 by Michele Meglio, PhD Student; Enrica Olivola, MD, PhD; Marco Santilli, BSC; Francesco Lena, MSC, PhD; Diego Centonze, MD, PhD; Matteo Bologna, MD, PhD; and Nicola Modugno, MD, PhD
by Michele Meglio, PhD Student; Enrica Olivola, MD, PhD; Marco Santilli, BSC; Francesco Lena, MSC, PhD; Diego Centonze, MD, PhD; Matteo Bologna, MD, PhD; and Nicola Modugno, MD, PhD
Dr. Meglio is with IRCCS Neuromed in the Unit of Neuro-Ophthalmology in Pozzilli, Italy; he’s a PhD student in Neuroscience at University of Rome Tor Vergata in Rome, Italy. Drs. Olivola, Santilli, Lena, Centonze, Bologna, and Modugno are with the IRCCS Neuromed in their Department of Neurology. Dr. Modugno is the head of the Parkinson Center in the same Institute. Prof. Centonze is Full Professor of Neurology at the University of Rome Tor Vergata and Director of the Department of Neurology at the IRCCS Neuromed. Dr. Bologna is with the Department of Human Neurosciences at Sapienza University in Rome, Italy.
FUNDING: This study was supported by Carl Zeiss Vision Italia S.p.A. – Castiglione Olona (Italy).
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. In Parkinson’s disease (PD), postural abnormalities such as lateral axial dystonia (LAD) are relatively common. Evidence suggests that both peripheral and central mechanisms contribute to these postural abnormalities. We previously reported an improvement in LAD following the use of prisms in two PD patients. Here, we further investigate the effects of prismatic lenses in a case series of nine patients with PD and LAD.
Methods. Nine patients underwent an orthoptic evaluation and were provided with prismatic lenses. Patients were evaluated at baseline and after one and three months of permanent prismatic lens use and again re-evaluated one month after the discontinuation of prismatic lens use.
Results. We found a linear relationship between disease duration and LAD severity. Compared to basal measurements, we observed a slight improvement in LAD. Furthermore, we found a significant reduction in self-perceived back pain due to the use of prismatic lenses. There was no significant association between the individual effects of prismatic lenses in patients with PD and their baseline LAD or other clinical and demographic features (all P>0.05).
Conclusion. The present pilot study provides novel data on the possible effectiveness of prismatic lenses for LAD treatment in PD patients.
Keywords: Lateral axial dystonia, pisa syndrome, Parkinson’s disease (PD), prisms, oculo-motor system
Innov Clin Neurosci. 2021;18(1–3):xx–xx
In Parkinson’s disease (PD), postural abnormalities such as lateral axial dystonia (LAD) are relatively common. LAD, also called Pisa Syndrome (PS), is defined as lateral trunk flexion that is worsened by walking and reduced by passive mobilization or supine positioning. The deformity concerns the coronal plane overall, although some degrees of forward trunk flexion and rotation could also be seen.1 The diagnosis of LAD is based on the clinical evaluation of the trunk lateral dislocation. A wall goniometer is often used to bring out a lateral flexion of at least 10 degrees, which is considered the main diagnostic sign.1,2 LAD can be distinguished from scoliosis by traditional radiography, noting the typical vertebral rotation. Moreover, it could be helpful to note that scoliosis does not resolve in the supine position.3,4 Typically LAD in PD develops chronically and progressively worsens.
To date, little data are available concerning the epidemiology of LAD in PD. A multicenter Italian study estimates a prevalence of 8.8 percent.5 The same study asserts that patients with LAD are older with a significantly longer disease duration. They also have a more severe illness, a veering gait, and a worse quality of life. A recent study has also shown a significant association between motor impairment and degree of lateral trunk bending, indicating that a rise in lateral bending increases the probability of greater motor impairment.6
The precise pathophysiology of LAD has not yet been clarified. It appears to result from both musculoskeletal and neurological abnormalities, including basal ganglia dysfunction, defective sensorimotor integration, and postural control abnormalities.1,7,8 More recently, it has been hypothesized that oculomotor dysfunction and altered visual processing are responsible for generating postural abnormalities in patients with parkinsonism.9
The treatment of LAD in PD remains a difficult issue today. Pharmacological and nonpharmacological interventions are considered an integrated part of the management plan to improve the quality of life of patients. LAD can develop subacutely after any type of medication change to manage PD symptoms. This is why it is critcal that the first step is a correct and weighed pharmacological management.10
Only a preliminary study suggests an improvement of the postural imbalance in PS trough deep brain stimulation on subthalamic nucleus.11 Spinal surgery should represent an option in carefully selected patients and only be used for complex spinal deformities that are not responsive to the conservative treatment.12
Treatment with botulinum toxin, proposed in selected patients with an evident and excessive muscolar hyperactivity, seems to be a valid option only to reinforce the rehabilitative management.13 Rehabilitative treatment remains the most used approach in most cases of LAD in PD, as well as customized postural exercises intervention.14,15
There are studies showing a beneficial effect of prismatic lenses on postural abnormalities in various neurological conditions.15,16 A prism is a transparent, solid triangular refracting medium with a base and apex; its apical angle determines its power. A prism of one prism diopter power (Δ) produces an apparent displacement of one centimeter to an object situated one meter away.17 Light entering the prism will deviate toward its base, an image appears shifted to the apex, and the eye examined or treated tend to deviate toward its apex.16 A base of a prism on a spectacle, and so the orientation of the displacement in space, can be vertical, horizontal, or oblique, in a range of 360 degrees.
Prisms are regularly used in ophthalmology, both for diagnostic and therapeutic purposes.They are also often used for the treatment of nonproperly ophthalmological disorders. In neurology, prisms have been used for a long time in the treatment of visual neglect and in visual field defects, such as cases of acquired brain injury.17
Moreover, the results of some studies regarding the use of prisms in the management of postural anomalies in other pathological conditions are interesting. A substantial improvement of the predominant postural imbalance in left-hemiparetic patients due to horizontal prism adaptation has been shown.18 Similarly, another study demonstrated a long-lasting reduction in postural asimmetry by prism adaptation in patients with right brain lesion without neglect.19
Another preliminary study found that low-power prisms at specific orientations in patients with adolescent idiopathic scoliosis could cause postural changes which could be potentially helpful in controlling scoliotic curves.20
Some studies seem to indicate an association between vertical heterophoria and balance control, showing that the application of vertical prisms on healthy subjects, even of a small power, can have complex effects on postural control.21,22
Objective
In a prior study, we saw an improvement in LAD following the use of low-power horizontal prismatic lenses in two patients with PD.23 This result provided support for a direction-specific effect of prism adaptation in postural abnormalities. Based on this report, we aimed to further investigate the effect of prismatic lenses on LAD in a larger sample of patients with PD.
Methods
- We enrolled nine subjects with PD and LAD (4 female, 5 male). Inclusion criteria were as follows:
- Subjects must be at least 18 years of age
- Subjects must have a diagnosis of PD based on the diagnostic criteria of the United Kingdom Brain Bank
- Subjects must have a Mini-Mental State Examination score greater than 14
- Subjects must have Hoehn-Yahr Stage 1 to 3
- Subjects must have LAD of at least 10 degrees
Individuals with the following exclusion criteria were excluded from the study:
- Spinal morphostructural changes
- Atypical parkinsonism
- Use of antagonist drugs in the six months prior to enrollment
- Major spinal surgery
- Other neurological diseases
- Eye movement or visual sensorial disorders before PD diagnosis
- A binocular vision acuity less than 7/10 or other ophthalmological diseases
The study protocol included the collection of clinical and pharmacological history. LAD was measured in degrees and photographed in frontal projections with the patient positioned against a wall goniometer. The presence and evaluation of lower back pain intensity was assessed using the visual analog scale (VAS).
An orthoptic evaluation was performed, and patients were provided with prisms. Power and orientation of prisms varied from subject to subject in terms of momentary patient feedback and binocular fusion stability.10 To optimize the use of prisms, ground-in prisms were used.10
We evaluated patients during their usual dopaminergic therapy: at baseline (T0), after one month (T1), and after three months (T2) of permanent prismatic lens use, and one month after treatment suspension (T3).
The study was reviewed and approved by the local ethics committee and all subjects provided written informed consent.
Results
The patient mean age ±1 standard deviation (SD) was 66.8±8.2 years (range 58–78 years). The mean disease duration was 8.0±3.70 years (Hoehn-Yahr Stage 1–3). The mean Unified Parkinson’s Disease Rating Scale Part III score ±1 SD was 31.3±8.5. The mean LAD was 22.8±6.7 degrees. Spearman’s rank correlation showed a linear relationship between disease duration and LAD severity (R=0.67; P=0.04), implying that patients with longer disease duration had more severe LAD.
Friedman analysis of variance (ANOVA) revealed a slight though nonsignificant change in the goniometric measurements of LAD patients at T1, T2, or T3 (F9,3=5.95, P=0.11) (Figure 1). Compared to basal measurements, we observed an average improvement in lateral deviation of about 19.5 percent (data pooled from T1, T2, and T3), ranging from -40 percent to 133 percent. However, there was no significant association between the individual percentage improvement after prismatic correction of oculomotor disorders and baseline LAD (R=-0.30; P=0.42) or other clinical and demographic features (all P>0.05).
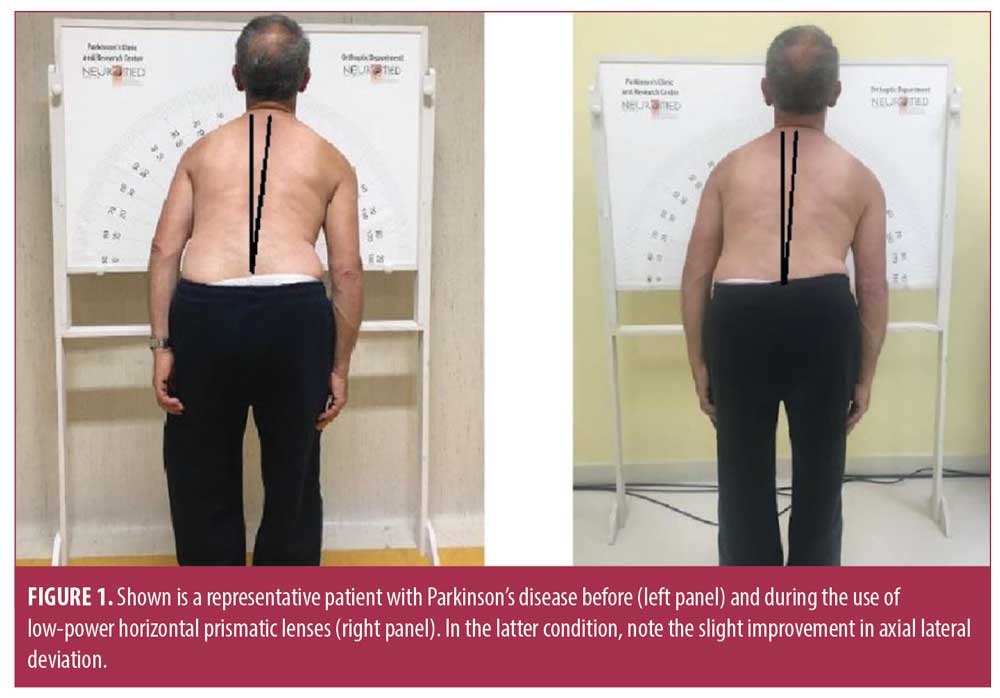
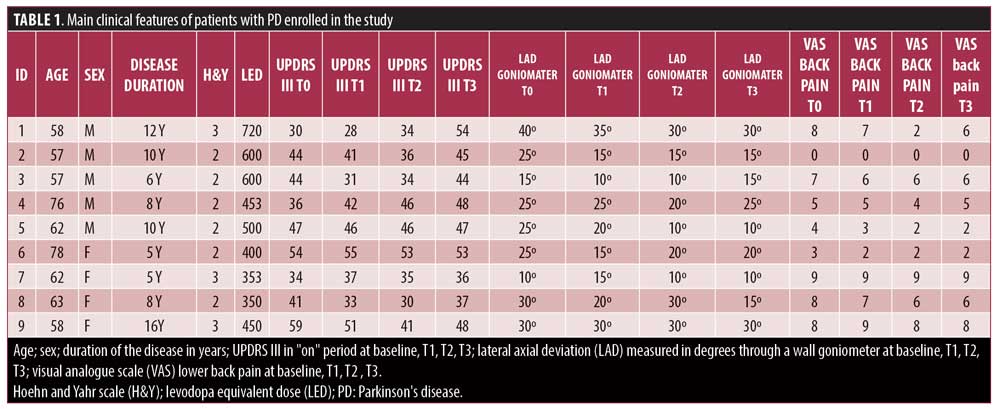
Friedman ANOVA revealed a significant change in VAS back pain in patients at T1, T2, and T3 (F9,3=12.62, P=0.005). Compared to basal measurements, we observed an average improvement in VAS back pain of about 18.6 percent (data pooled from T1, T2, and T3). However, there was a trend toward a significant association between the individual percentage improvement of baseline axial deviation and VAS back pain changes (R=-0.57; P=0.09) after prismatic correction of oculomotor disorders in patients. The main clinical features of the patients are summarized in Table 1.
Discussion
The present pilot study conducted on a case series of nine patients with PD and LAD represents the extension of a previous study in which we reported an improvement in LAD following the use of low-power horizontal prismatic lenses in two patients with PD.23
In the present study, as suggested in the previous study, we confirmed a trend toward LAD improvement in patients with PD of about 20 percent, though this result achieved low statistical significance. We can exclude that the paucity of statistical significance of our data was due to morphological alterations of the spinal cord, as this was an exclusion criterion during the preliminary investigation. We can also exclude that the data of statistical significance was due to clinical evaluation and not objective assessment of LAD using computerized techniques since the two evaluation methods recently have been found to be equivalent.24 Conversely, we believe that the results regarding this improvement is attributable to the clinical variability of patients and the relatively small sample size, which likely influenced the statistical power of the tests used. It should also be noted that the variability in response to prismatic lens use could also be attributed to various pathophysiological mechanisms that might characterize LAD, which, to a large extent, are not yet fully known.
We can highlight the significant improvement in lower back pain perceived by patients, considering this as an index of something that changes. This result, therefore, is consistent with the trend toward LAD improvement.
Limitations. Two major limitations of this study are the small sample size of patients with PD and the lack of a control group with placebo treatment. Extending the study to a larger population and dividing by degrees of the severity of LAD could provide more data and aid in achieving statistical significance. We believe that dividing the sample into stages could prove that patients who benefit from prismatic treatment are those in the initial stage of the disease, with mild LAD. This would be an important finding because it would be possible for patients to obtain a significant improvement.
Conclusion
The results of this pilot study should be considered as a starting point on the plausible specific effect that orientation and power of a prism causes on the modification on postural deficits in PD, as well as, on the role that the oculomotor system would have in the genesis of postural alterations. These results might be useful in designing a further randomized, blinded, and methodical clinical trial with a larger patient sample.
Acknowledgements
The authors would like thank Dr. Lorenzo Meglio for help in manufacturing the glasses used for the study.
References
- Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011;10:538–549.
- Tinazzi M, Juergenson I, Squintani G, et al. Pisa syndrome in Parkinson’s disease: an electrophysiological and imaging study. J Neurol. 2013;260:2138–2184.
- Vrtovec T, Pernus F, Likar B. A review of methods for quantitative evaluation of spinal curvature. Eur Spine.
2009;18:593–607. - Tinazzi M, Fasano A Geroin C, et al. Italian Pisa syndrome study group. Pisa syndrome in Parkinson’s disease: an observational multicenter Italian study. Neurology. 2015;85:1769–1779.
- Geroin C, Artusi CA, Gandolfi M, Zanolin E, Ceravolo R, et al. Does the degree of trunk bending predict patient disability, motor impairment, falls, and back pain in Parkinson’s disease? Front Neurol. 2020;11:207.
- Geroin C, Smania N, Schena F, et al. Does the Pisa syndrome affect postural control, balance, and gait in patients with Parkinson’s disease. An observational cross-sectional study. Parkinsonism Relat Disord. 2015; 21:736–741.
- Di Lazzaro G, Schirinzi T, Giambrone MP, et al. Pisa syndrome in Parkinson’s disease: evidence for bilateral vestubulospinal dysfunction. Parkinson’s Dis. 2018;8673486.
- Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson’s disease with camptocormia. J Neurol Neurosurg Psychiatry.
2006;77(11): 1223–1228 . - Cannas A, Solla P, Floris G, et al. Reversible Pisa syndrome in patients with Parkinson’s disease on dopaminergic therapy. J Neurol. 2009;256:390–395.
- Unemura A, Oka Y, Ohkita K, Yamawaki T, Yamada K. Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson’s disease. J neurosurg. 2010;112:1283–1288.
- Ha Y, Oh JK, Smith JS, et al. Impact of movement disorders on management of spinal deformity in the elderly. Neurosurgery. 2015 ;77(suppl4):S173–S185.
- Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951–957.
- Tinazzi M, Geroin C, Gandolfi M, et al. Pisa syndrome in Parkinson’s disease: an integrated approach from pathophysiology to management. Mov Disord.
2016;31:1785–1795 - Lena F, Iezzi E, Etoom M, et al. Effects of postural exercises in patients with Parkinson’s disease and Pisa syndrome: a pilot study. NeuroRehab. 2017;41:423–428.
- Padula WV, Nelson CA, Benabib R,Yilmaz T, Krevisky S. Modifying postural adaptation following a CVA through prismatic shift of visuo-spatial egocenter. Brain Inj. 2009;23:566–576.
- Kapoula Z, Gaertner C, Matheron E. Spherical lenses and prisms lead to postural instability in both dyslexic and non dyslexic adolescents. PLoS One. 2012;7(11).
- Antony J. Prisms in clinical practice. Kerala J Ophthalmol.2017;29:79–85.
- Bansal S, Han E, Ciuffreda KJ. Use of yoked prisms in patients with acquired brain injury: a retrospective analysis. Brain Inj. 2014;28(11):1441–1446.
- Tilikete C, Rode G, Rossetti Y, et al. Prism adaptation to rightward optical deviation improves postural imbalance in left-hemiparetic patients. Curr Biol. 2001;11:524–528.
- Hugues A, Di Marco J, Lunven M, et al. Long-lasting reduction in postural asymmetry by prism adaptation after right brain lesion without neglect. Cogn Process. 2015;16 (suppl 1):S371–S375.
- Wong MS, Mak FT, Luk KDK, Evans JH, Brown B. Effect of using prismatic eye lenses on the posture of patients with adolescent idiopathic scoliosis measured by 3-D motion analysis. Prosthet Orthot Int.
2002;26(2):139–153. - Matheron E, Lê TT, Yang Q, Kapoula Z. Effects of a two-diopter vertical prism on posture. Neurosci Lett. 2007;23;423(3):236–234.
- Matheron E, Kapoula Z. Vertical phoria and postural control in upright stance in healthy young subjects. Clin Neurophysiol. 2008;119: 2314–2320.
- Santilli M, Meglio M, Varanese S, et al. Improvement of lateral axial dystonia following prismatic correction of oculomotor control disorder in Parkinson’s disease. J Neurol. 2016;263:403–440.
- Tinazzi M, Gandolfi M, Artusi CA, et al. Validity of the wall goniometer as a screening tool to detect postural abnormalities in Parkinson’s disease. Parkinsonism Relat Disord.
2019;69:159–165.





