
By Rui Lopes, MD, and Bernardo Dias Pereira, MD
Dr. Lopes is with the Faculty of Medicine at the University of Porto in Porto, Portugal. Dr. Pereira is with the Department of Endocrinology and Nutrition at the Hospital do Divino Espírito Santo de Ponta Delgada in Azores, Portugal, and the Pre-clinical Section of the Medicine Course, Faculty of Sciences and Technology, at the University of Azores in Ponta Delgada, Portugal.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The authors report no conflicts of interest for the published content.
Innov Clin Neurosci. 2018;15(5–6):30–33
Abstract: Delirium, acute confusional states, and secondary psychosis have been associated with several medical conditions, including endocrine disorders. In the context of diabetes mellitus (DM), it has been mostly related to hypoglycemia and rarely occurs in association with hyperglycemia, outside of the context of a hyperglycemic hyperosmolar state or diabetic ketoacidosis. Here, we describe a case of delirium and psychotic symptoms associated with hyperglycemia in a patient with poorly controlled Type 2 DM as an attempt to alert clinicians to this rare association. We also review the pathophysiological mechanisms that might lead to the onset of delirium in the context of hyperglycemia.
Delirium or acute confusional state is a heterogeneous neuropsychiatric disorder that commonly occurs in acute care hospitals and is related to significant morbidity and mortality.1 It is characterized by changes in awareness and attention, cognitive disturbance, psychomotor abnormalities, emotional disturbances (e.g., depression, anxiety, irritability, or euphoria), and a disrupted sleep–wake cycle.2 Depending on psychomotor activity, it can present as a hyperactive, hypoactive, or mixed subtype.3 The onset of delirium is usually rapid/acute (hours) or insidious/subacute (days), and there could be a fluctuating course during the day. It can often be attributed to severe acute or chronic medical illness, changes in electrolyte balance (e.g., hyponatremia, hypercalcemia), metabolic or endocrine disturbances, infection, surgery, adverse medication effects, or alcohol and drug withdrawal syndrome.1,2
Early recognition of delirium in general medical practice is essential for prompt evaluation, diagnosis, and treatment.1 As the etiopathogenesis of delirium can be multifactorial, it is essential to recognize poorly controlled diabetes mellitus (DM) as a potential trigger. Cases of delirium secondary to hypoglycemia in patients with DM are not rare,4–7 whereas reports of secondary delirium in the context of hyperglycemia (not related to a hyperglycemic hyperosmolar state or diabetic ketoacidosis) are scarce, and these have been reported mainly in patients with Type 1 DM (DM1).8,9 Here, we present a case of delirium and psychotic symptoms triggered by hyperglycemia in a patient with poorly controlled Type 2 DM (DM2) and without previous psychiatric illness.
Case Presentation
We present the case of an 80-year-old woman, single with no children, who lived alone, and, up until presentation, was able to perform activities of daily living self-sufficiently. She reported to have the support of friends and neighbors, and she lived in the small community of São Miguel Island, Azores, Portugal. She had completed two years of college before dropping out during her third year, and her last job was as an elder care-worker. She had no personal or family psychiatric antecedents and no history of alcohol or illicit drug addictions. The patient was diagnosed with DM2 two years previously and was treated with oral antihyperglycemic drugs (OADs), but she voluntarily discontinued these agents six months prior to admission to our hospital. She was referred to the emergency room, accompanied by a psychologist and a social worker (from the community home-team of the local health center), with an approximately one-month-long history of behavioral changes, including disorganization, confusion, agitation, irritability and impulsivity, insomnia, social isolation, and illogical thinking with delusional ideas of persecution. Additionally, the patient started neglecting her hygiene, lived in poor conditions (e.g., there were dirty dishes covered with remnants of spoiled food in her home), and presented with superficial burns on her eyebrows and hair, which had occurred while she was cooking. According to information provided by neighbors, the month before presentation, she began behaving like “totally different person.” She began taking walks during the night, making street disturbances (e.g., dropping rubbish in the small village river), talking “strangely” to herself, lying to others, and threatening neighbors (verbally and physically). Sometimes she displayed excessive familiarity with strangers (e.g., talking profusely to them or allowing them to enter her house and steal from her). She also reportedly went grocery shopping to an excessive degree, spending a great deal of money on food that she insisted was necessary for her to make dinner. These behaviors were completely unlike the patient’s typical behaviors, tended to fluctuate in severity during the course of the day, and resulted in a mild-to-moderate impairment of everyday activities.
During evaluation, the medical team noted that the patient’s appearance was unkempt, and though alert, she was disoriented to time, space, and situation. Her attention was challenging to maintain, and presented a waxing and waning course. She showed signs of irritability and impulsiveness, often getting up from her chair claiming she needed to leave because it was time to go home “to make dinner.” Her mood was anxious and rapidly shifted, and she exhibited disorganized and confabulatory speech, difficulty in remembering details of recent events, and perseverance in answers. She described delusional ideas of persecution that were poorly systematized. She did not appear to have hallucinations, but she had no insight or critical appraisal for her clinical situation or the need for medical treatment.
Physical examination was remarkable for the presence of herpes zoster virus (HZV), which appeared as healing lesions in the right T6/7 dermatomes and had erupted two weeks prior to hospital admission. No other physical (e.g., fever) or neurological (e.g., meningism, headache, neck stiffness, or other focal abnormality) signs or symptoms were found.
Biochemical investigation revealed serum glucose of 367mg/dL; (normal: 70–110mg/dL) and a glycated hemoglobin level (HbA1c) of 10.1 percent (normal: <6.5%). Full blood count with platelets, thyroid, parathyroid, hepatic and renal functions, electrolytes, lipid profile, folic acid, vitamin B12, and C-reactive protein were within the normal range. Summary analysis of urine revealed glycosuria (1,000mg/dL) and the absence of ketonuria. Toxicological screening for illicit drugs in urine and alcohol in blood; testing for viral markers of human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV); and syphilis serology were all negative. Electrocardiography, chest radiography, and a brain computed tomography (CT) scan showed no relevant abnormalities.
A brief neuropsychological assessment was performed, and the patient scored 18 out of 30 points on the Portuguese version of the Mini-Mental State Evaluation (MMSE; abnormal score: ?22 points, according to the level of education of the patient; the MMSE utilized here was standardized for the Portuguese population).10,11 She exhibited abnormalities on the cognitive domains of orientation (time and space), attention and calculation, recall, and visuoconstruction ability. During the assessment, she was easily distracted and had difficulty directing and maintaining focus.
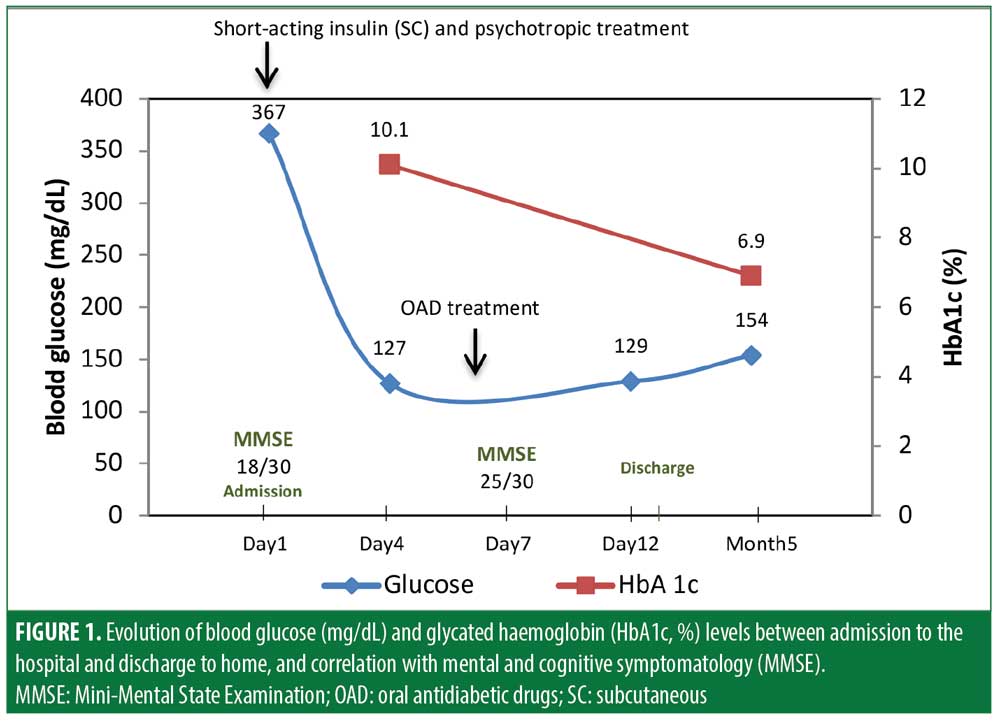
Our presumptive diagnosis was an acute confusional state with psychotic symptoms associated with hyperglycemia in the context of poorly controlled DM2. The patient was admitted to our psychiatric ward for psychopathological and metabolic treatment. As criteria for hyperglycemic hyperosmolar state and diabetic ketoacidosis were not present, we initiated a sliding scale protocol of short-acting insulin (subcutaneous), as required every six hours, and psychotropic medication (oxazepam, 15mg, 3x/day; haloperidol, 1mg, 3x/day). For the first four days, the patient’s symptoms of delirium tended to fluctuate in severity during the course of the day and were characterized by worsening spatial–temporal disorientation in the evenings. Over the course of 5 days, we observed a gradual improvement in her glycemic profile, with blood glucose dropping to a pre-prandial range of 120mg/dL to 130mg/dL after one week (Figure 1).We also observed a simultaneous remission of her psychotic symptoms and improvement in insight regarding her health status. On Day 6, we initiated metformin 850mg and vildagliptin 50mg twice a day, while the haloperidol and oxazepam were gradually reduced. We also prescribed gabapentin 200mg three times daily for postherpetic neuralgia. On Day 7, her MMSE score was 25/30 points, and we observed a substantial improvement in orientation, attention, and retention cognitive domains,10,11 also reflecting global improvement in her overall mental state. Thirteen days after admission, the patient demonstrated she was capable of using a glucose meter and managing her DM therapeutic regimen. We referred her for gerontopsychiatry and internal medicine consultations. We prescribed the same OAD treatment regimen instituted at the ward and increased her gabapentin to 400mg three times daily. We also prescribed oxazepam 15mg to take as needed for insomnia. At her five-month follow-up appointment, she reported maintaining an oriented mental state, normal sleep–wake cycle, and independence in daily activities of living (personal and instrumental). She also reported having no subjective cognitive problems. Her glycemic control was within the desired range (HbA1c: 6.9%).
Discussion
This clinical vignette highlights the importance of considering hyperglycemia (in the absence of a hyperglycemic hyperosmolar state or diabetic ketoacidosis) as a causal factor for delirium and psychotic symptoms in patients with DM2. To our knowledge, there are only two previous reports of delirium associated with hyperglycemia, but both involved patients with DM1.8,9 In our patient, we excluded other organic causes by conducting a thorough clinical assessment of the patient, including analytical evaluations and imaging (i.e., brain CT). Illicit substance-/medication-induced delirium and other medical/neurologic conditions were also excluded at the time of delirium. We believe that the reversal of symptoms following effective control of serum glucose with an OAD regimen, along with the administration of low doses of anxiolytic and antipsychotic medications, support our diagnosis of delirium and psychosis triggered by hyperglycemia.2
Differential diagnosis of dementia is essential, as dementia can have different therapeutic and prognostic implications.12 There is ample evidence that patients with dementia are at increased risk of developing delirium.12 However, in our patient, the absence of clinical signs of dementia and cognitive deterioration in the months following hospital discharge, as well as the reported full recovery to her previous psychosocial functioning and level of independence, excluded dementia as the cause of her delirium.
Epileptic activity, such as in nonconvulsive status epilepticus, might also result in prolonged altered mental state and delirium.13 However, an electroencephalogram was not performed in our evaluation, since a normal neurological assessment and the absence of a history suggestive of epilepsy excluded this possibility.
Pain can also be a precipitating factor for delirium.1 We believe this was unlikely in our case because neuropathic pain secondary to postherpetic neuralgia occurred after global improvement of the patient’s mental state. Although it is used more frequently for neuropathic pain and epilepsy, gabapentin has been shown to be a useful treatment for several psychiatric disorders, such as anxiety, bipolar, and substance use-related disorders.14 The prescription of gabapentin for postherpetic neuralgia after our patient’s global improvement likely did not have had a precise role in the resolution of her delirium and psychotic symptoms, though it might have contributed to the control of anxiety symptoms and maintenance of the asymptomatic status of our patient following discharge.
Additionally, the presence of HZV-related lesions that erupted two weeks before admission and HZV postherpetic neuralgia might also suggest the possibility of concomitant HZV encephalitis, which could have contributed to the clinical presentation of delirium and psychotic symptoms in our patient through direct injury of the central nervous system.15 Characteristically, encephalitis presents with fever, meningism, headache, and a combination of other signs and symptoms, such as changes in consciousness (ranging from drowsiness to coma), delirium, confusion, abrupt changes of behavior, psychotic symptoms (hallucinations and/or delusions), epileptic activity, and other focal neurological symptoms. Although a lumbar puncture is essential to exclude encephalitis, we did not perform one in our patient due to unremarkable findings of the physical and neurological examinations (on admission to the hospital and in the ward) and the absence of abnormalities on the brain CT scan. Furthermore, the full clinical recovery seen after implementation of insulin treatment and psychotropic medication made the diagnosis of encephalitis unlikely in our patient.
Psychotic symptoms are a clinical feature that can often be present in delirium.16 Accordingly, the delusional ideas of persecution in our patient were incoherent, poorly systematized, and remitted quickly after treatment. There were no clinical features suggestive of acute or transient psychotic disorders, primary psychotic disorders (such as schizophrenia), late-onset psychosis, or affective disorders with psychotic symptoms. Additionally, we also excluded acute stress disorder, dissociative state, and malingering.
DM has been recognized as a risk factor for the development of delirium in internal medicine wards17 and postoperative procedures.18 Interestingly, hyperglycemic levels have been associated with a higher risk of hyperactive delirium in critically ill patients.19 Furthermore, perioperative hyperglycemia has been documented as an independent risk factor for delirium,20,21 while preoperative glucose control has been associated with a reduced incidence of postoperative delirium and cognitive dysfunction.22 Although the multifactorial etiopathogenesis of delirium is well-established and is believed to involve neurotransmission and neuroinflammation abnormalities,1 the exact mechanisms of DM-related delirium are not fully understood. In acute severe hyperglycemic hyperosmolar state or diabetic ketoacidosis, delirium or even coma might develop because of severe global brain dysfunction. Accordingly, changes in electrolyte balance, metabolic abnormalities, and nutritional deficiency or excess (including glucose, complex B vitamins, water, and antioxidants) have been associated with the development of delirium through direct influence on normal brain functions.23 Therefore, both hyperglycemia and hypoglycemia secondary to DM could hypothetically be associated with delirious states due to brain dysfunction. Interestingly, white and gray matter microstructural abnormalities (which can include vascular pathology) and cognitive dysfunction have been found consistently in both DM1 and DM2 patients.24
A recent study with human neuronal culture cells of the brain revealed hyperglycemia-induced inflammation and neurodegeneration through increased production of neuronal reactive oxygen species.25 Hypothetically, accumulated neuroinflammation and neurodegeneration resulting from oxidative stress induced by chronic hyperglycemia might result in progressive structural abnormalities of the brain. In patients with DM, these abnormalities could lead to brain dysfunction and lower cognitive reserve, increasing the risk of delirium.
Studies in rodent diabetes models have also found several biochemical, structural, ultrastructural, and neurotransmitter abnormalities associated with brain dysfunctioning.26 Interestingly, it has also been documented in streptozotocin-induced diabetic rats that hyperglycemia can have a higher detrimental impact than recurrent hypoglycemia on normal brain functions, including cognition (e.g., impaired spatial memory).27 Although studies on animal models and in-vitro culture cells cannot be directly translated to humans, results from those studies suggest a connection between hyperglycemia and brain dysfunctioning that warrants further investigation. Studies that explore the exact links between hypo- and hyperglycemia of poorly controlled DM and delirium, cognitive dysfunction, and dementia are needed.
Conclusion
Our case presentation should alert clinicians to the possibility of psychopathological changes caused by hyperglycemia in patients with poorly controlled DM2 (outside the setting of a hyperosmolar hyperglycemic state). Psychopathological decompensation in patients with DM can also lead to nonadherence with the OAD treatment regimen, which can then create a vicious cycle of medical and psychiatric illnesses. Increased awareness of a secondary form of delirium and psychotic symptoms elicited by hyperglycemia in the context of poorly controlled DM2 could lead to suitable and timely treatment with antipsychotic medication and, more importantly, targeted antihyperglycemic agents.
References
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–1466.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):
248–254. - Knezevi? A, Hrnjakovic L. [Hypoglycemia—a delirious state with vestibular hallucinations]. Med Pregl. 1991;
44(5–6):231–232. Article in Croatian. - Fishbain DA, Rotundo D. Frequency of hypoglycemic delirium in a psychiatric emergency service. Psychosomatics. 1988;29(3):346–348.
- Shehadeh N, Kassem J, Tchaban I, et al. High incidence of hypoglycemic episodes with neurologic manifestations in children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11 Suppl 1:183–187.
- Knezevi? A. [Hypoglycemia and states of confusion]. Med Pregl. 1990; 43:221–223. Article in Croatian.
- Maharajh HD, Konings M. Fire setting in a patient with hyperglycaemic delirium. J Forensic Sci. 2006;51(4):940.
- Sahoo S, Mehra A, Grover S. Acute hyperglycemia associated with psychotic symptoms in a patient with Type 1 diabetes mellitus: a case report. Innov Clin Neurosci. 2016;13(11–12):25–27.
- Folstein MF, Folstein SE, McHugh PR. Mini-mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- Morgado J, Rocha CS, Maruta C, et al. Novos valores normativos do mini-mental state examination. Sinapse. 2009;9(2):10–16.
- Morandi A, Davis D, Bellelli G, et al. The diagnosis of delirium superimposed on dementia: an emerging challenge. J Am Med Dir Assoc. 2017;18(1):12–18.
- Wu CJ. Acute confusional state in type 2 diabetic patient: non-convulsive status epilepticus. Geriatr Gerontol Int. 2009;9(1):89–91.
- Berlin RK, Butler PM, Perloff MD. Gabapentin therapy in psychiatric disorders: a systematic review. Prim Care Companion CNS Disord. 2015;17(5).
- Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system—Prognosis, diagnostics and treatment. J Infect. 2015;71(3):281–293.
- Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519–522.
- Fortini A, Morettini A, Tavernese G, et al. Delirium in elderly patients hospitalized in internal medicine wards. Intern Emerg Med. 2014;9(4):435–441.
- Bucerius J, Gummert JF, Walther T, et al. Impact of diabetes mellitus on cardiac surgery outcome. Thorac Cardiovasc Surg. 2003;51(1):11–16.
- Heymann A, Sander M, Krahne D, et al. Hyperactive delirium and blood glucose control in critically ill patients. J Int Med Res. 2007;35(5):666–677.
- Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):
862–866. - Yildizeli B, Ozyurtkan MO, Batirel HF, et al. Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg. 2005;79(3):1004–1009.
- Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242.
- Sanford AM, Flaherty JH. Do nutrients play a role in delirium? Curr Opin Clin Nutr Metab Care. 2014;17(1):45–50.
- Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:
60–71. - Kumar P, Raman T, Swain MM, et al. Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented tuberous sclerosis complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol. 2017;54:
238–254. - Sickmann HM, Waagepetersen HS. Effects of diabetes on brain metabolism—is brain glycogen a significant player? Metab Brain Dis. 2015;30(1):335–343.
- Malone JI, Hanna S, Saporta S, et al. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes. 2008;9(6):
531–539.





