Changes over time were estimated with repeated measures, mixed-effects (ME) models. The repeated measures model included visit (as a categorical variable), as a fixed effect. Either an unstructured or autoregressive AR(1) covariance matrix was used to fit the within-subject repeated measures effect for the models. P-values for pairwise comparisons were adjusted with Tukey’s method, and P-values less than 0.05 were considered to be statistically significant. Statistical analyses were conducted using SPSS version 19.0 (IBM Corp., Armonk, New York, United States).
Results
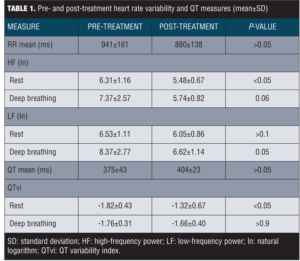
Physiological data. Data on cardiac measures are summarized in Table 1. There was no significant difference between pre- and post-treatment RR mean or QT interval mean (P-values for both >0.05). Analyses of the LF (ln) data at rest showed no significant change from pre- to post-treatment, F(1,10)=2.1, P>0.1. There was a marginally significant change in LF (ln) during deep breathing from pre- to post-treatment, F(1,9)=5.4, P=0.05. Further, we observed a significant decrease in HF (ln) at rest from pre- to post-treatment, F(1,10)=7.6, P<0.05. A marginally significant change was observed for HF (ln) during deep breathing from pre- to post-treatment, F(1,9)=4.7, P=0.06. Analysis of pre- and post-treatment QTvi revealed a significant increase in QTvi during rest, F(1,10)=8.0, P<0.05. No significant change was observed for QTvi during deep breathing from pre- to post-treatment, F(1,9)<1, P>0.9.
Safety and efficacy data. During the study, there were nine nonserious adverse events reported, including five events (three cases of insomnia, one case of loose bowels, and one case of bruxism) determined to be possibly related to study drug and four events (one case of worsening asthma, one case of cough, one case of sleepwalking, and one case of irritability) determined to be unrelated to study drug. There was one reported serious adverse event (suicidal ideation) that was determined to be possibly related to study drug. In addition, there was some attrition secondary to adherence issues.
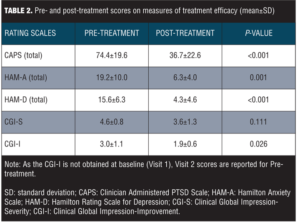
Mean scores on measures of treatment efficacy pre- and post-treatment are reported in Table 2. Scores on the CAPS, HAM-A, and HAM-D decreased significantly from Visit 1 to Visit 7 (all P-values <0.05). CGI-S scores at Visit 1 and Visit 7 did not differ significantly (P=0.11), while CGI-I scores showed a significant reduction from Visit 2 to Visit 7 (P<0.05).
Discussion
The primary aim of the current study was to examine the effects of escitalopram treatment on cardiac vagal and sympathetic function in veterans with PTSD and comorbid depression. It was hypothesized that this treatment would result in a normalization of HRV and a decrease (or no change) in QT variability. Contrary to our hypothesis, however, we observed a significant decrease in HF HRV and increased QTvi in our sample. On the secondary outcomes measures, treatment with escitalopram was associated with improvement in PTSD and depressive symptoms. Data pertaining to drug safety further indicates that the study drug was well tolerated.
Autonomic dysregulation, including reduced HRV, has been well documented in PTSD and depression.[3–7,30,31] The reduction in HF HRV observed in this study suggests that treatment by escitalopram may lead to further reduction in parasympathetic (vagal) control of heart rate and reduced flexibility in autonomic responding. This finding of decreased HF HRV is consistent with previous work indicating that antidepressant use can reduce HRV and cardiac vagal control,[32,33] which has been associated with increased risk of cardiovascular disease and mortality.
One might speculate that the observed increase in QTvi is probably due to anticholinergic effect of the drug. The mean post-treatment QT interval was higher but did not reach significance in this study. This probably indicates a decrease in vagal tone and shift toward sympathetic dominance. On the other hand, the increase in QTvi might also reflect a possible effect of escitalopram on the QT interval. In 2011, the United States Food and Drug Administration (FDA) released a safety announcement regarding QT interval prolongation seen at higher doses of citalopram,20 with a revised warning released the following year,[34] and there have been other reports of QT prolongation in patients treated with citalopram or escitalopram.[21,22,35,36] Normal QTc values are less than 430 milliseconds (ms) in men and less than 450ms in women. For risk of sudden cardiac death, borderline QTc in men is 431 to 450ms, and in women 451 to 470 ms. An abnormal QTc in men is a QTc greater than 450ms, and in women, greater than 470ms. The FDA analysis estimated that 40mg citalopram would increase QTc interval by 12.6ms. As drug-induced QT prolongation is more likely to be clinically significant in patients with cardiac risk factors,[37] clinicians should be aware of such risk factors when prescribing these medications and provide additional monitoring as needed, particularly for patients who are using other QT-prolonging agents. Such measures include avoiding use of escitalopram or citalopram in patients with long QT interval and monitoring and correcting hypokalemia and hypomagnesaemia.
Limitations. There are a number of limitations that should be acknowledged in this study. First, due to the small sample size, further research is needed to validate these findings. Second, as there was no placebo or control group for comparison in this study, we cannot rule out the possibility that factors other than escitalopram treatment contributed to our findings. Also, we did not obtain QT corrected (QTc) from the initial 12-lead ECG recording according to Bazett’s formula.[38] Instead, our study was designed to calculate QTvi. The procedure of calculating QTvi utilized in our study is not an accurate way to calculate QTc as it is set by the person choosing a lead that has a good “R” wave. QTc (a measure of the time between the start of the Q wave and the end of the T wave adjusted for heart rate) is usually digitally calculated by EKG machines. However, none of the commercially available software guarantees reliable estimates of this interval due to technical difficulties in identifying the end of the T-wave before it merges with the baseline. We calculated QTvi by Berger’s formula which, like QTc, is a measure of cardiac repolarization lability and marker of sudden death and malignant ventricular arrhythmias. Ours is the first study to report QTvi changes following escitalopram therapy in PTSD.
Conclusion
In conclusion, we found that escitalopram treatment was associated with a significant reduction in clinical symptoms in veterans with PTSD and comorbid depression, though we also observed decreased HRV and increased QT variability in our sample. Increased QT variability and decreased HRV have each been associated with a greater risk of cardiovascular disease and mortality, and it is conceivable that the two in combination may increase this risk even further. Given the increased risk of mortality in patients with depression and anxiety, it will be important for future research to further address the long-term health implications of antidepressant treatment for PTSD and depression.
References
1. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060.
2. Thomas JL, Wilk JE, Riviere LA, et al. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614-623.
3. Cohen H, Kotler M, Matar MA, et al. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997;41(5):627–629.
4. Cohen H, Kotler M, Matar MA, et al. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry. 1998;44(10):1054–1059.
5. Cohen H, Benjamin J, Geva AB, et al. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000a;96(1):1–13.
6. Cohen H, Kotler M, Matar M, Kaplan Z. Normalization of heart rate variability in post-traumatic stress disorder patients following fluoxetine treatment: preliminary results. Isr Med Assoc J. 2000;2(4):296–301.
7. Shah AJ, Lampert R, Goldberg J, et al. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 2013;73:1103–1110.
8. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381.
9. Binah O, Rosen MR. Mechanisms of ventricular arrhythmias. Circulation. 1992;85(1 Suppl):I25–31.
10. Dobson CP, Kim A, Haigney M. QT variability index. Prog Cardiovasc Dis. 2013;56(2):186–194.
11. Atiga WL, Calkins H, Lawrence JH, et al. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9(9):899–908.
12. Yeragani VK, Pohl R, Jampala VC, et al. Increased QT variability in patients with panic disorder and depression. Psychiatry Res. 2000;93(3):225–235.
13. Yeragani VK, Pohl R, Balon R, et al. Twenty-four-hour QT interval variability: increased QT variability during sleep in patients with panic disorder. Neuropsychobiology. 2002;46(1):1–6.
14. Yeragani VK, Berger R, Pohl R, Balon R. Effect of age on diurnal changes of 24-hour QT interval variability. Pediatr Cardiol. 2005;26(1):39–44.
15. Yeragani VK, Pohl R, Jampala VC, et al. Effect of posture and isoproterenol on beat-to-beat heart rate and QT variability. Neuropsychobiology. 2000a;41(3):113–123.
16. Pohl R, Balon R, Jayaraman A, et al. Effect of fluoxetine, pemoline and placebo on heart period and QT variability in normal humans. J Psychosom Res. 2003;55:247–251.
17. Kubzansky LD, Koenen KC, Spiro A 3rd, et al. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64(1):109–116.
18. Robert S, Hamner MB, Ulmer HG, et al. Open-label trial of escitalopram in the treatment of posttraumatic stress disorder. J Clin Psychiatry. 2006;67(10):1522–1526.
19. Höschl C, Svestka J. Escitalopram for the treatment of major depression and anxiety disorders. Expert Rev Neurother. 2008;8(4):537–552.
20. United States Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). Published August 24, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm269086.htm. Accessed June 2, 2015.
21. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs. 2006;7(3):256–263.
22. Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. Br Med J. 2013;346:f288.
23. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
24. Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90.
25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: The American Psychiatric Press, Inc.; 2001.
26. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55.
27. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;139:297–305.
28. Guy W. ECDEU Assessment Manual for Psychopharmacology Revised (DHEW Publication ADM 76-338). Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs;1976.
29. Berger RD, Kasper EK, Baughman KL, et al. Beat to beat QT interval variability. Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565.
30. Stein PK, Carney RM, Freedland KE, et al. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. 2000;48(4–5):493–500.
31. Koschke M, Boettger MK, Schulz S, et al. Autonomy of autonomic dysfunction in major depression. Psychosom Med. 2009;71(8):852–860.
32. Licht CM, de Geus EJ, Zitman FG, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry. 2008;65(12):1358–1367.
33. Licht CM, de Geus EJ, van Dyck R, Penninx BW. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry. 2010;68(9):861–868.
34. U.S. Food and Drug Administration. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. Published March 28, 2012. http://www.fda.gov/drugs/drugsafety/ucm297391.htm. Accessed June 2, 2015.
35. Zivin K, Pfeiffer PN, Bohnert AS. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40mg. Am J Psychiatry. 2013;170(6):642–650.
36. Hanash JA, Hansen BH, Hansen JF, et al. Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the DEpression in patients with Coronary ARtery Disease (DECARD) trial. J Cardiovasc Pharmacol. 2012;60:397–405.
37. Vieweg WV, Hasnain M, Howland RH, et al. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med. 2012;125(9):859–868.
38. Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–370.