anticholinergic cognitive burden scale score of 3 or greater is considered clinically relevant,14 we stratified the non-clozapine-treated participants into two groups: a high ACB score group (≥3) versus a low ACB score group (<3), which resulted in three study groups (clozapine-treated, low ACB non-clozapine-treated, and high ACB non-clozapine-treated) forming the independent variables. We compared the clozapine group to the non-clozapine total group, the clozapine group to the two non-clozapine groups separately, and the two non-clozapine groups to each other on all cognitive and clinical variables. Because the clozapine group included a considerable number of participants who were also on an additional antipsychotics, we also compared the clozapine-only group with the clozapine combination group.
Descriptive statistics were computed as mean and standard deviation (SD) for continuous variables and as absolute and relative frequencies for categorical variables. All variables of the three groups were compared by means of t-tests (for 2-group comparison) or by one-way analysis of variance (ANOVA) (for 3-group comparison) for continuous variables and by means of Chi-square test for categorical variables. To further explore these associations, linear regression was performed with the MCCB composite, with its domains as the dependent variables and treatment with clozapine and non-clozapine antipsychotics as the independent variables. We controlled for years of education, race (White, Black, other), days on clozapine or non-clozapine antipsychotic treatment, substance use (No, Yes), smoking status (No, Yes), LOS (defined as date of consent minus date of admission), and total CPZ equivalents (to control for total cumulative antipsychotic exposure). We did not include age or sex as covariates in the analyses because the MCCB T-scores were a priori corrected for these demographic variables. All analyses were conducted using SAS (version 9.4; SAS Institute Inc., Cary, North Carolina).
Results
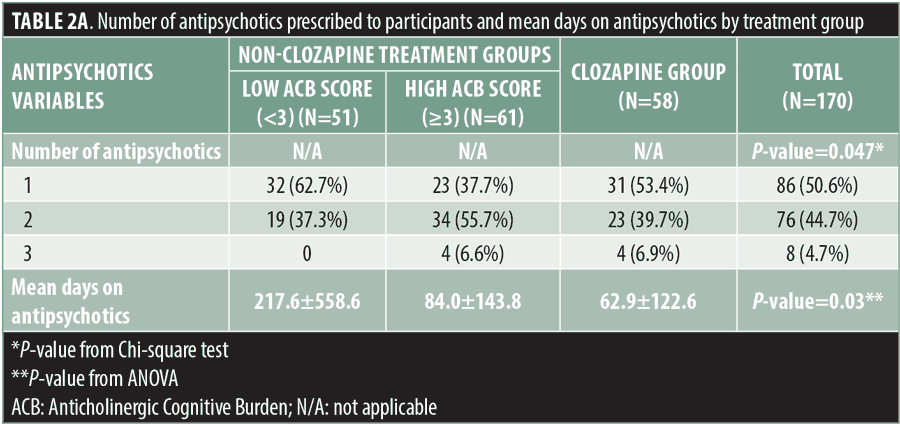
The initial sample consisted of 172 participants, two of whom were excluded due to inaccurate medication information. The remaining 170 participants were organized into two groups: clozapine group (n=58) and non-clozapine antipsychotic group (n=112). The majority were male (82%), Black (54%), had received a high school education or less (72%), and had a history of substance use (68%) (Table 1). Compared to the total non-clozapine-treated participants, the clozapine-treated participants were younger (age in years: 35.7±11.5 vs. 42.0±12.4; p<0.002), had more years of education (education in years: 12.3±1.8 vs. 11.5±2.1; p<0.02), and were prescribed a higher total CPZ equivalent daily antipsychotic dose (868.7±338.2 vs. 542.9±353.6; p<0.0001). Furthermore, 51.2 percent of patients were prescribed one antipsychotic, 43.5 percent received two antipsychotics, and 4.7 percent received three different antipsychotics concomitantly. The four most frequently prescribed antipsychotics were clozapine (34%), haloperidol (29%), olanzapine (20%), and risperidone (18%). All participants had been on stable antipsychotic treatment for at least two months before baseline assessment.
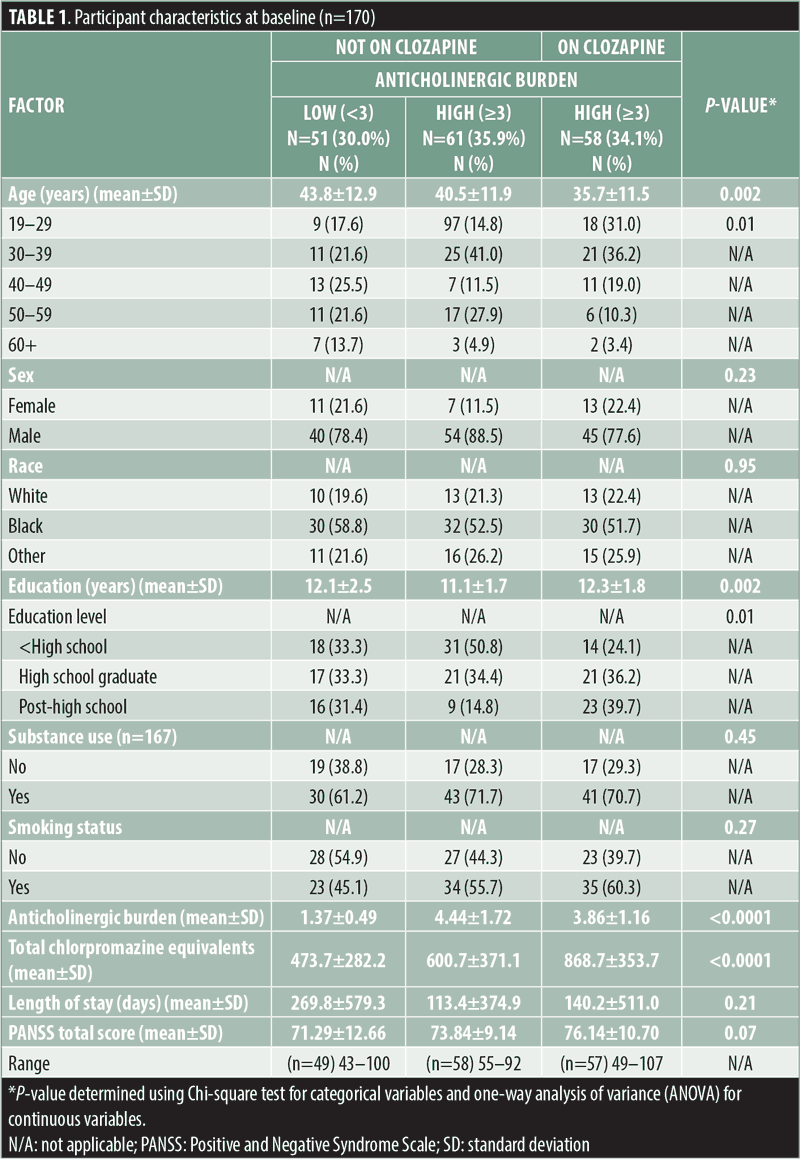
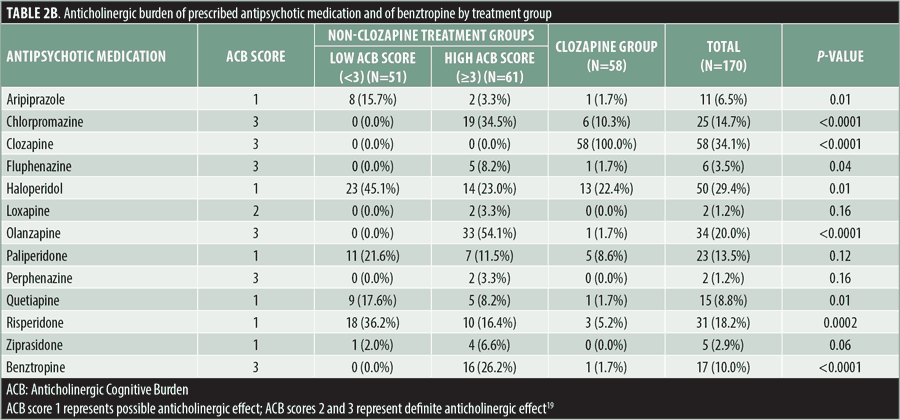
The mean ACB score for the entire sample was 3.32±1.81. The mean score of ACB of the clozapine group was 3.86±1.16, while the non-clozapine group’s mean ACB score was 3.04±2.02. The non-clozapine treatment group was further divided into two subgroups according to the anticholinergic burden of their respective antipsychotic medications: high ACB score (≥3; n=61, mean=4.44±1.72) and low ACB score (<3; n=51, mean=1.37±0.49). Among the non-clozapine treatment group with low ACB scores, 37 percent were on two drugs; 59 percent of the non-clozapine treatment group with high ACB scores were on 2 to 3 drugs, and 47 percent of the clozapine treatment group were on 2 to 3 drugs (Tables 2a and 2b). The mean CPZ equivalent doses were 473.7±282.2mg for the low ACB score non-clozapine treatment group, 600.7±371.1mg for the high ACB score non-clozapine group, and 868.7±375.9mg for the clozapine treatment group, and they were significantly different between groups (p≤0.0001). The mean CPZ equivalent doses were significantly correlated with the corresponding ACB scores (r=0.36; p<0.0001). As expected, the anticholinergic burden was significantly higher in the clozapine treatment group compared with the non-clozapine treatment group (3.86±1.16 vs. 3.04±2.02; p=0.001) (Table 1).
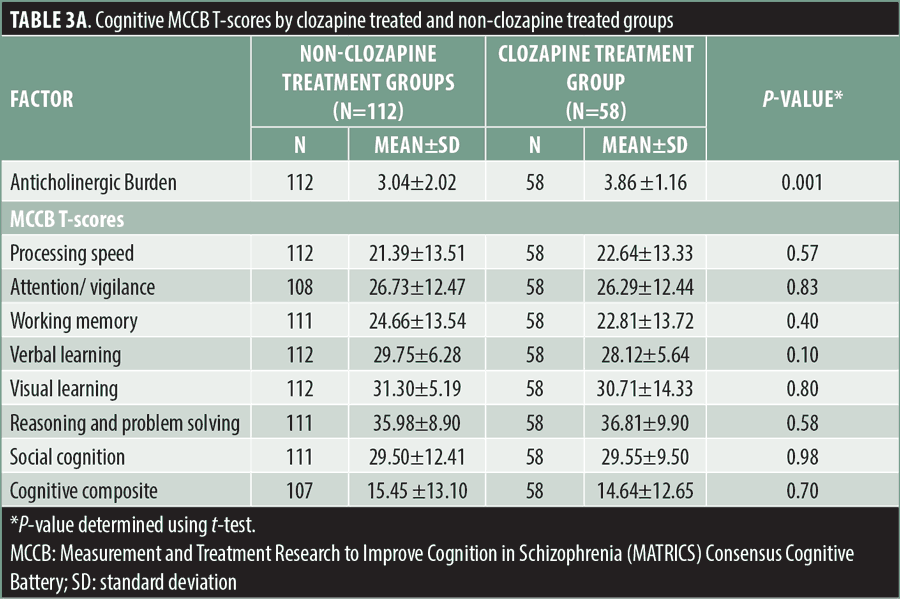
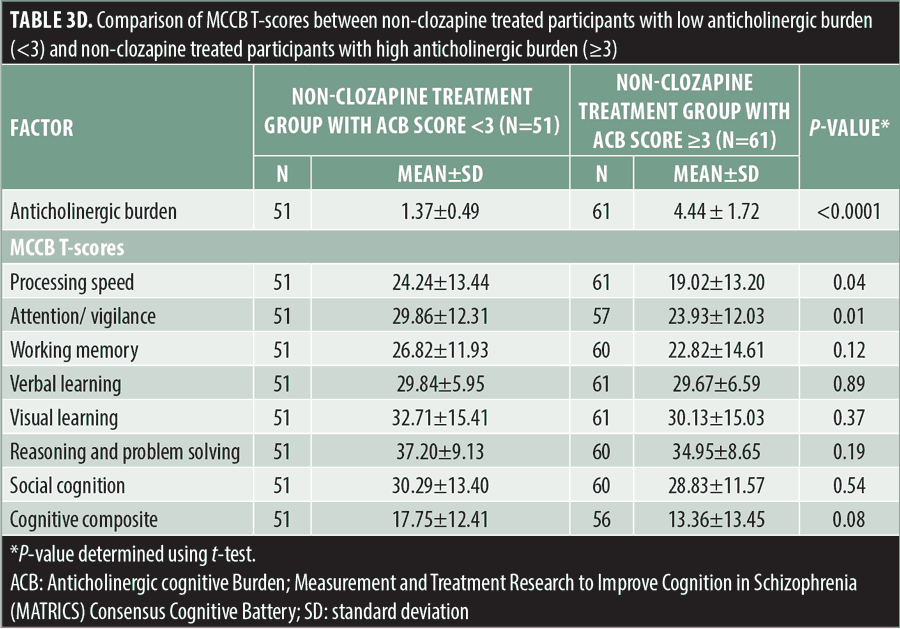
Neurocognition. No significant differences were observed in the MCCB neurocognitive composite T-scores or domain T-scores between the clozapine and total non-clozapine treatment groups (Table 3a). No significant differences were observed in mean MCCB T-scores between either the high ACB or low ACB non-clozapine-treated groups, compared to the clozapine treatment group (Tables 3b and 3c). In contrast, the high ACB non-clozapine treatment group scored significantly lower on the MCCB processing speed (p=0.04) and attention/vigilance (p=0.01) domains than the low ACB non-clozapine treatment group (Table 3d).
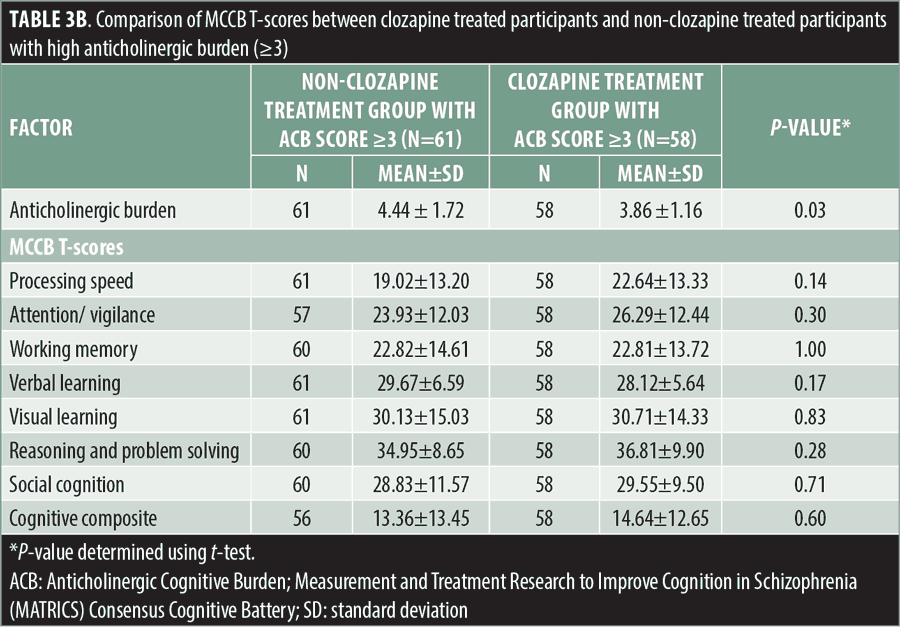
Since 47 percent of the participants in the clozapine treatment group were on 2 to 3 drugs, which potentially diluted the clozapine effect, we stratified the clozapine group into those receiving only clozapine (n=31) and those receiving clozapine and another antipsychotic (n=27). No significant difference in any of the MCCB cognitive domains between these two groups (all p-values >0.66) was seen using linear regression. Similarly, the MCCB cognitive domain scores of the non-clozapine treatment group (n=112) and the participants receiving only clozapine (n=31) were compared. Again, no significant difference between any of the MCCB cognitive domain scores of these two groups was detected (all p-values >0.22). However, we found significantly higher ACB scores in the high ACB score non-clozapine treatment group compared to the clozapine-only group (4.44±1.72 vs. 3.86±1.16; p=0.03).
To assess whether clozapine rather than the ACB score was associated with neurocognition, we compared the clozapine treatment group with the total non-clozapine treatment group, controlling for ACB. We found that clozapine was not significantly associated with any of the MCCB neurocognitive domains. However, we also found that the neurocognitive domain of attention/vigilance was significantly negatively associated with ACB scores, regardless of whether we included clozapine use in the regression model. Specifically, including clozapine in the regression model, attention/vigilance was significantly negatively associated with ACB analyzed as either a continuous variable (β=–1.59; 95% confidence interval [CI]:–2.78, –0.40; p=0.01) or a categorical variable with two categories: low ACB score and high ACB score (β=–5.93; 95% CI:–10.81, –1.06; p=0.02).
When comparing the clozapine treatment group to the non-clozapine treatment group with high ACB, there was no significant difference on any of the MCCB cognitive domains. Similarly, when comparing the clozapine treatment group with the non-clozapine treatment group with low ACB, no significant difference on any of the MCCB cognitive domains were found when clozapine was included in the regression model (clozapine p-values >0.28; ACB p-values >0.32). In contrast, when clozapine was excluded from the regression model and regardless of whether ACB score was analyzed as either a continuous variable (β=–2.11; 95% CI:–4.17, –0.06; p=0.04) or a categorical variable (β=–6.45; 95% CI: –12.37, –0.53; p=0.03), attention/vigilance was significantly negatively associated with ACB score.
Restricting the study sample to participants who were not treated with clozapine, the neurocognitive domain of attention/vigilance was significantly associated with ACB scores. Specifically, both the continuous ACB scores (β=–1.79; 95% CI:–3.13, –0.45; p=0.01) and categorical (high) ACB scores (β=–7.10; 95% CI: –12.14, –2.06; p=0.01) were inversely significantly associated with attention/vigilance.
Clozapine/norclozapine ratio and cognitive functions. When examining the relationship of the clozapine/norclozapine ratio with the neurocognitive domains and the composite score among participants on clozapine, it was not significantly associated with any of the MCCB T-scores (all p-values>0.21, except verbal learning, p-value=0.08).
Discussion
The present study sought to examine the effects of clozapine, compared to non-clozapine antipsychotics, on neurocognition in chronic schizophrenia in a naturalistic and cross-sectional study design. We hypothesized that clozapine would be associated with significant impairments in neurocognitive functions given its high level of anticholinergic burden. However, we found no direct increase in negative effects of clozapine on neurocognition compared to non-clozapine treatment, as measured by the MCCB and its domains.
These findings are not surprising, as participants who were treated with non-clozapine antipsychotics in the high ACB group had an even higher mean ACB score than patients treated with clozapine. Even when dichotomizing the group based on participants’ respective ACB scores, we still found no difference in cognitive functions between the clozapine treatment group and the non-clozapine treatment groups. This might relate to the protective cognitive effect of the pro-muscarinic activity of norclozapine16,17 or the younger age and higher education level of our clozapine treatment group, compared to the non-clozapine treatment groups. In contrast, we observed that among participants treated with non-clozapine antipsychotics, participants with high ACB scores scored lower on all MCCB neurocognitive domains compared to those with low ACB scores, with processing speed and attention/vigilance achieving statistical significance.
Overall, individuals treated with medications carrying a high anticholinergic burden performed more poorly in some neurocognitive functions. Similarly, an independent predictor for performing worse in attention/vigilance functioning among the non-clozapine treatment patients was a high ACB score. Similar to our findings, Rehse et al,3 in a retrospective record-based analysis of 104 patients with schizophrenia, all of whom were receiving antipsychotics, either mono- or polypharmacy, including clozapine, observed no detrimental cognitive effects from clozapine, irrespective of its high anticholinergic load. These results might need to be considered in the context of the naturalistic design of our study, whereby the clozapine and non-clozapine treatment groups significantly differed in age, educational level, and dosage of their respective antipsychotic medications.
While the CPZ equivalent dose was the highest among the clozapine treatment group, compared to the two non-clozapine treatment groups, it was associated with a lower ACB score than the high ACB non-clozapine treatment group. This might, in part, explain why we did not find a greater negative effect on cognition by clozapine. Our results indicated that the cognitive impact of the ACB score is an important mediator in negative cognitive effects in participants treated with antipsychotics carrying a high level of ACB.
Our negative results on the relationship of the clozapine/norclozapine ratio on cognition differ from those of Rajji et al,18 who reported that higher clozapine/norclozapine ratios were associated with poorer working memory. Similarly, Molins et al19 reported that higher clozapine/norclozapine ratios were associated with impaired executive function, and more recently, McArdle et al20 reported that the only significant correlation between cognitive tests and the clozapine/norclozapine ratio was for poorer performance on the Symbol Digit Modalities test. In contrast to our study, McArdle et al20 did not find any overall correlation between ACB score and neurocognition in their study.
Our findings implicate the anticholinergic burden of antipsychotic drugs as the primary contributor to the cognitive impairment in participants treated with antipsychotics. This effect has been shown in previous studies where higher levels of serum anticholinergic activity (SAA) were found to be associated with poorer verbal recall,4,21 poorer verbal working memory, verbal learning, and memory.22 Similar to our findings, antipsychotics with high anticholinergic properties were found to primarily impair attention and processing speed but had no effects on other aspects of cognition, including intelligence, working memory, executive functioning, and motor speed.23 However, others have found more extensive negative cognitive effects. Ang et al24 found that higher cumulative anticholinergic burden was associated with more extensive negative effects on cognition, such as executive functioning, memory/fluency, processing speed, and global cognition.
We also examined whether the treatment response status of the clozapine-treated patients affected their cognitive functions. We subdivided our 58 clozapine-treated patients using a cut off total PANSS score of less than 75,25 which resulted in a nontreatment-resistant group (n=29) and an ultra-treatment-resistant group (n=29). We compared the two groups in terms of their cognitive functions and found that only working memory was significantly different, being higher in the nontreatment-resistant group; however, there was no significant difference in their respective total ACB scores. The nontreatment-resistant group performed significantly better than the treatment-resistant group (n=24, mean=68.17 vs. n=25, mean=61.32; p=0.007) on the Personal Social Performance scale (PSP), yet there was no difference in the Clinical Global Impression scale between the two groups.
Limitations. We note several limitations to our study. First, our design was a naturalistic, nonrandomized design comparing clozapine with non-clozapine treatment groups not matched for age, severity, or length of illness, which limits the robustness of our findings. However, the three groups were all classified as treatment-resistant schizophrenia, and their PANSS ratings were comparable. Another limitation is that our sample represents individuals with chronic schizophrenia and low levels of functioning in a long-term tertiary care inpatient setting, so results might not apply to cognitively higher functioning individuals in other settings.26 We also noted the positive correlation of antipsychotic dosage and ACB score, which might have prevented us from elucidating the separate effects on cognition by anticholinergic burden on the one hand and the antipsychotic dose on the other hand. To disentangle the effect of clozapine dosage from the effect of anticholinergic burden on cognition among the clozapine treatment group, the variable clozapine dose was added to the linear regression. None of the MCCB cognitive domains were significantly associated with clozapine dose. Among the non-clozapine treatment group, we examined the association between total CPZ equivalents, as a substitute for antipsychotic dosage, and cognition. We found that none of the MCCB cognitive domains were significantly associated with the total CPZ equivalents. Another limitation was that we did not adjust for multiple comparisons, since such a conservative approach could have obscured clinically meaningful differences. Moreover, we believe that the individual cognitive domains tap into different areas of cognition. Finally, in assessing anticholinergic burden, we relied on the method proposed by Boustani et al,27 which might not have been as accurate as the measurement of SAA, which were not available to us. The strengths of our study include a large sample size, well-defined and reliable cognitive and clinical evaluations, and a clear definition of anticholinergic burden of antipsychotic medications involved with a significant duration of antipsychotic exposure.
Conclusion
Our results contribute significantly to clarifying clozapine’s effects, compared to effects of non-clozapine treatment, on neurocognitive functions in individuals with treatment-resistant schizophrenia. Our study indicates that clozapine might not be different from other antipsychotics in its effects on neurocognitive functions. However, our results showed negative effects on processing speed and attention/vigilance in participants treated with non-clozapine antipsychotics with a high anticholinergic burden. It appears that memory, executive function, and working memory were not affected. We found that this negative effect was related to the total ACB score. Given that ACB score was significantly correlated with total antipsychotic load, clinicians should be aware of these negative cognitive effects when using high antipsychotic dosages, whether with clozapine or with non-clozapine antipsychotics.
References
- Meltzer HY. Risperidone and clozapine for treatment-resistant schizophrenia. Am J Psychiatry. 1999;156:1126–1127.
- Rajji TK, Uchida H, Ismail Z, et al. Clozapine and global cognition in schizophrenia. J Clin Psychopharmacol. 2010;30(4):431–436.
- Rehse M, Bartolovic M, Baum K, et al. Anticholinergic loads on cognitive functions in patients with schizophrenia. Schizophr Res Treatment. 2016;2016:8213165.
- Strauss ME, Reynolds KS, Jayaram G, Tune LE. Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res. 1990;3(2):127–129.
- McGurk SR, Carter C, Goldman R, et al. The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry. 2005;162(5):1013–1016.
- Hoff AL, Faustman WO, Wieneke M, et al. The effects of clozapine on symptom reduction, neurocognitive function, and clinical management in treatment-refractory state hospital schizophrenic inpatients. Neuropsychopharmacology. 1996;15(4):361–369.
- Buchanan RW, Holstein C, Breier A. The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol Psychiatry. 1994;36(11):717–725.
- Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(Suppl B):82–87.
- Potkin SG, Fleming K, Jin Y, Gulasekaram B. Clozapine enhances neurocognition and clinical symptomatology more than standard neuroleptics. J Clin Psychopharmacol. 2001;21(5):479–483.
- Lindenmayer JP, Ozog VA, Khan A, et al. Predictors of response to cognitive remediation in service recipients with severe mental illness. Psychiatr Rehabil J. 2017;40(1):61–69.
- Lindenmayer JP, Fregenti S, Kang G, et al. The relationship of cognitive improvement after cognitive remediation with social functioning in patients with schizophrenia and severe cognitive deficits. Schizophr Res. 2017;185: 154–160.
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213.
- Wilkinson, GS. The Wide Range Achievement Test–Third Edition (WRAT–3). Wilmington, DE: Wide Range, Inc., 1993.
- Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69(7):1485–1496.
- Campbell N, Maidment I, et al. The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc. 2013;61(S1):S142–S143.
- Weiner DM, Meltzer HY, Veinbergs I, et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology (Berl). 2004;177(1–2):207–216.
- Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012;62(3): 1544–1553.
- Rajji TK, Mulsant BH, Davies S, et al. Prediction of working memory performance in schizophrenia by plasma ratio of clozapine to N-desmethylclozapine. Am J Psychiatry. 2015;172(6):579–585.
- Molins C, Carceller-Sindreu M, Navarro H, et al. Plasma ratio of clozapine to N-desmethylclozapine can predict cognitive performance in treatment-resistant psychotic patients. Psychiatry Res. 2017;258:153–157.
- McArdle PA, De Mel V, DeMonte V, et al. An investigation into the relationship between clozapine treatment and cognitive performance in patients with treatment resistant schizophrenia. Schizophr Res. 2019;206:450–451.
- Perlick D, Stastny P, Katz I, et al. Memory deficits and anticholinergic levels in chronic schizophrenia. Am J Psychiatry. 1986;143(2):230–232.
- Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–1062.
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161(1):116–124.
- Ang MS, Abdul Rashid NA, Lam M, et al. The impact of medication anticholinergic burden on cognitive performance in people with schizophrenia. J Clin Psychopharmacol. 2017;37(6):651–656.
- Campana M, Falkai P, Siskind D, Hasan A, Wagner E. Characteristics and definitions of ultra-treatment-resistant schizophrenia–a systematic review and meta-analysis. Schizophr Res. 2021;228:218–226.
- Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126:124–131.
- Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320.




