
Innov Clin Neurosci. 2018;15(3–4):11–14
Dear Editor:
Schizophrenia is a chronic and progressive psychiatric disorder requiring long-term treatment with antipsychotics. The use of long-acting injectable antipsychotics (LAI) has been shown to increase adherence, reduce risk of relapse, and support recovery for patients with schizophrenia by achieving an optimal dopamine (D)2 receptor occupancy.1 Among several oral antipsychotics, a significant association between D2 receptor occupancy levels and serum prolactin levels has been demonstrated.2 We reported that a fluctuation range of prolactin levels after switching to paliperidone palmitate (PP) might be useful to confirm optimal D2 receptor occupancy in maintenance treatment with LAI.3 In our subsequent report, safety switching to PP was supported by sequential changes of prolactin levels matched to time profiles of serum concentrations of active moiety in a pharmacokinetic analysis study.4
Aripiprazole once-monthly (AOM) is also an atypical LAI. Though the pharmacological mechanisms of PP and AOM are different, they are both active at D2 receptors. Here, we investigate the efficacy and tolerability of AOM in patients who were switched from oral aripiprazole, as well as examine its relationship to prolactin levels based on a 12-month prospective-designed method. The ethics committee of Kusatsu Hospital, Hiroshima, approved this study.
Research outline. Patients with schizophrenia who were switched to AOM 400mg were enrolled under the following inclusion criteria: treated with aripiprazole daily for at least the previous two months, ongoing AOM treatment for more than 12 months, not having changed antipsychotic treatment, and fulfilled recommended dose switching method. A total of 10 male patients and 15 female patients, with a mean age of 43.5 and 39.5 years, respectively, entered the study after providing written informed consent. The 18-item Brief Psychiatric Rating Scale (BPRS) total scores, 9-item Drug-induced Extrapyramidal Symptoms Scale (DIEPSS) total scores, and the fasting serum prolactin levels divided by sex and daily oral aripiprazole dosage (switched from 24mg [n=8] or 30mg [n=2] in male subjects and from18 mg [n=2], 24mg [n=9] or 30mg [n=4] in female subjects) were assessed at baseline and at one, three, six, and 12 months. Multiple comparisons were performed with repeated measured analysis of variance (ANOVA), followed by post-hoc analysis.
The BPRS scores were significantly decreased at six and 12 months in female subjects, while no statistical changes were found in the BPRS scores of the male subjects or in the DIEPSS scores in both sexes from baseline to the last observed points. The prolactin levels increased at one month and three months and decreased at 12 months in the female subjects; prolactin levels consistently decreased in male subjects, but not significantly. In male subjects on the 24mg dose, the prolactin levels significantly decreased at 12 months from baseline. The time-sequential changes of the values are presented in Table 1 and Figure 1.
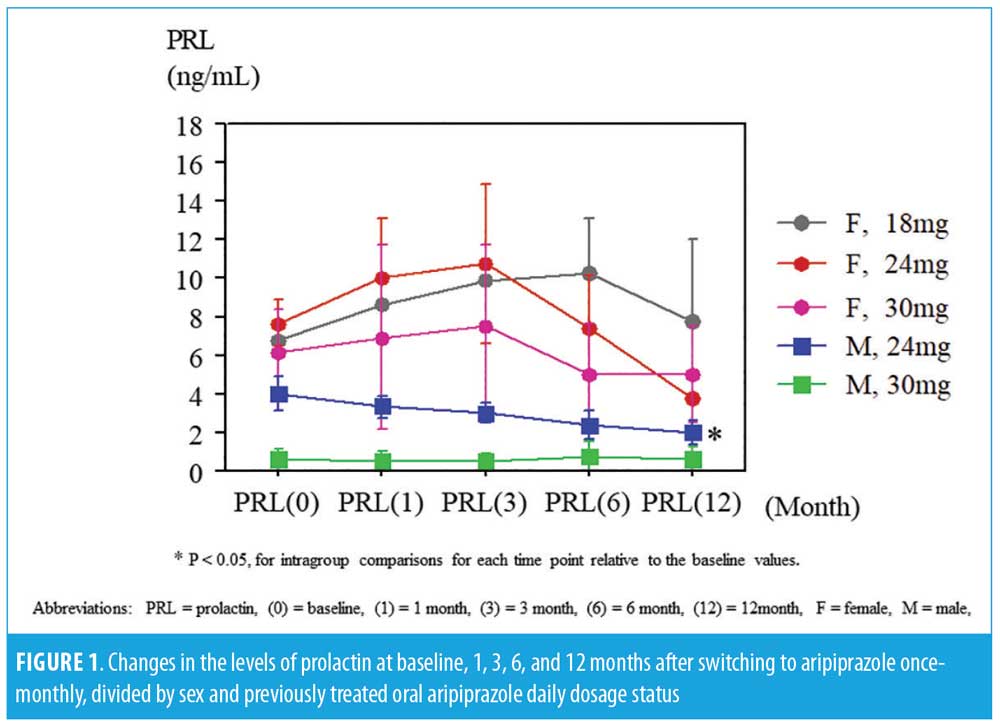
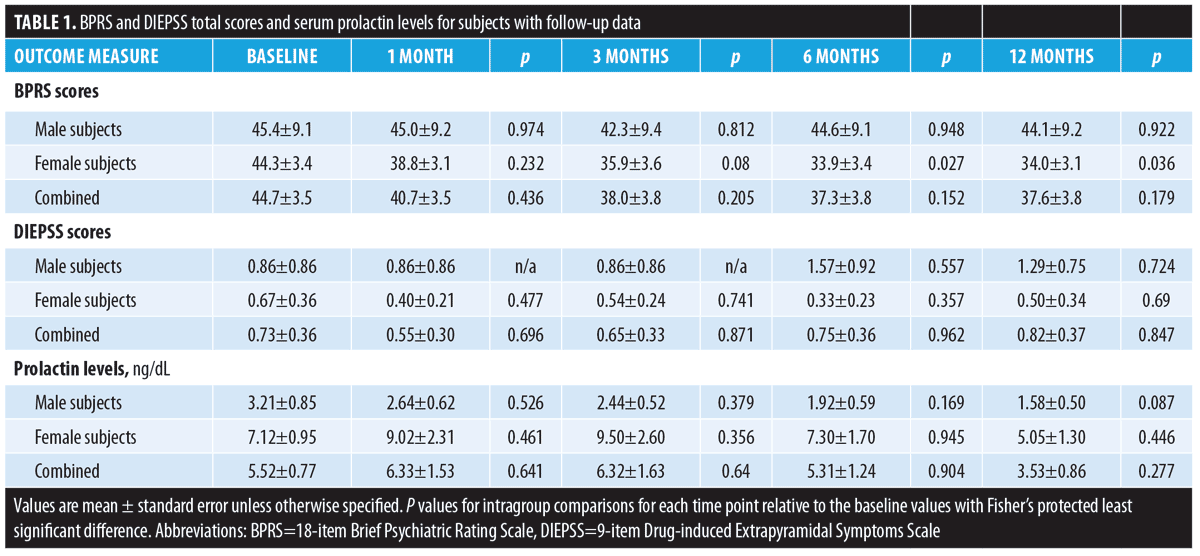
Discussion. The strength of our study demonstrates that switching to AOM is safe. The prolactin levels of our patients might correlate with D2 receptor blockade suggested by previous studies. Administration of AOM 400mg has a pharmacokinetic profile consistent with that of oral doses of aripiprazole
(10–30mg/day);5 however, a steady state serum concentration is attained after the fourth injection based on approved label method.6 The peak to trough fluctuation in serum concentration of aripiprazole is considered to be predominantly low,7 and this dopamine partial agonist causes dose-dependent hypoprolactinemia in male subjects.8 So, it is more likely that the prolactin levels are transiently elevated in female subjects and finally reduced or stabilized in all subjects.
A comparative analysis from two placebo-controlled relapse prevention studies supported maintenance of effect and safety with PP compared with oral paliperidone extended release.9 Also, a double-blind, randomized, noninferiority study reported that AOM 400mg was noninferior to oral aripiprazole 10mg or 30mg/day and had a safety profile comparable to that of oral aripiprazole.10 In a meta-analysis of randomized, controlled studies comparing the same antipsychotics, it was demonstrated that LAIs and oral antipsychotics did not differ in serious individual adverse events, while LAIs were associated with significantly lower prolactin change.11
Although the non-double-blind method, small sample size, and insufficient follow-up intervals were limitations in this study, serum prolactin levels in patients switched from daily to once-monthly aripiprazole might be consistently useful to estimate D2 receptor occupancy and confirm safety treatment with LAI in a clinical setting.
References
- Iyo M, Tadokoro S, Kanahara N, et al. Optimal extent of dopamine D2 receptor occupancy by antipsychotics for treatment of dopamine supersensitivity psychosis and late-onset psychosis. J Clin Psychopharmacol. 2013;33: 398–404.
- Arakawa R, Okumura M, Ito H, et al. Positron emission tomography measurement of dopamine D receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia. J Clin Psychiatry. 2010;71:1131–7.
- Nakamura M, Nagamine T, Sato G, et al. Prolactin levels after switching to paliperidone palmitate in patients with schizophrenia. Innov Clin Neurosci. 2016;13:28–30.
- Nakamura M, Nagamine T. Serum prolactin levels might become a useful marker for switching strategy to paliperidone palmitate in male schizophrenia patient. Asia Pac Psychiatry. 2018 Mar; 10(1). doi: 10.1111/appy.12300.
- Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44:179–187.
- Mallikaarjun S, Kane JM, Bricmont P, et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple dose study. Schizophr Res. 2013;150:281–288.
- Wakamatsu A, Aoki K, Sakiyama Y, et al. Predicting pharmacokinetic stability by multiple oral administration of atypical antipsychotics. Innov Clin Neurosci. 2013;10:23–30.
- Nakamura M, Nagamine T. Hypoprolactinemia and extrapyramidal symptoms in male schizophrenia or psychotic affective disorder patients treated with aripiprazole. Clin Neuropsychopharmacol Ther. 2012;3:18–22.
- Markowitz M, Fu DJ, Levitan B, et al. Long-acting injectable paliperidone palmitate versus oral paliperidone extended release: a comparative analysis from two placebo-controlled relapse prevention studies. Ann Gen Psychiatry. 2013;12:22.
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205:135–44.
- Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176:220–230.
With regard,
Masaru Nakamura, MD, PhD and Takahiko Nagamine, MD, PhD
Dr. Nakamura is with the Department of Psychiatric Internal Medicine, Kosekai-Kusatsu Hospital, Hiroshima, Japan. Dr. Nagamine is with the Department of Psychiatric Internal Medicine, Sunlight Brain Research Center, Yamaguchi, Japan.
Funding/financial disclosures: No funding was provided for the preparation of this letter. The authors have no conflicts of interest relevant to the content of this letter.




