 by Alok Sharma, MCh; Nandini Gokulchandran, MD; Pooja Kulkarni, MSc; Sandhya Kiran Mullangi, MPT; Khushboo Bhagawanani, BPT; Vishal Ganar, MPhil; Hemangi Sane, MD; and Prerna Badhe, MD
by Alok Sharma, MCh; Nandini Gokulchandran, MD; Pooja Kulkarni, MSc; Sandhya Kiran Mullangi, MPT; Khushboo Bhagawanani, BPT; Vishal Ganar, MPhil; Hemangi Sane, MD; and Prerna Badhe, MD
Drs. Sharma and Gokulchandran are with the Department of Medical Services and Clinical Research at NeuroGen Brain and Spine Institute in Navi Mumbai, India. Ms. Kulkani and Drs. Mullangi and Sane are with the Department of Research and Development at NeuroGen Brain and Spine Institute in Navi Mumbai, India. Dr. Bhagawanani and Mr. Ganar are with the Department of Neurorehabilitation at NeuroGen Brain and Spine Institute in Navi Mumbai, India. Dr. Badhe is with the Department of Regenerative Laboratory Services at NeuroGen Brain and Spine Institute in Navi Mumbai, India.
FUNDING: No funding was provided for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Cerebral palsy (CP) is a chronic childhood disorder that is characterized by a group of motor and cognitive impairments, resulting in abnormal movement patterns, loss of motor control, incoordination, and unbalanced posture. It can also have an impact on fine motor skills, gross motor skills, and oral motor functioning. Currently, the treatment of CP is palliative and does not cure the disease pathology. Hence, there is a need for an intervention that might be able to alter the core pathology. Autologous bone marrow mononuclear cells (BMMNC) transplantation is one of the novel treatment strategies in recent years. In this study, we presented the case of a 4-year-old male child with spastic diplegic CP who underwent two intrathecal transplantations at interval of seven months with autologous BMMNC along with neurorehabilitation program. During an overall 16-month follow-up, significant improvements were observed in motor control, coordination, balance, sitting tolerance, and memory. The abnormal ‘W’ sitting posture and scissoring gait pattern of the patient resolved. Started sitting with good head, trunk, and pelvic alignment and attained regular gait pattern; the patient started to walk independently without support as well. On objective scale, Gross Motor Functional Measure score improved from 60.67 to 81.78. The patient’s Gross Motor Functional Classification System grade improved from Level 3 to Level 2, and Functional Independent Measure score improved from 97 to 99. A comparative positron emission tomography–computed tomography (PET CT) brain scan was performed before and seven months after the first intervention, which revealed improvement in the metabolism of the anterior cingulate lobe, parietal cortex, medial temporal cortex, thalamus, basal ganglia, and cerebellum. No adverse events were recorded throughout the study. Thus, multiple cellular therapies, along with neurorehabilitation, might be a novel safe, feasible option to enhance recovery in CP.
Keywords: Cerebral palsy, autologous BMMNCs, neurorehabilitation, positron emission tomography–computed tomography (PET CT) brain scan
Innov Clin Neurosci. 2020;17(10–12):
Cerebral palsy (CP) is defined as a heterogeneous group of nonprogressive developmental disorders. It is a lifelong condition with disability that increases with age,1 with an incidence rate of 2.1 per 1000 live births.2 CP is a result of many prenatal, perinatal, and postnatal risk factors; hypoxic ischemia during the perinatal period is considered as one of the major risk factors. Periventricular leukomalacia (PVL) is the foremost underlying pathogenesis of CP.3 The clinical presentation of CP mainly depends upon the location and extent of the cerebral structures involved. It is characterized by symptoms such as muscle spasticity, involuntary movements, delayed milestones, decreased mobility, behavioral problems. Based on the type of neuromuscular deficit, five major diseased patterns of CP are spastic, flaccid, ataxia, athetoid, and mixed.4 CP affects the movement and posture causing severe neurodisability. Permanent disability in children with CP limits their participation in the society, which leads to increased family and social burden. Various treatment approaches, such as pharmacological, surgical, and multidisciplinary rehabilitation programs, are available, but they are symptomatic and do not concentrate on the neuropathology of CP.
Autologous bone marrow mononuclear cells (BMMNC) transplantation gives a new scope in the management of cerebral palsy. Mechanism of action of these cells majorly involves direct cell replacement and indirect cell repair via paracrine effect. The ability of neural plasticity, neural repair, and neurogenesis restores the functioning of the injured cells and allows normal adjacent cells to compensate for the functions of injured cells.5 Few clinical studies have shown the safety and effects of cellular therapy in various types of cerebral palsies,6,7 but these articles failed to discuss the results broadly; comparison brain positron emission tomography–computed tomography (PET CT) imaging study was not used, and the long-term follow-up was not done, which provides strong evidence on effect of cellular therapy in cerebral palsy. In this report, we treated a case of a child with spastic diplegic CP and with multiple cellular therapies after showing limited response to rehabilitation. Results of clinical improvements were correlated with objective scales, such as Gross Motor Functional Measure (GMFM), Gross Motor Function Classification System (GMFCS), and Functional Independence Measure (FIM) and (PET CT) brain imaging studies.
Case Presentation
Herein, we present a case of a 4-year-old male child who was diagnosed with CP at the age of 1 year. The child was born full term by cesarean section delivery, with birth weight of 2.75kgs and a history of delayed birth cry; he also had history of three episodes of seizures, hence he was on anti-epileptic drugs. The chief complaints were that he was unable to stand and walk independently and had tightness in both lower limbs. His milestones were delayed, as he attained rolling by 4 months, head control by 6 months, sitting with support by 8 months, sitting independently by 1 year, crawling by a little over 1 year, and standing and walking with support by 2.5 years. He underwent surgery for the right tendoachillis (TA) release at the age of 3 and was on continuous physiotherapy for two years but still had major deficits and could not walk independently.
On complete neuropsychological assessment, speech, command following, social interaction, and play behavior were good. Sitting tolerance, attention, concentration, memory, and ability to learn new tasks were fair and not age appropriate. He also had behavioral issues, such as throwing objects when angry. Additionally, all superficial and deep sensations were intact, and tone of both lower limbs was hypertonic and hyperreflexia; Babinski’s sign was positive; bilateral adductors, iliacus, psoas major, hamstrings, and calf muscles were tight; both hips were internally rotated with equinovarus foot and had scissor gait. The tone of the right upper limb was normotonic with good voluntary control and that of left upper limb was hypertonic and voluntary control was less, compared to the right upper limb. Posture, balance, and coordination were poor. Functionally, he was moderately dependent for his activities of daily living. His GMFM score was 60.67, GMFCS level was 3, and FIM score was 97.
Magnetic resonance imaging (MRI) brain suggested mildly altered signal intensity in bilateral periventricular white matter with evidence of the marked paucity of cerebral white matter and posterior limbs of bilateral internal capsules. Mild cerebral atrophy was suggestive of in utero infection with the possibility of perinatal hypoxic insult. PET CT of the brain revealed hypometabolism in anterior cingulate gyrus, parietal cortex, medial temporal cortices, basal ganglia, thalamus, and cerebellum. Sleep electroencephalogram (EEG) record suggested right side epileptic dysfunction.
Procedure. The selection of the patient was based upon the World’s Medical Association Helsinki declaration.8 The protocol was reviewed, and ethical approval was taken from the Institutional Ethics Committee (IEC) and specific to our institution. The procedure was clearly explained to the parents and informed written consent was obtained. Before the intervention, the child underwent all neurological, biochemical, and preoperative investigations to assess his fitness for anesthesia. Human granulocyte colony stimulating factor (G-CSF) injections were administered subcutaneously, 72 hours, and 24 hours prior to autologous BMMNC aspiration. Additionally, 80mL of bone marrow was aspirated from the right side anterior superior iliac spine in supine position under local anesthesia; the aspirated bone marrow was collected in heparinized tubes; mononuclear cells were separated from bone marrow and checked for viability, which was found to be 98 percent; CD34+ cells count percentage were calculated using fluorescence-activated cell sorting (FACS) analysis, which was found to be 1.69 percent. Approximately 7.36 ×108 BMMNCs were administered intrathecally through lumbar puncture between L4 and L5 spinal segments. Simultaneously, 300mg solumedrol in 500mL of Ringer lactate solution was also administered intravenously to decrease the inflammatory effects and to enhance the viability of the infused BMMNC.
After the transplantation procedure, the patient underwent a customized rehabilitation program, which included physiotherapy, occupation therapy, aquatic therapy, and special education along with dietary advice for one week. Physiotherapy exercises concentrated on stretching and strengthening exercises for the limbs and trunk, while occupational therapy concentrated on balance, coordination exercises, and trained activities of daily living. Aquatic therapy focused on strengthening exercises, postural control, balance, and gait training. Cognitive and vocational skills were trained by special education. The patient was advised to continue the rehabilitation as a home program after discharge.
There was a follow-up at seven months, where the patient was reassessed for postintervention clinical changes on objective scales and brain PET CT scan. Based on his overall improvements, he underwent a second BMMNC transplantation to further augment recovery. Approximately, 5.12 ×108 BMMNCs with 98 percent viability were administered intrathecally, and CD34+ cells were found to be 0.014 percent. Another follow-up took place at nine months after the second transplantation.
Results
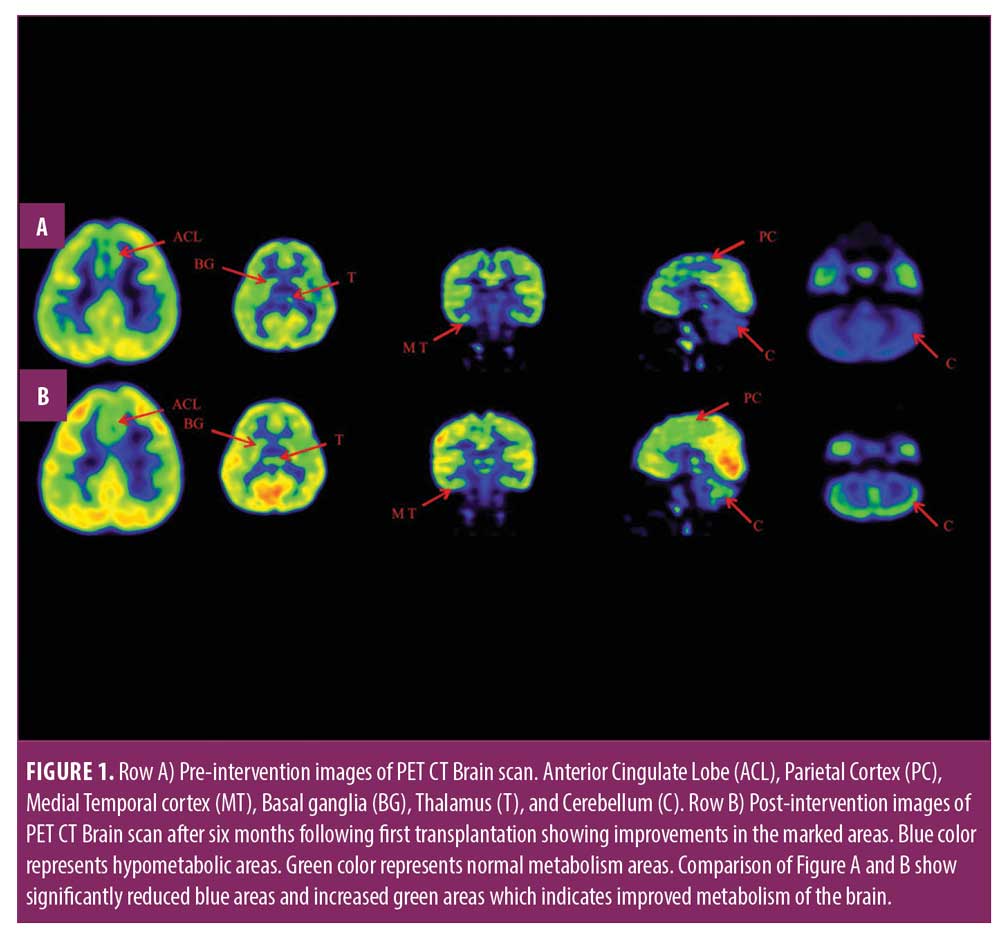
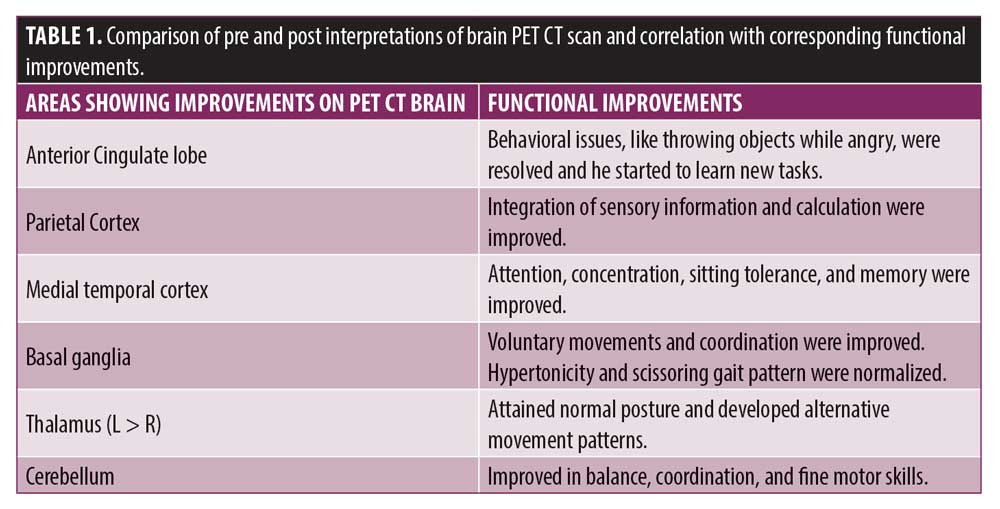
Adverse events after the first cellular therapy, the patient had mild pain at the site of insertion, which was subsided within 24 hours. A complete reassessment was done at seven months; no other adverse events and episodes of seizures were recorded throughout the course. He showed improvements in standing and walking balance, abnormal “W” sitting posture and scissoring pattern of gait had reduced, and protective and equilibrium reactions while standing and walking improved. Compared to his condition before, his weight shifts improved in sagittal and frontal planes; alignment of head, trunk, and pelvis also improved, and tightness in the hamstrings and calf muscles reduced bilaterally. The patient started performing bimanual tasks. Cognitively, his attention span and sitting tolerance were improved as well, as he attended his school regularly. GMFM score improved from 60.67 to 67.75; GMFCS grade improved from Level 3 to Level 2; and FIM score improved from 97 to 99. On comparing results of a brain PET CT scan performed before and during seven months after the first cellular therapy, significant improvement was recorded in metabolic activity in the anterior cingulate lobe, parietal cortex, medial temporal cortex, thalamus, basal ganglia, and cerebellum (Table 1).
Taking into consideration the improvements observed over the course of seven months after the first cellular therapy, he underwent a second transplantation.
The subsequent follow-up at six months after the second cellulartherapy (13 months after first transplantation) showed additional improvement in balance and coordination while sitting and standing. “W” sitting posture and scissoring gait pattern were reduced further. The patient started to maintain balance while walking on uneven surfaces and stepping with minimal support, and his GMFM score improved from 67.75 to 73.33 and the scores of GMFCS and FIM were maintained.
Nine months after the second cellular therapy (16 months post first transplantation), abnormal “w” sitting posture and scissoring gait pattern were resolved. He was sitting with good head, trunk, and pelvic alignment and attained regular gait pattern. Functionally, he started to walk independently and with minimal assistance on uneven surfaces. The patient also started stair climbing independently with minimal railing support, and his GMFM score improved from 73.33 to 81.78 and GMFCS and FIM scores were maintained.
Discussion
CP is a nonprogressive neurological disorder that is caused by brain injury or malformation that occurs while the child’s brain is under development that primarily affects body movements and muscle coordination. The extent of brain damage and involvement of brain structures decides the prognosis of the disease. The primary goal of management is to improve new motor learning pathways in the brain. Symptomatic pharmacological management and external rehabilitation approaches have a limited effect on the underlying pathology of the brain in CP,9 though cellular therapy has shown a promising outcome as a treatment strategy for CP. Various cells have been studied for their safety and efficacy.10–15 To study the effect of multiple cellular therapy in CP, we administered autologous BMMNCs intrathecally, as they are safe, easily obtainable, and involve no tumorigenicity.16 Mechanism of action of transplanted cells includes direct cell replacement and indirect cell repair via paracrine mechanisms,16 while transplanted BMMNCs migrate to the site of injury, get self-renewed, differentiate into host cells, and replace the injured neuronal cells. These self-renewed neurons improve synaptic connectivity and signal conduction in CP. BMMNCs also promote the repair process by enhancing neuroprotection and angiogenesis, decreasing inflammation, preventing apoptosis, releasing chemotactic factors, assisting in extracellular matrix tissue remodeling, and activating resident cells.17 The release of neurotrophic factors, such as nerve growth factors, and glial derived growth factors aid in survival and regeneration of the neurons18 and reduce the levels of tissue necrotic factors-α, Interleukin-1β, and 6, which promotes neuronal connectivity and signal transduction.19,20 Enhanced angiogenesis results in the formation of new blood vessels and improved blood supply to the damaged cerebral tissue, which reverses hypoxia and tissue necrosis.21 Intrathecal route of transplantation was used, as it is a safe and minimally invasive procedure that drives cells to the brain without causing any neural tissue damage;22 also, transplantation of autologous cells minimizes the risk of immune rejection.23,24 Rehabilitation exercises accelerate the recovery process by helping the mobilization of local stem cells, improving angiogenesis and release of cytokines and nerve growth factors after cellular therapy.25
Solopova et al26 conducted a study on neurorehabilitation in spastic CP, and they reported exercise maintains the optimal length of the muscles and joints range of motion, which finally establishes new standing and walking stereotypes. Thus, a combined therapeutic approach of cellular therapy and neurorehabilitation facilitates the neuroregeneration and fastens the recovery process in CP. In this case report, we evaluated the safety and long-term effects of multiple cellular therapies in spastic diplegic CP.
Here, postcellular therapy outcomes were monitored on objective scales, such as FIM, GMFM, and GMFCS and comparative PET CT scan brain neuroimaging studies. Over the course of 16 months, GMFM score improved from 60.67 to 81.88, where the domains of sitting improved from 59 to 60, crawling and kneeling from 32 to 40, standing from 6 to 27 and walking, and running and jumping from 8 to 32. GMFCS grade improved from Level 3 to Level 2, as the child was able to sit in the chair with both hands free, walk and run without assistance on even surfaces, and stair climb holding on a railing. FIM score improved from 91 to 99, which revealed improvements in his functional status.
The improvement in objective scales was supported by improved brain metabolism observed in comparative brain PET CT studies. Brain tissue requires glucose for normal functioning. In the brain, the active absorption of glucose is always directly proportional to its metabolism. PET CT records brain metabolism by using fluorodeoxyglucose (FDG) uptake. Low uptake of FDG indicates hypometabolism and hypofunction of neurons in the brain, while increased uptake denotes an improvement in metabolism and neuron function.27,28 Therefore, it is an accurate testing tool to detect cellular effects of neurorestoration in cellular therapy. Postcellular therapy, a PET CT brain scan showed increased uptake of FDG in the anterior cingulate lobe, parietal cortex, medial temporal lobe, basal ganglia, thalami, and cerebellum compared to the pre-scan. Improved metabolic activity in these areas of the brain resulted in improvement of various functions corresponding to them, as shown in Table 1. Improved sensory integration of movements and calculation were associated with improved metabolism in parietal cortex. Increased FDG uptake in anterior cingulate lobe was correlated with improved behavioral issues, such as throwing objects while angry, were resolved, and he started to learn new tasks. Attention, concentration, sitting tolerance, and memory were allied with augmented uptake in medial temporal cortex. Enhanced metabolism in thalamus related to acquired normal postural and alternative movement patterns of the limbs. Improved metabolism in basal ganglia was correlated with normalized tone and gait pattern. Improvement in balance, coordination, and fine motor skills were associated with increased FDG uptake in cerebellum.
Limitations. One of the limitations of this case report is there was no control to compare the effect of only rehabilitation. But since the child was undergoing rehabilitation for two years before cellular therapy with plateaued recovery, the improvements later signify vital role of cell transplantation. Larger, multicentric, randomized trials with multiple cell transplantations are required for comprehensive analysis.
Conclusion
This study demonstrates that multiple cellular therapies along with neurorehabilitation is effective in improving the gross motor functions and functional independence in CP. Multiple transplantations of autologous BMMNCs followed by intense neurorehabilitation fastens the process of neuroregeneration, which in turn reflects positive outcomes on the level of disability and quality of life of the patient. PET CT scans can effectively be used to monitor changes occurring at cellular level after the intervention. Thus, multiple cellular therapy is safe, feasible, and can be effectively used as an augmentative treatment in combination with neurorehabilitation for CP.
References
- Dodge NN. Cerebral palsy: medical aspects. Pediatr Clin N Am. 2008;55(5):1189–1207.
- Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55(6):509–519.
- Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20(12):940–949.
- Sankar C, Mundkur N. Cerebral palsy-definition, classification, etiology and early diagnosis. Indian J Pediatr. 2005;72(10):865–868.
- Ottoboni L, Merlini A, Martino G. Neural stem cell plasticity: advantages in therapy for the injured central nervous system. Front Cell Dev Biol. 2017;5:52.
- Sharma A, Gokulchandran N, Badhe P, et al. Multidisciplinary approach of cellular therapy with neurorehabilitation in a case of mixed cerebral palsy. World J Biol Med Science. 2017;4(3):70–74.
- Sharma A, Sane H, Pai S, et al. Intrathecal administration of autologous bone marrow mononuclear cells in a case of cerebral palsy coexisting with autistic features. Phys Med Rehabil Int. 2017;4(1):1110–1112.
- Ravinetto R, Guenzi PD, Massat P, Gaidano G. Globalisation of clinical trials and ethics of benefit sharing. Lancet Haematol. 2014;1(2):54–56.
- Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: Clinical care and neurological rehabilitation. Lancet Neurol.
2011;10(9):844–852. - Nguyen LT, Nguyen AT, Vu CD, et al. Outcomes of autologous bone marrow mononuclear cells for cerebral palsy: An open label uncontrolled clinical trial. BMC Pediatr. 2017;17(1):104–109.
- Sharma A, Gokulchandran N, Chopra G, et al. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21(1):79–90.
- Min K, Song J, Kang JY, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double‐blind, randomized, placebo‐controlled trial. Stem Cells. 2013;31(3):581–591.
- Luan Z, Qu S, Du K, et al. Neural stem/progenitor cell transplantation for cortical visual impairment in neonatal brain injured patients. Cell Transplant. 2013;22(1):
101–112. - Chen G, Wang Y, Xu Z, et al. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J Transl Med. 2013;11(1):21–31.
- Hassan MA, Gabr H, Fathi S, et al. Stem cell transplantation in Egyptian patients with cerebral palsy. Egypt J Neurol Psychiatr Neurosurg. 2012;49(2):117–122.
- Purandare C, Shitole DG, Belle V, et al. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep Transplant. 2012;2012:1–6.
- Chahine NH, Wehbe TW, Hilal RA, et al. Treatment of cerebral palsy with stem cells: a report of 17 cases. Int J Stem Cells. 2016;9(1):90–96.
- Xiao N, Le QT. Neurotrophic factors and their potential applications in tissue regeneration. Arch Immunol Ther Exp (Warsz). 2016;64(2):89–99.
- Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361(9351):47–49.
- Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30(1):140–149.
- Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117–1133.
- Callera F, Do Nascimento RX. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: a preliminary safety study. Exp Hematol. 2006;34(2):
130–131. - Deda H, Inci MC, Kürekçi AE, et al. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10(6):565–574.
- Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16(3):461–466.
- Fabel K, Wolf S, Ehninger D, et al. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:1–7.
- Solopova IA, Moshonkina TR, Umnov VV, et al. Neurorehabilitation of patients with cerebral palsy. Hum Physiol. 2015;41(4):448–454.
- Zürcher NR, Bhanot A, McDougle CJ, Hooker JM. A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: Current state and future research opportunities. NeurosciBiobehav Rev. 2015;52:56–73.
- Sandu N, Spiriev T, Schaller B. Stem cell transplantation in neuroscience: The role of molecular imaging. Stem Cell Rev Rep. 2012;8(4):1265–1266.





