Innov Clin Neurosci. 2025;22(10–12):57–71.
by Jayant Totlani, DO; Muhammad Arsalan Bashir, MBBS; Laurel Tay, BA; Ashley Ngor, BS;
Alexander J. Steiner, PsyD; Drew Hirsch, BS; Lorena Contreras, MA; Sabrina Renteria, MD; Itai Danovitch, MD; Robert N. Pechnick, PhD; Waguih William IsHak, MD; and Sarah Kim, MD
Dr. Totlani is with the Virginia Commonwealth University Health System in Richmond, Virginia and Cedars-Sinai Medical Center in Los Angeles, California. Dr. Bashir, Ms. Tay, Ms. Ngor, Mr. Hirsch, Ms. Contreras, Dr. Renteria, Dr. Danovitch, and Dr. Kim are with Cedars-Sinai Medical Center in Los Angeles, California. Dr. Steiner is with Executive Mental Health, Inc. in Los Angeles, California. Dr. Pechnick is with Western University of Health Sciences in Pomona, California. Dr. IsHak with Cedars-Sinai Medical Center in Los Angeles, California and the David Geffen School of Medicine at UCLA in Los Angeles, California.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors have no conflicts of interest to report regarding the content of this manuscript.
ABSTRACT: Objective: Neurocognitive disorders (NCDs), which include delirium, major and mild NCDs such as Alzheimer’s disease (AD), and other forms of dementia, constitute a significant and growing public health burden, affecting tens of millions of individuals worldwide. This systematic review aims to examine the medications approved by the United States Food and Drug Administration (FDA) for NCDs from 2008 to 2024, as well as those in the pipeline in Phase III, and to describe the mechanism of action, clinical indications, dosing, evidence for efficacy, and adverse effects. Methods: We searched the literature using the PubMed database for studies published from January 1, 2008, to December 31, 2024, focusing on FDA-approved psychiatric medications and Phase III pipeline medications, using the keywords “neurocognitive” OR “dementia” OR “Alzheimer*” AND “psychopharm*” OR “medic*” OR “pharm*.” Two reviewers performed an independent assessment of the resulting publications and reached a consensus on the eligible studies to include in the systematic review. Results: From 2008 to 2024, the FDA approved eight medications for major and mild NCDs, including monoclonal antibodies, acetylcholinesterase inhibitors, N-methyl-D-aspartate (NMDA) receptor antagonists, and an atypical antipsychotic. Additionally, we identified 22 pipeline medications currently in Phase III clinical trials for NCDs as of December 31, 2024, including biologics and neuroprotective agents, among others. No medications for delirium were FDA-approved or in Phase III, although agents for a variety of encephalopathies have been developed. Conclusion: Significant advancements in the pharmacological management of NCDs have been made during this period, including developing disease-modifying therapies for AD. However, available medications primarily provide symptomatic relief, and challenges persist in the implementation of disease-modifying treatments due to adverse effects and high costs of care. The pipeline of Phase III clinical trials includes many emerging agents with novel mechanisms of action, and ongoing trials will prove essential to confirm the efficacy and safety of these therapies. Keywords: Neurocognitive disorders, cognitive disorders, dementia, Alzheimer’s disease, psychiatric medications, FDA-approved
Introduction
Neurocognitive disorders (NCDs) encompass a broad spectrum of acquired health conditions characterized by a progressive deterioration in cognition, function, and behavioral patterns relative to an individual’s baseline level of function.1 Cognitive symptoms (such as problems with memory, language, recognition, orientation, and executive function) and neuropsychiatric symptoms (such as apathy, agitation, psychosis, sadness, and anxiety) are the two primary categories of symptoms caused by neurocognitive disorders.2 These symptoms occur in up to 90 percent of people with NCDs, and they usually get worse as the underlying contributory etiology progresses.3 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) classifies NCDs as delirium; major and mild NCDs associated with a variety of etiological factors, such as NCD due to Alzheimer’s Disease (AD), NCD with Lewy bodies, frontotemporal NCD, vascular NCD, and NCDs due to traumatic brain injury (TBI); Parkinson’s disease (PD); Huntington’s disease; prion disease; and multiple etiologies.4 Delirium involves an acute disturbance in attentional processing/cognition developing over a short period of time (hours or days) as a direct physiological consequence of another medical condition, substance intoxication/withdrawal, exposure to a toxin(s), or multiple etiologies.4 Although delirium is often mistaken for encephalopathy, it is considered the clinical manifestation of acute encephalopathy that is caused by a variety of underlying etiologies, as described by a consensus of 10 professional societies.5 Major and mild NCDs are stratified by both severity of cognitive and functional impairment (eg, mild versus major), etiological subtypes, and duration of symptom onset.6 NCDs are associated with a decline of one or multiple cognitive domains with concurrent declines in both basic and instrumental activities of daily living.7 Both nonpharmacological and pharmacological approaches are available for the treatment of cognitive and neuropsychiatric symptoms of NCDs.
This systematic review provides a review of the pharmacological treatment options for major and mild NCDs in the past 16 years (from 2008 to 2024). We highlight the reported mechanisms of action, indication, effectiveness, dosage, and adverse effects of each pharmacological agent aimed at reducing the disease burden. This review aims to disseminate the current landscape of varying treatment choices, address the effectiveness and efficacy gap, and identify new developing therapeutic approaches for improving the management and clinical outcomes of NCDs.
Methods
A systematic literature search was conducted using the PubMed database to identify relevant studies published between January 1, 2008, and December 31, 2024. The search strategy was designed to capture studies focusing on United States (US) Food and Drug Administration (FDA) approved psychiatric medications as well as Phase III investigational drugs in the pipeline with potential neurocognitive effects. The search terms included “neurocognitive” OR “dementia” OR “Alzheimer*” AND “psychopharm*” OR “medic*” OR “pharm*.”
Two independent reviewers conducted a comprehensive screening of the retrieved citations. Titles and abstracts were initially reviewed to exclude irrelevant studies, duplicates, preclinical research, and non-English publications. Full-text articles of potentially eligible studies were then obtained and assessed for inclusion based on predefined eligibility criteria. Discrepancies between the reviewers were resolved through discussion and, when necessary, by consulting a third reviewer. Key findings, including overviews of medications, mechanism(s) of action, route of administration, dosage, evidence for efficacy, and adverse effects, were extracted from included articles. The extracted data were tabulated to summarize relevant findings.
Results
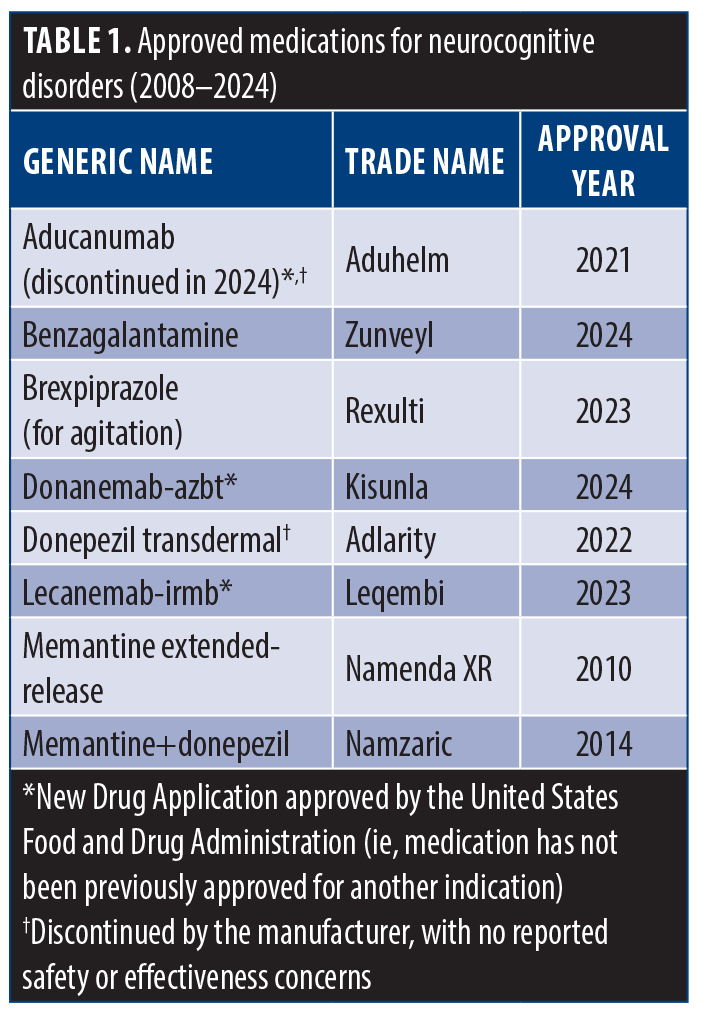
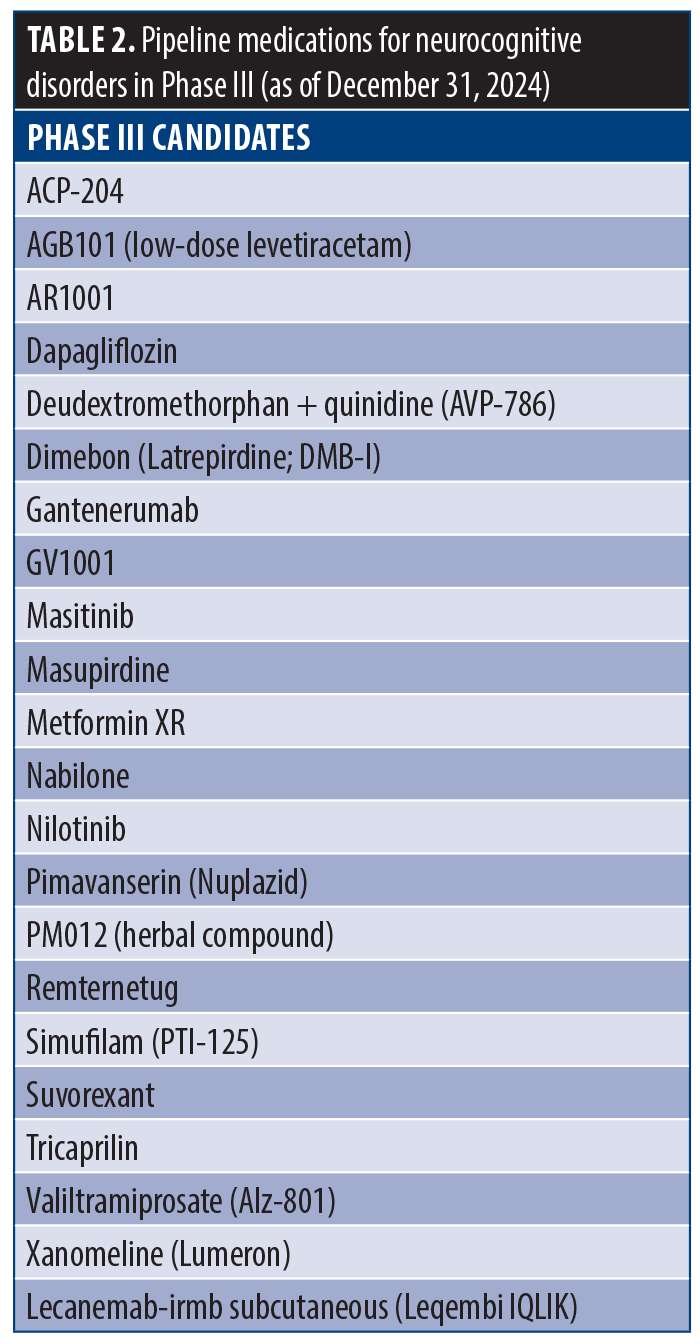
From 2008 to 2024, eight medications were approved for the treatment of major and mild NCDs (Table 1), including aducanumab, benzagalantamine, brexpiprazole, donanemab, donepezil transdermal, lecanemab, memantine extended-release, and memantine+donepezil. Additionally, 22 drugs are currently in Phase III clinical trials (Table 2). No medications specifically for delirium were FDA-approved or in Phase III. However, medications for a variety of encephalopathies have been available, such as lactulose for hepatic encephalopathy and thiamine for Wernicke’s encephalopathy, as well as pipeline gene therapy agents for epileptic encephalopathy (eg, CAP-002) and acute hepatic encephalopathy (eg, L-ornithine phenylacetate).
Aducanumab, a monoclonal antibody targeting beta-amyloid (Aβ) plaques, was granted fast-track approval due to its ability to reduce Aβ accumulation, a purported biomarker in AD. Its efficacy was limited to early and mild stages of AD.8 However, in 2024, Biogen discontinued its production, citing company reprioritization rather than concerns regarding safety or efficacy.9 Lecanemab, another monoclonal antibody, received FDA approval for its ability to reduce amyloid markers and slow cognitive decline in early AD.10 Donanemab-azbt from the same class received approval in 2024.
Benzagalantamine, an acetylcholinesterase inhibitor approved in July 2024, demonstrated bioequivalence to galantamine with reduced gastrointestinal side effects. Brexpiprazole, approved for the treatment of agitation associated with AD-related dementia, functions as a partial agonist at D2 and 5-HT1A receptors while antagonizing 5-HT2A receptors, leading to improved patient quality of life and safety.11 Donepezil transdermal inhibits acetylcholinesterase and is used for mild-to-severe dementia due to AD. It inhibits acetylcholinesterase and offers the advantage of once-weekly administration, enhancing patient compliance.12 Memantine extended-release, approved for moderate-to-severe AD-related dementia, selectively blocks N-methyl-D-aspartate (NMDA) receptors without disrupting normal receptor function.13 The combination therapy of memantine and donepezil is indicated for moderate-to-severe dementia, with memantine regulating glutamate activity and donepezil inhibiting acetylcholinesterase, resulting in reduced hospitalizations and improved caregiver support.14
Current FDA-approved pharmacotherapies for major and mild NCDs primarily address symptomatic management rather than disease modification.15 Cost and availability remain significant barriers to access.16 Aducanumab, the first approved anti-amyloid therapy, demonstrated efficacy in reducing clinical disease progression; however, adverse events, including amyloid-related imaging abnormalities (ARIA), were reported, manifesting as brain edema, microhemorrhages, and milder symptoms such as headache, dizziness, confusion, and nausea.8 The high cost of aducanumab raised concerns regarding cost effectiveness and safety.17 Following its discontinuation in January 2024, lecanemab emerged as the second FDA-approved anti-amyloid drug, showing promising results in early AD, though further trials are needed to assess its efficacy in advanced disease stages.18 Donanemeb-azbt followed by receiving approval in 2024.
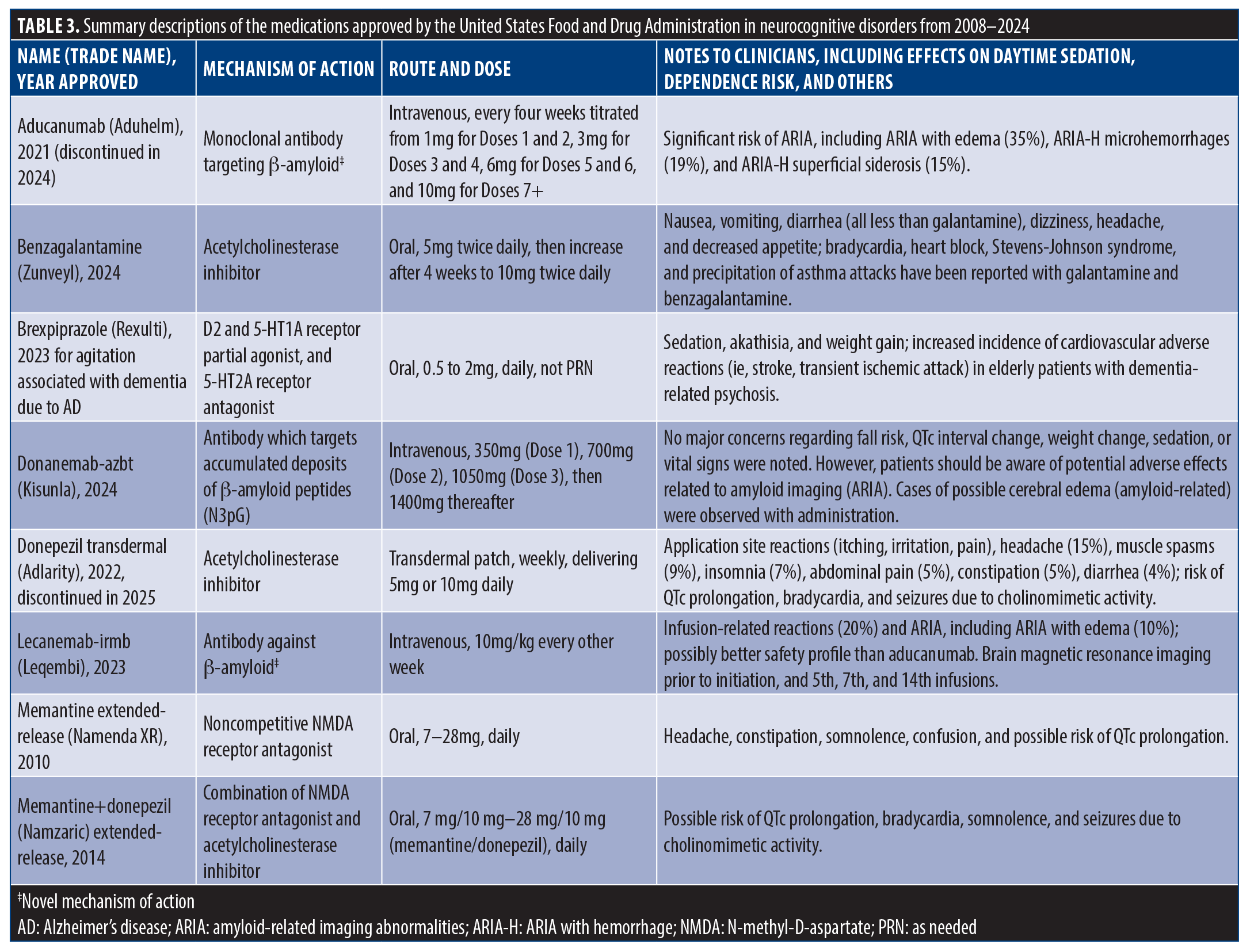
Aducanumab (Aduhelm). Overview. Aducanumab is an intravenous (IV) infusion Aβ-targeting antibody used for AD that was approved in 2021 and discontinued in 2024. There has been major controversy surrounding this medication, with differing opinions among professionals regarding the validity of both trials and different analyses of why both trials had opposing outcomes. Although the manufacturer decided to discontinue it in 2024, due to the reprioritization of the company’s AD treatments, it is presented here as the first-in-class approved FDA medication for AD.
Indication. Aducanumab was indicated for AD.
Dose and route. Aducanumab is administered via IV infusion every four weeks titrated from 1mg for Doses 1 and 2, 3mg for Doses 3 and 4, 6mg for Doses 5 and 6, and 10mg for Doses 7 and up.
Evidence. Two 78-week placebo-controlled trials were conducted evaluating the efficacy of aducanumab in early AD, ENGAGE (n=959) and EMERGE (n=877). Divergent outcomes were observed. The ENGAGE trial did not demonstrate a significant benefit, whereas the EMERGE trial reported a clinical benefit in the high-dose group (titrated to 10mg/kg).
The primary outcome measure was the change in the Clinical Dementia Rating–Sum of Boxes (CDR-SB) score, which measures both cognition and functioning and ranges from 0 (no dementia) to 18 (severe dementia). In the EMERGE trial, a difference of –0.39 (a 22% decrease) for high-dose aducanumab from placebo was detected, whereas in the ENGAGE trial, there was a difference of 0.03 (a 2% increase) from placebo.8 However, the results of biomarker substudies confirmed target engagement and time/dose-dependent reduction in pathophysiological markers of AD, such as tau positron emission tomography (PET) imaging signals, cerebrospinal fluid (CSF) phosphorylated tau (p-tau), and plasma p-tau181.19
The discordance between ENGAGE and EMERGE has been debated in the literature.20,21 Concerns regarding demographic representation have also been raised. Manly et al22 highlighted the limited diversity within the study cohorts, with Black and Hispanic participants comprising only 0.6 percent and three percent of the total sample, respectively, underscoring systemic disparities in clinical trial enrollment. The lack of racial and ethnic diversity in both trials presents a major difficulty with generalizing study findings to the larger US population, a highly heterogeneous population at large. Additionally, preclinical research supports the potential mechanistic benefits of aducanumab. Bastrup et al23 reported significant gene expression alterations in mitochondrial metabolism, cytoskeletal proteins, stress response pathways, and protein trafficking within the hippocampus in animal models, suggesting possible neuroprotective effects through epigenetic modulation.
Practical implementation issues. This is the first monoclonal antibody targeting Aβ approved for AD. These are highly controversial results, and the relatively high prevalence of amyloid-related imaging abnormalities limits clinical use for many patients.
Adverse effects. ARIA with edema or effusion (ARIA-E), ARIA with hemorrhage (ARIA-H), headache, nausea, and visual problems were reported.
Benzagalantamine (Zunveyl). Overview. Benzgalantamine is a delayed-release acetylcholinesterase inhibitor used for treating mild-to-moderate AD in adults. It acts by increasing acetylcholine levels through competitive inhibition of acetylcholinesterase, aiming to enhance cholinergic function, which is often diminished in AD.
Indication. Benzgalantamine is indicated for AD (mild-to-moderate dementia).
Dose and route. The starting dose is 5mg taken orally twice daily, which can be increased to 10mg twice daily after a minimum of four weeks based on tolerability. If further adjustment is necessary, a maximum dose of 15mg twice daily can be reached after an additional four weeks at the 10mg dosage. Benzgalantamine should be taken with adequate fluid intake and may be administered with or without food. Tablets should be swallowed whole and not split, crushed, or chewed. For patients with moderate hepatic or renal impairment, the dosage should not exceed 10mg twice daily, and it is not recommended for those with severe renal impairment.
Evidence. Efficacy data for benzgalantamine are based on trials with its active metabolite, galantamine, showing statistically significant improvements in cognitive assessments such as the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). Trials have demonstrated its benefits at dosages of 16 to 24mg/day, where cognitive improvements were measured over 13 to 26 weeks, and the majority of participants responded well at maintenance dosages.24
Practical implementation issues. Benzgalantamine is contraindicated in patients with hypersensitivity to benzgalantamine or galantamine. It requires careful monitoring for gastrointestinal issues, cardiovascular conditions (such as bradycardia or atrioventricular block), and serious skin reactions, including Stevens-Johnson syndrome. Patients with respiratory conditions, especially severe asthma or chronic obstructive pulmonary disease (COPD), should be monitored closely. Concomitant use of alcohol may lead to a rapid release of medication into the body despite its delayed-release form, with potential negative effects.
Adverse effects. The most common adverse events associated with benzgalantamine include nausea, vomiting, diarrhea, dizziness, headache, and decreased appetite. Rare but serious reactions may involve cardiac events (eg, bradycardia, syncope), gastrointestinal bleeding, and skin reactions like Stevens-Johnson syndrome.
Brexpiprazole (Rexulti). Overview. Brexpiprazole is an oral atypical antipsychotic, a D2 and 5-HT1A receptor partial agonist, and a 5-HT2A receptor antagonist which is FDA-approved for the treatment of schizophrenia as an adjunct in the treatment of major depressive disorder (MDD) and reduced agitation in patients with dementia.
Indication. Brexpiprazole is indicated for schizophrenia (FDA-approved in 2015), as an adjunct in MDD (FDA-approved in 2015), and for agitation in patients with dementia (FDA-approved in May 2023) as standing medication (not as needed).
Evidence. Two studies observed the effectiveness of 0.5mg, 1mg, and 2mg per day in the treatment of agitation in patients with AD dementia.25 Efficacy was measured using the Cohen-Mansfield Agitation Inventory (CMAI) and the Clinical Global Impression-Severity of Illness scale (CGI-S). Results showed that 2mg/day was the ideal dosage, with greater improvements in Study 1 compared to placebo. Notably, however, statistically significant differences were not observed in Study 2 for all three treatment doses. Study 1 showed efficacy with a 2mg dose, whereas Study 2 observed a limited significant effect compared to placebo. Higher dosage effectiveness should be experimented with to explore a minimum consistent treatment threshold.
Practical implementation issues. Benzgalantamine is similar to aripiprazole in pharmacodynamic profile without adverse effects on cognition.26
Adverse effects. Common adverse effects include headache, akathisia, weight gain, and somnolence.27 Mortality risk with antipsychotics in dementia is highlighted with a black box warning and confirmed by trial data analyses.28
Donanemab-azbt (Kisunla). Overview. Donamemab-azbt is a Aβ-directed antibody that targets accumulated deposits of Aβ peptides (N3pG). It was approved by the FDA in July 2024 for the treatment of AD.
Indication. Donanemab is indicated for the treatment of mild cognitive impairment (MCI) or mild dementia due to AD.
Dose and route. Donanemab is administered via IV infusion over 30 minutes of 700mg every four weeks for three doses, then 1,400mg every four weeks.
Evidence. In clinical trials, patients who received donamemab-azbt had a statistically significant reduction in clinical decline on the Integrated Alzheimer’s Disease Rating Scale (iADRS) compared to placebo at Week 76, as well as on the iADRS component scales, ADAS-Cog, Alzheimer’s Disease Cooperative Study–Instrumental Activities of Daily Living (ADCS-iADL) scale, and CDR-SB.
Practical implementation issues. The Alzheimer’s Association cited a cost of $32,000 per year per patient for donamemab-azbt.29
Adverse effects. The following adverse events were observed in more than 10 percent of patients: ARIA-E, ARIA-H (microhemorrhage), ARIA-H, superficial siderosis, and headache.
Donepezil transdermal (Adlarity). Overview. Donepezil transdermal is a transdermal patch indicated for the treatment of dementia due to AD. Donepezil was initially approved by the FDA for the treatment of mild-to-severe AD in 1996, but the FDA approved the transdermal patch for the treatment of AD in March 2022. The patch was discontinued by the manufacturer in 2025.
Indication. Donepezil transdermal is indicated for the treatment of dementia due to AD.
Evidence. Several clinical studies have observed the efficacy of transdermal donepezil in the treatment of AD and accompanying dementia.30
One study observed donepezil treatment in patients with mild-to-moderate AD (n=473) for 30 weeks (24 weeks treatment, 6 weeks washout), with participants randomly assigned daily doses of 5mg, 10mg, or placebo. Efficacy was evaluated using the ADAS-Cog. Results showed significant clinical improvement in ADAS-Cog scores (5mg/day, +2.8; 10mg/day, +3.1) and change in baseline (5mg/day, about –1.5; 10mg/day, about –1) compared to placebo (baseline, +2). In an randomized, double-blind, placebo-controlled study observing patients with moderate-to-severe AD (n= 248), participants were administered donepezil (5mg for the first 28 days, then 10mg/day after) or placebo. The efficacy of donepezil treatment on symptoms of moderate-to-severe AD was evaluated using Severe Impairment Battery (SIB) and the ADCS-ADL-Severe scale (observing daily functionality of patients). Results showed that there was a significant improvement in mean SIB scores (5.9) between patients administered donepezil (10mg/day, +4) and placebo (–1.9) at six months. ADCS-ADL-Severe scores of donepezil-treated participants were significantly better compared to placebo (mean difference: 1.8) (ClinicalTrials.gov ID: NCT03197740). Clinical trials involving direct observation of the efficacy of transdermal patches of donepezil are recommended rather than in comparison to oral tablets. Due to the progressive nature of AD, participants who stopped taking doses during the six-week washout period showed similar results to placebo in terms of symptom resurgence. Demographics for previous trials showed the majority of participants being of Caucasian descent.
Practical implementation issues. The treatment of AD using donepezil transdermal patches is deemed comparable to donepezil tablet prescriptions.
Adverse effects. More than five percent of participants reported symptoms such as nausea, diarrhea, insomnia, muscle cramps, vomiting, fatigue, and anorexia.30
Lecanemab-irmb (Leqembi). Overview. Lecanemab-irmb IV injection is a monoclonal antibody treatment targeting Aβ protofibrils to slow disease progression in early AD. It is designed to support neuronal function by reducing amyloid plaque and mitigating the neurotoxic effects of protofibrils.31
Indication. Lecanemab-irmb is indicated for AD, specifically MCI or mild dementia stages of AD.
Dose and route. The dose is 10mg/kg IV infusion every other week.
Evidence. Change in CDR-SB was used to measure efficacy in the treatment versus placebo group. The treatment group showed a statistically significant difference with a 1.66 reduction compared to a 1.21 decline (difference: 0.45) in the placebo group after 18 months of treatment.10
Practical implementation issues. The medication is expected to cost $26,500 per year/per patient.
Adverse effects. Common adverse effects include mild-to-moderate local injection site reactions (eg, redness, irritation, swelling) and ARIA, which may manifest as asymptomatic brain edema or microhemorrhage. Severe ARIA cases are rare but monitored, especially in Apolipoprotein E (APOE) ε4 homozygotes, due to their higher risk.32
Memantine extended-release (Namenda XR). Overview. Memantine extended-release is an NMDA receptor antagonist approved for the treatment of moderate-to-severe AD. It is postulated to work by mitigating neurotoxicity due to excessive NMDA receptor activation. Memantine does not slow the progression of AD, but helps alleviate symptoms.
Indication. Memantine extended-release is indicated for the treatment of moderate-to-severe dementia of the AD type.
Dose and route. The initial dose for Memantine extended-release is 7mg, taken once daily. The dose is titrated weekly in 7mg increments to reach the target dose of 28mg once daily. For patients with severe renal impairment (creatinine clearance of 5–29mL/minute), the recommended target dose is 14mg once daily. Memantine extended-release can be taken with or without food and may be swallowed whole or opened and sprinkled on applesauce.
Evidence. Memantine extended-release was evaluated in a 24-week, randomized, double-blind, placebo-controlled clinical trial in patients with moderate-to-severe AD. In patients receiving an acetylcholinesterase inhibitor in combination with memantine extended-release (28mg/day), results showed significant improvements in cognitive function (measured by the SIB) and global clinical status (measured by the Clinician’s Interview-Based Impression of Change [CIBIC+]) compared to placebo plus acetylcholinesterase inhibitor.33
Practical implementation issues. Memantine extended-release is contraindicated in patients with hypersensitivity to memantine or any excipients in the formulation. Caution should be used in patients with severe hepatic or renal impairment and conditions that raise urine pH, as these may decrease memantine clearance, leading to elevated plasma levels. Memantine should not be used concomitantly with other NMDA antagonists (eg, amantadine, ketamine) without careful evaluation due to potential pharmacodynamic interactions.
Adverse Effects. The most common adverse effects (occurring in at least 5% of patients and at a higher rate than placebo) are headache, diarrhea, and dizziness. Other adverse effects include back pain, hypertension, constipation, vomiting, somnolence, and urinary incontinence. Serious adverse reactions, although rare, include bradycardia, syncope, and seizure.
Memantine+donepezil (Namzaric). Overview. Memantine+donepezil is a combination of memantine, an NMDA receptor antagonist, and donepezil, an acetylcholinesterase inhibitor. This dual treatment is designed to treat moderate-to-severe AD by addressing both the glutamatergic system (via memantine) and cholinergic system (via donepezil), enhancing cognitive function and slowing disease progression.
Indication. Memantine+donepezil is indicated for the treatment of moderate-to-severe AD in patients who are stabilized on both memantine and donepezil.
Dose and route. Memantine+donepezil is administered orally once daily, available in dosages ranging from 7mg/10mg to 28mg/10mg (memantine/donepezil). It can be swallowed whole, or the capsules may be opened and sprinkled on applesauce for patients who have difficulty swallowing.
Evidence. The efficacy of memantine and donepezil combination treatment has been supported by clinical trials showing superior outcomes in cognition, global assessment, activities of daily living, and neuropsychiatric symptoms compared to either agent alone or placebo. The combination was particularly effective in improving scores on the ADAS-Cog, SIB, and CGI of Change (CGI-C). Studies have demonstrated that combination treatment results in better maintenance of cognitive function, reduced progression of symptoms, and enhanced quality of life compared to monotherapy with either donepezil or memantine alone.34
Practical implementation issues. While memantine+donepezil offers a convenient once-daily dosing regimen and the potential to slow symptom progression, it is important to monitor patients for adverse events, particularly those related to cholinomimetic activity. Patients with a history of QTc prolongation or bradycardia should be evaluated carefully before initiating treatment. Additionally, caution should be exercised in patients with renal impairment, with a maximum recommended dose of 14mg/10mg for those with severe renal insufficiency.34
Adverse effects. The most common adverse events reported in patients taking memantine+donepezil include dizziness, headache, diarrhea, vomiting, and anorexia. Serious but less common adverse events include bradycardia, syncope, and seizures. Memantine may also cause confusion, agitation, and hallucinations, while donepezil is associated with gastrointestinal side effects like nausea and dyspepsia.
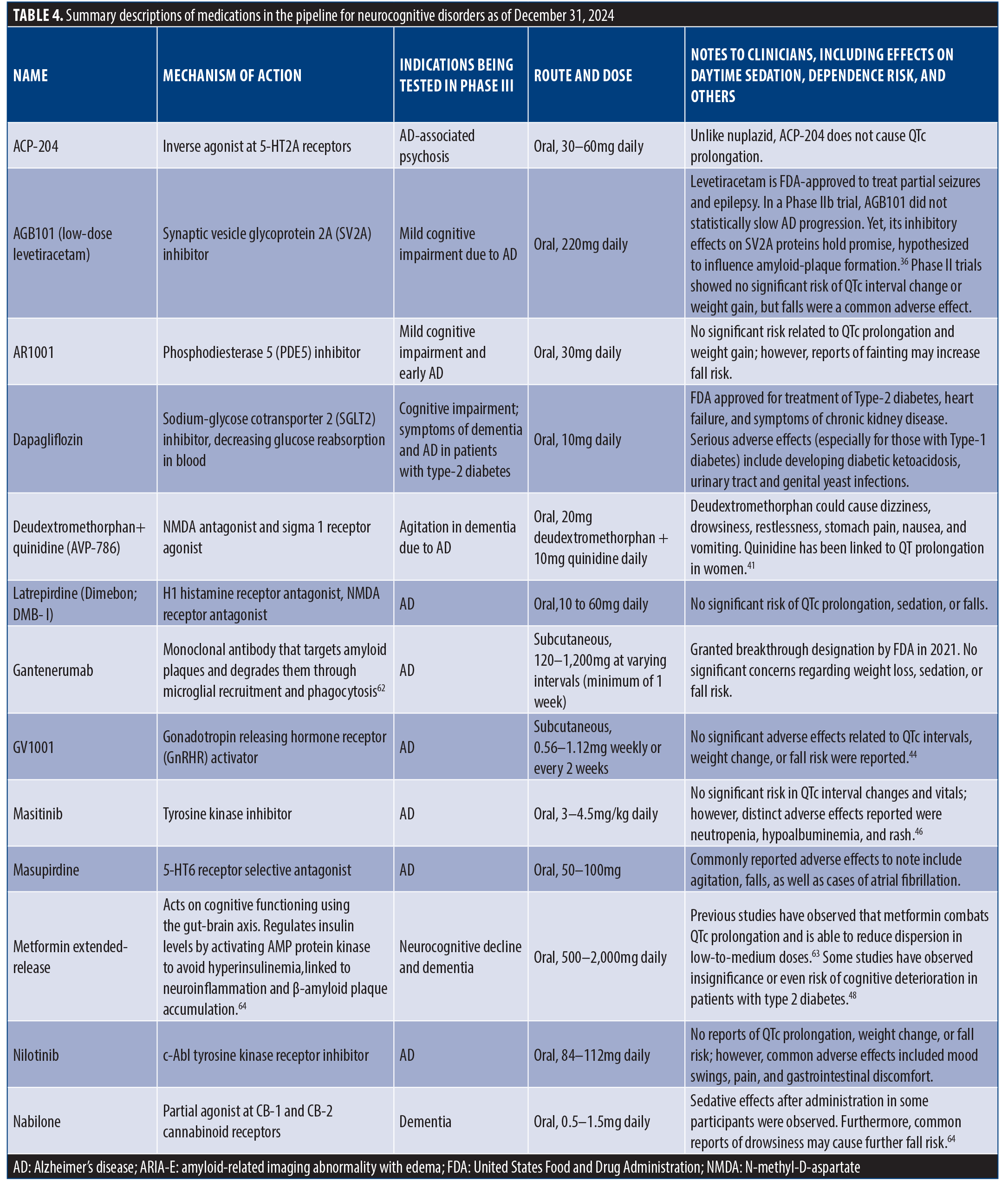
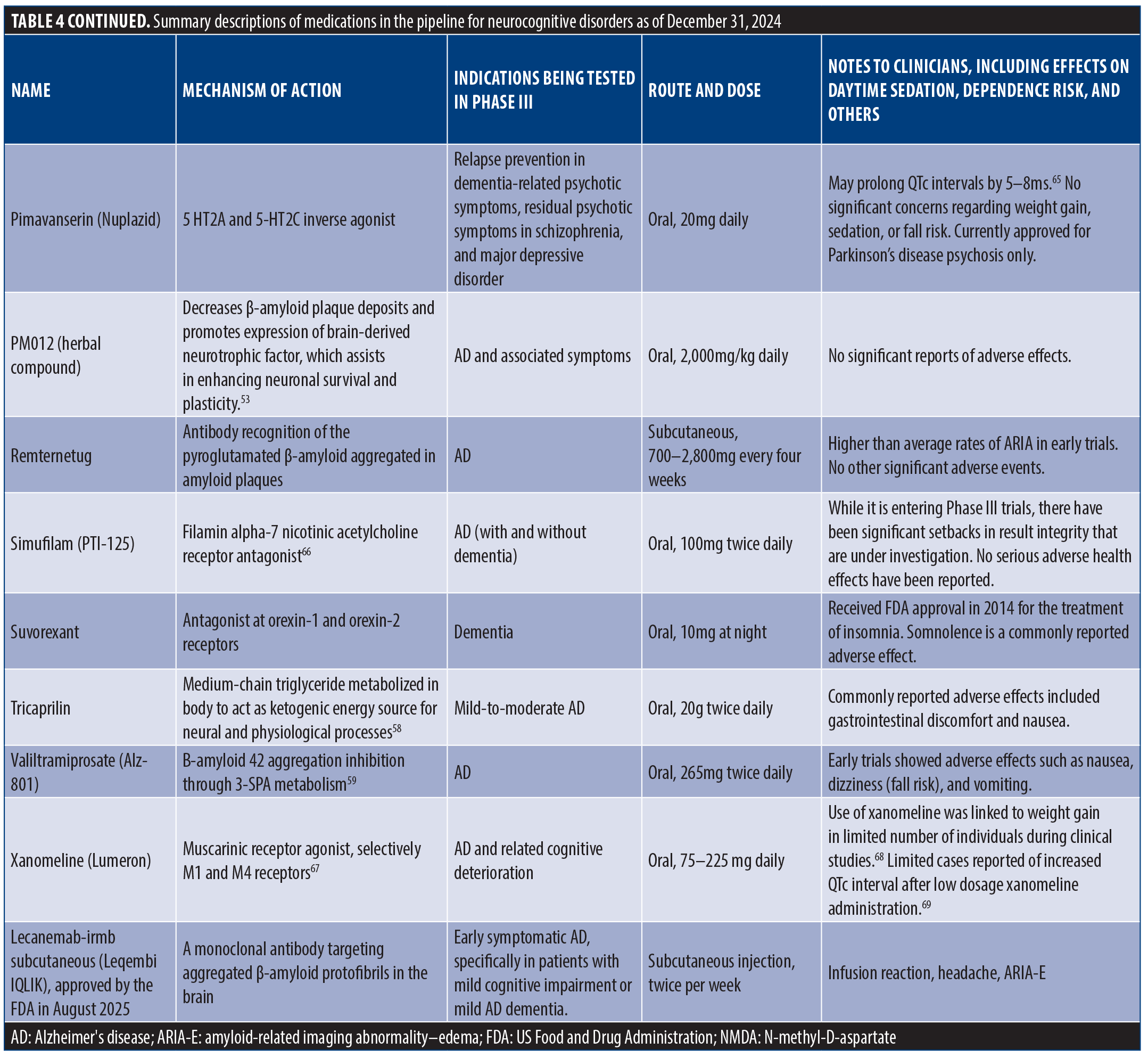
Detailed Descriptions Of Medications In The Pipeline For Neurocognitive Disorders As Of December 31, 2024 (Table 4).
Twenty-two new medications have been investigated for the treatment of major and mild NCDs and are currently in, or will be undergoing, Phase III trials. These medications are generally indicated for the treatment of AD and associated agitation and cognitive impairment, yet they are diverse in their unique mechanisms of action. Deudextromethorphan adjunct treatment with quinidine (AVP-786) inhibits NMDA receptors. Gantenerumab uses monoclonal antibodies to target amyloid plaques. Additionally, nabilone (cannabinoid receptor agonist), pimavanserin (serotonergic receptor agonist), suvorexant (orexinergic receptor antagonist), and xanomeline (muscarinic receptor antagonist) offer promising and distinct approaches for treating symptoms of dementia and AD. See Table 4 for more details on each medication in the approval pipeline for NCDs.
ACP-204. Overview. ACP-204 acts as an inverse agonist at the 5-HT2A serotonin receptor. It is currently being developed for the treatment of psychosis occurring during the course of AD.35
Dose and route. ACP-204 is administered orally, 30 to 60mg daily.
Preliminary findings. Data unavailable.
Adverse effects. None have been reported, no QTc prolongation.35
AGB101 (low-dose levetiracetam). Overview. AGB101 is an extended-release formulation of low-dose levetiracetam designed to reduce excessive neural activity in the hippocampus (considered to be a predictor of disease progression) in order to slow the advancement of AD in patients with MCI. AGB101 is the first therapeutic formulated specifically to address hippocampal overactivity in the context of AD.
Indication. AGB101 is indicated for patients with MCI due to AD, specifically those demonstrating amyloid pathology and heightened hippocampal activity. It targets early-stage AD in efforts to delay progression towards dementia.
Dose and route. The recommended dose is a single, daily oral dose of 220mg. This extended-release formulation ensures sustained plasma levels throughout the day, aligning with evidence that such levels are effective in reducing hippocampal overactivity. No titration is necessary for starting or stopping the treatment.
Preliminary findings. The HOPE4MCI trial, a double-blind, placebo-controlled study, evaluated the effectiveness of AGB101 over 78 weeks. Results showed no statistically significant difference between AGB101 and placebo in the primary endpoint, which was the change in CDR-SB scores. However, a trend suggested possible benefits for APOE ε4 noncarriers, with a relative reduction in progression indicators, warranting further studies focused on this subgroup.36
Adverse effects. AGB101’s safety profile was generally favorable, with most adverse effects being mild and consistent with previous findings in low-dose levetiracetam studies. The most common adverse events included urinary tract infections, falls, and anxiety. There were a few serious adverse events, including trial discontinuations and two reported deaths, though none were directly linked to the treatment.36
AR1001. Overview. AR1001 is a phosphodiesterase 5 (PDE5) inhibitor initially developed for erectile dysfunction but is now being investigated for AD treatment. Developed by Aribio, a South Korean biopharmaceutical company, AR1001 is notable for its superior blood-brain barrier (BBB) penetrance and greater PDE5 inhibition potency than sildenafil. It might enhance cognitive function by increasing cyclic guanosine monophosphate (cGMP) signaling and activating cellular pathways associated with neuroprotection.
Indication. AR1001 is indicated for mild-to-moderate AD, targeting early cognitive impairment to slow down disease progression. Preliminary data suggest it may be more beneficial in early cognitive decline cases.
Dose and route. The dose in clinical trials has been either 10mg or 30mg administered once daily in tablet form.
Preliminary findings. In a Phase II, 26-week, randomized, placebo-controlled trial (n=210) for mild-to-moderate AD, AR1001 showed a slight reduction in cognitive decline on the ADAS-Cog-13 scale for those on the 30mg dose, with limited effect seen in the placebo group. Improvements were more pronounced in patients not using other AD medications, indicating possible negative interactions. No significant functional improvement was noted in moderate cases, and post-hoc analyses showed modest cognitive gains in mild AD patients taking AR1001 as a monotherapy. Further studies are underway to evaluate associated biomarkers and conduct a Phase III trial.37
Adverse effects. AR1001 was well tolerated in the Phase II trial, with mild or moderate adverse effects, such as urinary tract infections (10.6%), diarrhea (7.1%), depression (6.4%), skin lacerations (5.7%), and headache (5%). Discontinuations due to side effects were minimal, with the placebo group experiencing the highest adverse event rate.37
Dapagliflozin. Overview. Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor initially developed to manage type 2 diabetes mellitus by reducing blood glucose levels. However, recent research has uncovered potential neuroprotective properties, suggesting that dapagliflozin may also play a role in treating AD. The neuroprotective mechanisms of dapagliflozin involve reducing antioxidative stress, antineuroinflammation, promotion of autophagy, antiapoptosis effects, acetylcholinesterase inhibition, and protection of endothelial cells from atherosclerosis and BBB damage.
Indication. Dapagliflozin is indicated for the treatment of type 2 diabetes to improve glycemic control, reduce the risk of hospitalization for heart failure, and delay the progression of chronic kidney disease. In addition, emerging research suggests that dapagliflozin could be beneficial for patients with AD by targeting mechanisms that contribute to neurodegeneration.
Dose and route. The standard dose of dapagliflozin for managing type 2 diabetes is 10mg once daily, taken orally. For its potential role in AD, clinical trials and further studies are required to establish the optimal dosing regimen, as its usage in this context remains experimental.38
Preliminary findings. Preclinical studies and early human trials suggest that dapagliflozin’s benefits in AD could stem from its antioxidative properties, reduction in neuroinflammatory markers, and enhancement of autophagic processes. In animal models, dapagliflozin has been shown to upregulate antioxidant pathways, increase neuronal energy balance, and decrease neurotoxic Aβ aggregation. Observational studies in populations with diabetes also indicate a lower dementia risk among SGLT2 inhibitor users, with dapagliflozin showing a potential reduction in AD incidence.38
Adverse effects. While generally well tolerated, dapagliflozin might have adverse effects, primarily related to its use in diabetes mellitus management, such as urinary and genital infections, hypovolemia, and hypoglycemia. In specific cases, it might also increase the risk of rare conditions such as Fournier’s gangrene. Its safety in patients with AD without diabetes remains under study.
Deudextromethorphan+quinidine (AVP-786). Overview. AVP-786 is an oral combination of the deuterated form of dextromethorphan (a central nervous system [CNS] agent approved to relieve cough) and quinidine, which is used to increase the concentration of dextromethorphan. The combination of dextromethorphan and quinidine has been approved under the name Nuedexta for the treatment of pseudobulbar affect. AVP-786 is an NMDA antagonist and sigma-1 receptor agonist that reduced agitation in patients with dementia in a Phase III trial; however, it failed to show similar results in subsequent trials. Agitation in patients with dementia was improved in only one of two Phase III trials.
Dose and route. AVP-786 is taken orally, at a dose of 20mg (deudextromethorphan) plus 10mg (quinidine) daily.
Preliminary findings. Previous preliminary studies have investigated the effectiveness of deudextromethorphan in the treatment of agitation and other co-occurring symptoms of AD. Although findings for standalone deudextromethorphan as a treatment for AD are limited, its use alongside other medicinal and therapeutic modalities has been investigated. One study observing the efficacy of adjunctive deudextromethorphan-quinidine on agitation in patients with AD found that patients administered deudextromethorphan-quinidine (n=152) showed greater improvement in AD-related symptoms, including decreased agitation and aggression, compared to placebo; effectiveness was measured using scores from the agitation and aggression domains of the Neuropsychiatric Inventory (NPI).39 In a single Phase III trial, AVP-786 improved agitation in patients with dementia. Previous studies on dextromethorphan found that administration of dextromethorphan and quinidine at a ratio of 20mg:10mg, respectively, led to significant improvement of pseudobulbar affect in patients with dementia, with reduction of pseudobulbar affect symptoms by 67.7 percent, clinician-reported improvements (CGI-C) by 77.5 percent, and patient/caregiver-reported improvements (Patient Global Impression of Change [PGI-C]) by 76.5 percent.40
Adverse effects. Deudextromethorphan can cause dizziness, drowsiness, restlessness, stomach pain, nausea, and vomiting. Quinidine has been linked to QT prolongation in women.41
Latrepirdine (Dimebon,DMB-I). Overview. Latrepirdine, is a nonselective antihistamine initially developed and marketed in Russia for allergy treatment. In recent years, it has been investigated as a potential treatment for AD and other neurodegenerative disorders. Latrepirdine is believed to have multiple mechanisms of action, including inhibition of acetylcholinesterase, mitochondrial protection, and modulation of NMDA receptors, which could contribute to its potential neuroprotective effects.
Indication. Latrepirdine has been studied for the treatment of AD and Huntington’s disease. It is not yet approved for any neurodegenerative condition and remains an investigational treatment.
Dose and route. In clinical trials for AD, latrepirdine was administered at doses of 20mg three times daily (60mg/day) for up to 52 weeks. The oral tablet form was the standard route of administration.42
Preliminary findings. Early trials, including a 2008 Phase II study published in The Lancet, suggested that latrepirdine provided significant improvements in cognition, behavior, and overall functioning in patients with mild-to-moderate AD. However, subsequent Phase III trials (such as the CONNECTION and CONCERT studies) failed to replicate these results. The CONCERT trial, which tested latrepirdine in combination with donepezil, showed no significant cognitive or functional benefits compared to placebo. In a meta-analysis of clinical trials, no conclusive evidence was found to support latrepirdine’s efficacy in improving cognition or daily functioning. However, some studies noted modest improvements in neuropsychiatric symptoms, such as behavior, based on the NPI. Nonetheless, the overall quality of evidence remains low due to inconsistencies between studies and the lack of definitive results.42
Adverse effects. Latrepirdine has generally been well tolerated, with adverse events comparable to placebo. The most common side effects include dry mouth, depressed mood, dizziness, and gastrointestinal disturbances such as nausea. Serious adverse events were rare, and no significant differences in dropout rates were observed between latrepirdine and placebo groups in clinical trials. Although it appears safe, its lack of efficacy has limited further development for AD.
Gantenerumab. Overview. Gantenerumab is a subcutaneous injection of monoclonal immunoglobulin G1 (IgG1) antibodies that targets and degrades amyloid plaque deposits through microglial recruitment. It was granted Breakthrough Therapy designation by the FDA in 2021 for promising treatment of AD. Most recent Phase III trials raised doubts about its efficacy in early AD.
Dose and route. Gantenerumab is adiministered in 105mg and 225mg doses by subcutaneous injection every four weeks
Preliminary findings. A Phase III study (n=799) found that gantenerumab administration over two years showed efficacy in patients with AD.43 Participants were randomly assigned into one of three groups (105mg gantenerumab, 225mg gantenerumab, or placebo). While the study was halted due to lack of effectiveness, there was potential for open-label investigation of higher doses.
A subsequent double-blind study (n=89) evaluated the efficacy of gantenerumab on older age geriatric patients (≥50 years) diagnosed with early-to-moderate signs of AD.62 Doses were titrated (up to 10 months) to a target dose of 1,200mg every four weeks. Results showed that after two years of high-dose gantenerumab administration, nearly 51 percent of participants experienced a significant reduction in Aβ plaque concentration. The most recent trials (GRADUATE 1 and GRADUATE 2), which included patients with early AD as well as patients with mild dementia and MCI, failed to show a statistical significance and have been terminated as a result (ClinicalTrials.gov ID: NCT03444870).
Adverse effects. Common adverse reactions include diarrhea, nausea, vomiting, and constipation.
GV1001. Overview. GV1001 is a peptide derived from a fragment of human telomerase reverse transcriptase. Initially developed as a cancer vaccine, it has shown potential neuroprotective effects against AD by reducing oxidative stress, neuroinflammation, and cell apoptosis caused by Aβ. It also stabilizes mitochondria and exhibits anti-aging properties, making it a candidate for disease modification in AD.
Indication. GV1001 is under investigation for moderate-to-severe AD, particularly for patients already receiving donepezil. Its multifaceted actions against AD pathology make it promising for improving cognitive and daily living functions in advanced AD.
Dose and route. GV1001 is administered subcutaneously, starting with weekly injections for four weeks, followed by biweekly injections up to 24 weeks. In the study, doses of 0.56mg and 1.12mg were compared against a placebo.
Preliminary findings. In a 24-week randomized, double-blind, placebo-controlled trial involving 96 patients with moderate-to-severe AD, the group receiving 1.12mg of GV1001 showed a significant improvement in the SIB scores compared to placebo, indicating better cognitive function preservation. Secondary measures like the NPI also showed improvement, though other scales did not achieve statistical significance. Results suggest GV1001 could offer cognitive benefits for AD patients on stable doses of donepezil.44
Adverse effects. GV1001 was well tolerated, with a similar safety profile to placebo. Mild-to-moderate adverse events, such as skin reactions at injection sites, were reported, but no severe adverse events were linked directly to GV1001. These findings support its safety in the trial population.
Masitinib. Overview. Masitinib is an oral tyrosine kinase inhibitor targeting mast cells and microglia, which are involved in neuroimmune responses. Originally developed for cancer treatment, it is now being investigated for its neuroprotective properties in AD. By inhibiting neuroinflammatory processes, masitinib may help manage the cognitive decline associated with AD.45,46
Indication. Masitinib is indicated as an adjunct treatment for mild-to-moderate AD, specifically in patients who are already on cholinesterase inhibitors or memantine. It is also studied for other neurodegenerative diseases, such as multiple sclerosis and amyotrophic lateral sclerosis.46
Dose and route. Masitinib is administered orally, with an initial dose of 4.5mg/kg/day. In some trials, the dose is titrated up to 6mg/kg/day, though higher doses have shown increased adverse events. The 4.5mg/kg/day dose is considered optimal for balancing efficacy and safety.46
Preliminary findings. In a Phase III trial for AD, masitinib (4.5mg/kg/day) showed a significant benefit in slowing cognitive decline over 24 weeks, as measured by the ADAS-Cog. Patients taking masitinib showed better cognitive stability compared to those on placebo, with a mean improvement of –2.15 on ADAS-Cog. Functional improvements were noted on the ADCS-ADL scale, though results were less consistent at higher doses.45,46
Adverse effects. Masitinib’s adverse effects include mild-to-moderate reactions, primarily maculopapular rash, neutropenia, and hypoalbuminemia. Severe adverse events were more common with the higher 6mg/kg/day dose. Common severe events included neutropenia and skin reactions. Nonfatal severe adverse event rates were higher in masitinib groups than placebo (incidence rate ratio [IRR]: 2.4), with treatment discontinuations due to these effects in some cases. The 4.5mg/kg/day dose is generally better tolerated.45,46
Masupirdine. Overview. Masupirdine (SUVN-502) is a serotonin-6 (5-HT6) receptor antagonist evaluated as an adjunct treatment for moderate AD. This receptor is significant in modulating neurotransmitters linked to cognition, making it a promising target for AD-related cognitive symptoms. The Phase II study focused on its efficacy and safety in patients also taking donepezil and memantine, common AD treatments.
Indication. Masupirdine is studied as an adjunct treatment for moderate AD, specifically to enhance cognitive outcomes when used with donepezil and memantine.
Dose and route. Patients received either 50mg or 100mg of masupirdine, taken orally once daily, compared to a placebo group.
Preliminary findings. A Phase II, double-blind, randomized study with 564 participants assessed masupirdine’s cognitive effects over 26 weeks. Results showed no statistically significant improvement in cognitive function (measured by ADAS-Cog-11 scores) compared to placebo. However, numerical differences favoring masupirdine were observed, particularly in the NPI scale, suggesting potential effects on behavioral symptoms.47
Adverse effects. A total of 968 treatment-emergent adverse events (TEAEs) were reported in 56.8 percent of patients, at similar rates across treatment arms (54.0% in masupirdine 50mg, 59.1% in 100mg, and 57.4% in placebo). TEAEs attributed to treatment were higher in the 100mg group (21.0%) than in the 50mg group (15.5%) and placebo group (11.2%). Common TEAEs included urinary tract infections, falls, diarrhea, headache, and elevated liver enzymes. Serious TEAEs (eg, pneumonia, sepsis, syncope, acute kidney injury, dehydration) were infrequent, with only dehydration considered treatment-related. A small number of TEAEs led to study discontinuation, with common causes being agitation, mental status changes, and elevated liver enzymes. Six fatalities occurred but were deemed unrelated to treatment.47
Metformin extended-release. Overview. Metformin, a biguanide, is widely used as a first-line treatment for type 2 diabetes due to its efficacy in lowering blood glucose by inhibiting hepatic gluconeogenesis. Besides its metabolic benefits, metformin has shown potential neuroprotective and cognitive benefits, possibly by enhancing insulin sensitivity and influencing the gut-brain axis. The drug may reduce the risk of dementia and neurodegenerative diseases, although the cognitive effects vary across studies.48
Indication. Primarily indicated for the management of type 2 diabetes, metformin is also explored for potential use in managing age-related cognitive decline and neurodegenerative diseases due to its observed effects on cognition and gut microbiota.
Dose and route. Metformin is typically administered orally at a dose of 500 to 2,000mg daily for diabetes mellitus management. Doses vary based on individual tolerance and treatment response, with higher doses generally split into two or more administrations throughout the day.
Preliminary findings. Studies suggest metformin treatment may be associated with reduced dementia incidence among individuals with type 2 diabetes. For example, a large study of US veterans with type 2 diabetes found lower dementia rates among metformin users than in those using other antidiabetic drugs. Additionally, metformin has shown associations with improved executive function, memory, and processing speed, although some studies report negligible effects on cognitive outcomes.48
Adverse effects. The most common adverse effects of metformin are gastrointestinal, including nausea, vomiting, and diarrhea. Long-term use may lower vitamin B12 levels, increasing the risk of deficiency-related cognitive impairment. Other less common side effects include lactic acidosis, especially in patients with renal impairment, and hypoglycemia when used with other glucose-lowering agents.48
Nabilone. Overview. Nabilone is a partial agonist at CB-1 and CB-2 cannabinoid receptors. It has been shown to reduce agitation in patients with dementia in a Phase III trial.
Dose and route. The starting dose of nabilone was 0.5mg in the evening, which was later increased to 0.5mg twice daily.
Preliminary findings. Nabilone was shown to reduce agitation in patients with dementia in a Phase III trial. In a previous case study of a 72-year-old patient with dementia and associated cognitive decline, administration of nabilone decreased behavioral imbalance and greatly improved associated regulation caused by dementia.49
Adverse effects. Possible adverse effects include sedation and affected cognition.50
Nilotinib. Overview. Nilotinib, a tyrosine kinase inhibitor traditionally approved for Philadelphia chromosome–positive chronic myeloid leukemia, has shown potential in reducing AD pathology. Low doses penetrate the BBB, promoting the degradation of Aβ and tau proteins. In AD research, nilotinib was tested for its safety, tolerability, and biomarker modulation in mild-to-moderate AD.
Indication. Nilotinib is primarily indicated for chronic myeloid leukemia. However, preclinical evidence suggested potential efficacy in AD, leading to investigations into its safety and efficacy in AD through clinical trials.
Dose and route. In the AD study, patients received an oral dose of 150mg daily for the first 26 weeks, followed by 300mg daily for an additional 26 weeks.
Preliminary findings. This Phase II study revealed that nilotinib was well tolerated, achieved detectable CSF levels, and reduced key biomarkers of AD. Specifically, it reduced CNS amyloid burden in the frontal lobe and lowered cerebrospinal fluid Aβ40 and Aβ42 levels. P-tau181 was also reduced at six and 12 months. Additionally, hippocampal volume loss was attenuated by 27 percent after 12 months in the nilotinib group compared to the placebo group.51
Adverse effects. Nilotinib was associated with mood swings, particularly agitation and irritability, with a higher incidence during the 300mg dosing phase. Other reported adverse effects included headache, gastrointestinal issues, skin and respiratory disorders, and occasional nervous system and musculoskeletal complaints. No QT prolongation or serious adverse events were observed, and adverse events were resolved without intervention.51
Pimavanserin (Nuplazid). Overview. Pimavanserin is an orally administered atypical antipsychotic medication that has been FDA-approved for the treatment of psychotic symptoms in PD. It is an inverse agonist at serotonin 5-HT2A and 5-HT2C receptors. It is being investigated in Phase III trials for relapse of dementia-related psychosis, schizophrenia with residual psychotic symptoms, and MDD.
Dose and route. Pimavanserin 34mg is taken orally once daily without titration.
Preliminary findings. In a Phase III trial, pimavanserin was successful in preventing relapse of psychosis in patients with dementia. A meta-analysis of randomized clinical trials of pimavanserin in the treatment of PD-related psychosis52 found that pimavanserin was extremely effective in alleviating symptoms of psychosis in patients, assessed by decreased scores in the Scale of Assessment of Positive Symptoms, Hallucinations, and Delusions. In the HARMONY Phase III trial, pimavanserin was successful in preventing relapse of psychosis in patients with dementia.52
Adverse effects. Common reported adverse effects included headache and somnolence.
PM012 (herbal compound). Overview. PM012 is a standardized herbal formula derived from traditional herbal medicines, primarily targeting cognitive improvement and neurogenesis. Composed of various herbal extracts, PM012 has demonstrated potential benefits in AD models by reducing Aβ deposits, promoting brain-derived neurotrophic factor expression, and enhancing spatial memory. PM012’s efficacy has been supported through animal studies involving AD models, where it ameliorated memory deficits and increased neurogenesis and glucose metabolism.53,54
Indication. PM012 is primarily investigated for AD and related cognitive impairments. It is suggested as a therapeutic option due to its effects on memory enhancement and neuroprotection, although it is still in the experimental phase.54
Dose and route. In preclinical studies, PM012 was administered orally to mice at doses of 100mg/kg and 400mg/kg. It was tested over periods of up to 26 weeks in rats at doses of 500, 1,000, and 2,000mg/kg per day to assess its safety and tolerability.54
Preliminary findings. Studies indicate that PM012 significantly reduces escape latency in spatial memory tests (Morris Water Maze) in AD-model mice, suggesting improved cognitive function. Additionally, PM012 reduced Aβ deposits in the hippocampus, upregulated brain-derived neurotrophic factors, and promoted neurogenesis, pointing toward its neuroprotective properties and potential as an AD treatment.53,54
Adverse effects. In toxicity studies, PM012 showed no serious adverse effects, even at high doses (up to 2,000mg/kg in rats). Minor observations included an increase in thymus weight in female rats, although this was deemed toxicologically insignificant. PM012 did not affect survival, body weight, or food/water consumption or cause noticeable histopathological changes in major organs.54
Remternetug. Overview. Remternetug is an investigational N3pH Aβ monoclonal antibody developed for AD. It is a successor to donanemab, also designed to reduce amyloid plaques. Remternetug works by targeting pyroglutamate Aβ, aiming to slow cognitive decline in patients with mild cognitive impairment or early AD.55
Dose and route. In the Phase I multiple ascending dose (MAD) study (NCT04451408), patients received IV doses ranging from 250mg to 2,800mg every four weeks.
Preliminary findings. Forty-one participants with MCI or mild-to-moderate dementia were randomly assigned to remternetug IV infusions ranging from 250 to 2,800mg every four weeks or placebo, and amyloid plaque levels were measured by florbetapir PET at baseline, Day 85, and Day 169. Remternetug showed dose-dependent lowering of amyloid plaque, with 75 percent of participants on higher doses showing significant amyloid clearance by Day 169.55
Adverse effects. ARIA was reported in 10 of 24 participants, with one symptomatic patient.55
Simufilam (PTI-125). Overview. Simufilam is an oral small-molecule drug developed for AD. It targets an altered conformation of filamin A (FLNA), a scaffolding protein associated with neuroinflammation and synaptic dysfunction in AD. By binding to and restoring FLNA’s normal shape, simufilam aims to disrupt toxic Aβ interactions, reduce tau hyperphosphorylation, and improve insulin receptor signaling, which are all linked to AD pathology.
Indication. Simufilam is intended for the treatment of AD, particularly targeting neurodegenerative processes involved in AD pathology, such as neuroinflammation and synaptic impairment.
Dose and route. In clinical trials, simufilam is administered orally at a dose of 100mg twice daily. The current studies include Phase III trials testing its efficacy and safety over extended periods in patients with AD.
Preliminary findings. Simufilam has shown promising results in preclinical and early clinical trials. It was found to reduce aberrant FLNA linkages with receptors implicated in AD pathology, such as the alpha-7 nicotinic acetylcholine receptor (α7nAChR) and toll-like receptor 4 (TLR4). This action decreases Aβ42’s toxic effects, reduces neuroinflammation, and inhibits tau hyperphosphorylation. Furthermore, simufilam has demonstrated potential in restoring insulin receptor sensitivity by reducing overactive mammalian target of rapamycin (mTOR) signaling and improving mTOR’s response to insulin, another key issue in AD.56
Adverse effects. Early-phase trials have reported no significant adverse effects related to simufilam. The drug has been well tolerated, with no serious adverse events directly linked to its use. Ongoing trials will provide further insight into its safety profile over longer durations and across a broader patient population.
Suvorexant. Overview. Suvorexant is an antagonist at orexin-1 and orexin-2 receptors that is approved for the treatment of insomnia. It is currently being investigated in a Phase III trial for improved sleep and restlessness in participants with dementia.
Dose and route. Suvorexant 10mg is taken orally once per day.
Preliminary findings. Suvorexant improved sleep and restlessness in participants with dementia in a Phase III trial. In a randomized, double-blind, four-week trial (n=285), suvorexant was effective in improving symptoms of insomnia in AD. Results obtained from the polysomnography-derived total sleep time (TST) measure showed a significant improvement of 73 minutes from baseline after four weeks of treatment compared to 45 minutes for placebo.57
Adverse effects. Reported adverse effects include mild-to-moderate sedation.58
Tricaprilin. Overview. Tricaprilin is a medium-chain triglyceride used as a ketogenic agent for managing mild-to-moderate AD. It releases octanoic acid, which the liver metabolizes into ketones that can serve as an alternative energy source for the brain, particularly useful in AD, where glucose metabolism may be impaired.
Indication. Tricaprilin is primarily indicated for mild-to-moderate AD, where it aims to support cognitive function through ketogenesis, compensating for reduced glucose uptake in the AD brain.
Dose and route. In clinical settings, a specific formulation of tricaprilin (AC-SD-03) was administered orally as a 50g dose mixed with water and taken after a meal. This dose provided 42,500µmol of tricaprilin, which undergoes digestion in the gastrointestinal tract.58
Preliminary findings. Initial studies showed that tricaprilin is effectively digested and rapidly converted into ketones, achieving sustained plasma levels of ketone bodies, beta-hydroxybutyrate, and acetoacetate. This conversion offers a nonglucose energy source to the brain, improving cognitive outcomes in AD patients. Findings from a Phase I clinical trial indicated consistent plasma ketone levels postadministration, supporting tricaprilin’s efficacy as a ketogenic agent.58
Adverse effects. Tricaprilin has shown a favorable safety profile, with no major adverse effects reported. Minor gastrointestinal discomfort and slight increases in certain liver enzymes were observed, but these effects were generally mild and transient.
Valiltramiprosate (ALZ-801). Overview. Valiltramiprosate, marketed as ALZ-801, is an oral prodrug of tramiprosate developed to treat AD. It works by stabilizing Aβ monomers, preventing their aggregation into toxic oligomers. Valiltramiprosate is designed to improve the pharmacokinetic limitations of tramiprosate, including high variability and gastrointestinal side effects, providing a more stable delivery and reduced gastrointestinal discomfort.
Indication. Valiltramiprosate is being investigated for mild-to-moderate AD, with a focus on patients carrying two pairs of the APOE4 allele, a group that exhibits significantly increased risk of high levels of amyloid plaques and elevated risk for AD progression.
Dose and route. In clinical trials, the recommended dose of valiltramiprosate is 265mg twice daily, taken orally with food to enhance gastrointestinal tolerability. This dosage achieves tramiprosate plasma levels comparable to those in previous trials.59
Preliminary findings. Valiltramiprosate has shown efficacy in reducing amyloid plaque buildup and improving cognitive outcomes in APOE4-positive patients with AD. Studies indicated that valiltramiprosate maintains a stable plasma profile of tramiprosate with reduced intersubject variability, and it preserves cognitive function better than the parent compound in this population.59
Adverse effects. The most common adverse effects reported were mild nausea and vomiting, which generally decreased with continued treatment. Valiltramiprosate has demonstrated a favorable safety profile, with no severe or dose-limiting toxicities reported, especially when administered with food to minimize gastrointestinal discomfort.
Xanomeline (Lumeron). Overview. Xanomeline is a muscarinic receptor agonist selective for M1 and M4 receptors. It is indicated for AD, as well as resulting cognitive impairment/deterioration. There is currently minimal information on the effectiveness of xanomeline in the treatment of AD compared to other conditions, such as schizophrenia, and it has yet to receive official approval by the FDA for the treatment of AD.
Dose and route. There are three doses of xanomeline: 75mg (low dose), 150mg (medium dose), and 225mg (high dose).
Preliminary findings. The efficacy of xanomeline in the treatment of AD was evaluated in a parallel-group study.60 Participants (n=343) were randomly assigned either 75mg, 150mg, 225mg, or placebo. Efficacy was evaluated through the use of the ADAS-Cog, Alzheimer’s Disease Symptomatology Scale (ADSS), CIBIC+, and Nurses’ Observational Scale for Geriatric Patients (NOSGER). Results showed that the administration of xanomeline was effective in treating AD symptoms, showing a dose-dependent relationship. Both ADAS-Cog and CIBIC+ showed significant improvement compared to placebo. NOSGER also reported significant improvement in geriatric patients with high-dose administration (p<0.02). It has proceeded to Phase III clinical trials for other conditions, such as schizophrenia, but has yet to receive any official approval by the FDA for AD.61
Adverse effects. Adverse effects include sweating (75.9%), nausea (51.7%), vomiting (42.5%), chills (36.8%), dyspepsia (24.1%), increased salivation (24.1%), and syncope (high doses).62
Lecanemab-irmb subcutaneous (Leqembi-IQLIK). Overview. Lecanemab-irmb subcutaneous injection is a monoclonal antibody treatment targeting Aβ protofibrils to slow disease progression in early AD. It was recently approved by the FDA on August 29, 2025.
Indication. Lecanemab-irmb subcutaneous injection is indicated for early stages of AD, specifically MCI or mild dementia.
Dose and route. In Phase III trials, lecanemab-irmb’s subcutaneous formulation is administered as two injections weekly. The subcutaneous route has shown enhanced pharmacokinetics (11% higher area under the curve) and 14-percent greater amyloid clearance than the biweekly IV formulation.
Preliminary findings. Subcutaneous lecanemab-irmb demonstrates a promising reduction in amyloid burden with a favorable safety profile. Data show that 76 percent of low-tau patients exhibited no clinical decline, and 60 percent showed improvement after 18 months. This outcome aligns with cognitive and functional preservation across measured scales.31
Adverse effects. Adverse effects include infusion reaction, headache, and ARIA-E.
Discussion
Summary of the findings. This systematic review examined the landscape of medications approved by the FDA for NCDs from 2008 to 2024, as well as those currently in Phase III clinical trials. During this period, we identified eight medications that received FDA approval, primarily targeting AD and related dementias. These approved medications include aducanumab, benzagalantamine, brexpiprazole, transdermal donepezil, lecanemab, extended-release memantine, donanemab, and a combination formulation of memantine and donepezil. While these treatments offer symptomatic relief and, in some cases, aim to modify disease pathology/course progression, they are often accompanied by well-established challenges such as adverse effects, high treatment costs, and limited efficacy in altering disease progression.
In addition to the approved medications, this review identified 22 pipeline drugs currently undergoing Phase III clinical trials. These investigational therapies exhibit a range of novel mechanisms of action, including targeting Aβ plaques, modulating neurotransmitter systems, and addressing neuroinflammation and mitochondrial dysfunction. The diversity of these approaches reflects an increased effort to improve therapeutic outcomes for patients with NCDs. Collectively, these findings reflect a degree of progress made in pharmacotherapy for NCDs.63 No medications for delirium were FDA-approved or in Phase III, although agents for a variety of encephalopathies have been developed.
Current state of treatment. Current guidelines for managing NCDs majorly advocate for both pharmacological and nonpharmacological approaches, tailored to the stage of the disease and the individual patient’s needs. Nonpharmacological treatment involves redirecting the patient through social interactions, engaging them in enjoyable activities, promoting behavioral activation, and effectively lowering conflict.9,64,65
First-line pharmacological treatment for mild-to-moderate AD typically includes acetylcholinesterase inhibitors such as donepezil and rivastigmine,66 while NMDA receptor antagonists such as memantine are recommended for moderate-to-severe stages.67 Professional organizations, such as the Alzheimer’s Association, recommend comprehensive treatment plans that integrate medication management with lifestyle interventions, caregiver support, and behavioral strategies to address neuropsychiatric symptoms (NPS).68 While it does not appear that any practice guidelines by professional organizations currently include disease-modifying therapies, such as monoclonal antibodies, as a recommended intervention,69 ongoing research and clinical trials continue to explore their potential efficacy and safety in different patient populations with AD, among other NCDs.
Drugs such as lecanemab and aducanumab, both monoclonal antibodies, target Aβ plaques and were among the first disease-modifying treatments approved to potentially slow disease progression in early AD. Donanemab-azbt, approved for MCI or dementia due to AD, demonstrated statistically significant reductions in clinical decline, presenting a promising alternative despite its high cost.29
Biologics represents a promising frontier in the treatment of NCDs by directly targeting the pathophysiological symptoms of diseases like AD. An advantage of biologics is its potential to modify disease progression rather than merely alleviating symptoms. However, their use is associated with significant challenges, including high costs,70 limited infrastructure for administering infusion-based therapies,71 and the need for regular monitoring for adverse events, such as ARIA.72 These treatments are most effective in the early stages of AD, requiring early and accurate diagnosis, which may not be feasible in resource-limited settings.73 Additionally, patient selection remains critical, as the risk-benefit ratio varies based on individual factors such as comorbidities and genetic predispositions.74 Future directions in biologics include the development of agents with improved safety profiles, more convenient delivery methods, and broader therapeutic targets, such as tau proteins and neuroinflammation.75 As biologics continue to evolve, their integration into clinical practice will require addressing these barriers to ensure equitable access and optimal outcomes.
The past 16 years have also witnessed improvements to existing medications. Benzgalantamine, as a delayed-release acetylcholinesterase inhibitor, offers improved dosing flexibility over galantamine. Donepezil transdermal patches were designed to enhance patient adherence to donepezil and are designed to be applied once weekly. In addition, combination therapies, including memantine+donepezil, may offer synergistic benefits by targeting multiple pathological mechanisms underlying AD.
Unique characteristics. Several unique characteristics emerged from the analysis of current and investigational medications for NCDs. One notable development is the shift toward disease-modifying therapies, exemplified by the approval of monoclonal antibodies such as aducanumab and lecanemab. These agents target the pathological accumulation of Aβ plaques, a hallmark of AD pathology, representing a significant departure from treatments focusing mainly on symptomatic relief.10
Another unique aspect is the introduction of novel drug delivery systems aimed at enhancing patient compliance and convenience. The approval of donepezil transdermal patches offers an innovative alternative to oral administration, potentially improving adherence in patients who may have difficulties swallowing pills or maintaining regular dosing schedules.75
The focus on neuropsychiatric symptoms, particularly agitation and aggression, has also garnered attention. The atypical antipsychotic brexpiprazole was approved for agitation associated wth AD dementia in 2023, marking the first antipsychotic approved for this indication.25,28 This decision by the FDA was not without controversy, as it has the potential to reverse efforts aiming to reduce the use of antipsychotics among older adults in long-term care homes.28 Nevertheless, agitation is reported in approximately 30 to 50 percent of patients living with dementia and is associated with increased healthcare utilization, caregiver burden, and poorer outcomes.76,77 Therefore, effective management of agitation would significantly improve favorable treatment outcomes.77
Furthermore, the repurposing of existing medications initially developed for other conditions demonstrates an innovative approach to a broader understanding of the disease and drug development. Agents such as dapagliflozin and metformin, originally indicated for type 2 diabetes, are being explored for their neuroprotective properties and potential cognitive benefits in NCDs.79
Lastly, investigational medications exhibit diverse mechanisms of action, targeting various aspects of neurodegenerative pathophysiology. These include modulating neurotransmitter systems (eg, masupirdine targeting the serotonergic system), reducing neuroinflammation (eg, masitinib), and enhancing neurogenesis (eg, PM012 herbal compound). This approach highlights an understanding that the pathophysiology of NCDs is complex and may require interventions at multiple biological levels.
New trends. Emerging trends identified in this review suggest fundamental changes in pharmacotherapeutic strategies for NCDs. One significant trend is the emphasis on early intervention. Medications such as lecanemab and other disease-modifying treatments continue to be investigated in patients with early or mild stages of AD before substantial neurodegeneration has occurred.10 Early intervention holds promise in slowing disease progression and preserving cognitive function for longer in patients with NCDs.
Another trend is the pursuit of enhanced safety profiles in new treatments. For instance, benzagalantamine offers similar efficacy to galantamine, a cholinesterase inhibitor, but with fewer gastrointestinal side effects, potentially improving patient tolerability and adherence to treatment regimens.24 The development of combination therapies represents a strategic approach to addressing the multifactorial nature of NCDs. The approval of a combination of memantine and donepezil reflects this trend, aiming to simultaneously modulate different neurotransmitter systems involved in cognitive function and neurodegeneration.13
There is also a growing interest in targeting alternative therapeutic pathways beyond the traditional amyloid and cholinergic hypotheses. Agents such as nabilone, a cannabinoid receptor agonist, and pimavanserin, a serotonergic receptor modulator,79 are being investigated for their potential to manage neuropsychiatric symptoms more effectively.49
Lastly, personalized medicine is becoming increasingly prominent, with some pipeline drugs focusing on genetic subtypes of AD. For example, treatments tailored for APOE4 carriers represent a move towards individualized therapeutic strategies, recognizing the genetic and molecular heterogeneity of neurocognitive disorders.80
Conclusion
The findings of this systematic review highlight significant advancements in the pharmacological management of NCDs over the past decade and a half. While current FDA-approved medications primarily offer symptomatic relief for AD and related dementias, limitations such as adverse effects, high costs, and modest efficacy in altering disease progression persist. The robust pipeline of investigational medications in Phase III clinical trials reflects a concerted effort to develop therapies with novel mechanisms of action, improved safety profiles, and innovative delivery methods. These emerging treatments hold promise not only for alleviating symptoms but also for modifying the underlying disease processes. Ongoing research and clinical trials will prove essential in validating the efficacy and safety of these potential therapies.
Ultimately, the evolution of pharmacotherapy in neurocognitive disorders aims to enhance patient outcomes and quality of life. The shift toward early detection/intervention, personalized medicine, and multifaceted treatment strategies offers hope for more effective management of these debilitating conditions. Continued interdisciplinary collaboration and investment in research will be crucial in translating these advancements into clinical practice. To date, there remains a dearth of studies investigating possible pharmacological treatments for NCDs other than AD, such as delirium, NCD with Lewy bodies, frontotemporal NCD, vascular NCD, and NCDs due to TBI, PD, Huntington’s disease, prion disease, and multiple etiologies. Future investigation efforts should continue to elucidate the mechanisms of action for varying degenerative conditions. In addition, continued development and utilization of nonpharmacological treatments should accompany the advancement of pharmacological treatments for optimal outcomes.
References
- Samtani S, Meka A, Siette J. Beyond memory: exploring the value of social cognition for older adults with neurocognitive disorders. Front Psychiatry. 2023;14:1209745.
- Fernández-Martínez M, Castro J, Molano A, et al. Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr Alzheimer Res. 2008;5(1):61–69.
- Tariot PN, Mack JL, Patterson MB, et al. The behavior rating scale for dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152(9):1349–1357.
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, Fifth edition, text revision (DSM-5-TR), 2022. https://psychiatryonline.org/pb-assets/dsm/update/DSM-5-TR_Neurocognitive-Disorders-Supplement_2022_APA_Publishing.pdf
- Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46(5):1020–1022.
- Maldonado J, Sher Y. Neurocognitive Disorders. In: Tasman’s Psychiatry. Springer; 2024. p. 1–60.
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734.
- Budd Haeberlein S, Aisen P, Barkhof F, et al. Two randomized Phase III studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210.
- Brasure M, Jutkowitz E, Fuchs E, et al. Nonpharmacologic interventions for agitation and aggression in dementia. Rockville (MD): Agency for Healthcare Research and Quality (US). 2016. Report No.: 16-EUC019-EF.
- Van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9–21.
- Liu KY, Stringer AE, Reeves SJ, Howard RJ. The neurochemistry of agitation in Alzheimer’s Disease: a systematic review. Ageing Res Rev. 2018;43:99–107.
- Tariot PN, Braeckman R, Oh C. Comparison of steady-state pharmacokinetics of donepezil transdermal delivery system with oral donepezil. J Alzheimers Dis. 2022;90(1):161–172.
- Deardorff WJ, Grossberg GT. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s Disease. Drug Des Devel Ther. 2016;10:3267–3279.
- Parsons CG, Danysz W, Dekundy A, Pulte I. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s Disease. Neurotox Res. 2013;24:358–369.
- Koskas P, Kohler S, Estrada J, et al. Effect of a multi-domain intervention on the quality of life in older adults with major neurocognitive disorder: A pilot study. Rev Neurol. 2022;178(4):355–362.
- Synnott PG, Whittington MD, Lin GA, et al. The effectiveness and value of aducanumab for Alzheimer’s disease: A summary from the Institute for Clinical and Economic Review’s California Technology Assessment Forum. J Manag Care Spec Pharm. 2021;27(11):1613–1617.
- Vaz M, Silva V, Monteiro C, Silvestre S. Role of aducanumab in the treatment of Alzheimer’s disease: challenges and opportunities. Clin Interv Aging. 2022;17:797–810.
- Qiao Y, Chi Y, Zhang Q, Ma Y. Safety and efficacy of lecanemab for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. Front Aging Neurosci. 2023;15:1169499.
- Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in two Phase III studies evaluating aducanumab in patients with early Alzheimer’s disease. JAMA Neurol. 2022;79(1):13–21.
- Cummings J, Aisen P, Lemere C, et al. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res Ther. 2021;13(1):98.
- Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33.
- Manly JJ, Glymour MM. What the aducanumab approval reveals about Alzheimer’s disease research. JAMA Neurol. 2021;78(11):1305–1306.
- Bastrup J, Hansen KH, Poulsen TBG, et al. Anti-Aβ Antibody Aducanumab Regulates the Proteome of Senile Plaques and Closely Surrounding Tissue in a Transgenic Mouse Model of Alzheimer’s Disease. J Alzheimers Dis. 2021;79(1):249–265.
- Alpha Cognition, Inc. (2024). Zunveyl (benzgalantamine) delayed-release tablets: Highlights of prescribing information. Retrieved from FDA on December 31, 2024.
- Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. Am J Geriatr Psychiatry. 2020;28(4):383–400.
- Das S, Barnwal P, Winston AB, et al. Brexpiprazole: so far so good. Ther Adv Psychopharmacol. 2016;6(1):39–54.
- Bruijnzeel D, Tandon R. Spotlight on brexpiprazole and its potential in the treatment of schizophrenia and as adjunctive therapy for the treatment of major depression. Drug Des Devel Ther. 2016;10:1641–1647.
- Whitaker R. How the FDA approved an antipsychotic that failed to show a meaningful benefit but raised the risk of death. BMJ. 2023;382:1801.
- Alzheimer’s Association. Donanemab Approved for Treatment of Early Alzheimer’s Disease. https://www.alz.org/alzheimers-dementia/treatments/donanemab. Retrieved on December 31, 2024.
- Corium Inc. (2022). ADLARITY prescribing information. https://corium.com/products/ADLARITY/ADLARITY_PI_ENGLISH_US.pdf. Retrieved on December 31, 2024.
- BioArctic AB. (2023). Press Release: New data from LEQEMBI® (lecanemab-irmb) Phase III Clarity AD study and subcutaneous formulation presented at CTAD, 2023.
- Eisai Co., Ltd. (2023). New Clinical Data Demonstrates Three Years of Continuous Treatment with Dual-Acting LEQEMBI. Retrieved on December 31, 2024.
- Forest Pharmaceuticals, Inc. (2010). Namenda XR (memantine hydrochloride) extended-release capsules: Prescribing information. Retrieved from the U.S. Food and Drug Administration website on December 31, 2024.
- Guo J, Wang Z, Liu R, et al. Memantine, donepezil, or combination therapy—what is the best therapy for Alzheimer’s disease? A network meta‐analysis. Brain Behav. 2020;10(11):e01831.
- Alzforum.org. Therapeutics: ACP-204. https://www.alzforum.org/therapeutics/acp-204. on December 31, 2024.
- Mohs R, Bakker A, Rosenzweig-Lipson S, et al. The HOPE4MCI study: a randomized double-blind assessment of AGB101 for the treatment of MCI due to AD. Alzheimers Dement (N Y). 2024;10(1):e12446.
- Alzheimer’s Disease Drug Discovery Foundation. (2022, January 21). Cognitive Vitality Reports: AR1001. Retrieved from https://www.alzdiscovery.org. On December 31, 2024.
- Chen P, Liang L, Dai Y, Hui S. The role and mechanism of dapagliflozin in Alzheimer’s Disease: a review. Medicine (Baltimore). 2024;103(39):e39687.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer’s dementia: a randomized clinical trial. JAMA. 2015;314(12):1242–1254.
- Doody RS, D’Amico S, Cutler AJ, et al. An open-label study to assess safety, tolerability, and effectiveness of dextromethorphan/quinidine for pseudobulbar affect in dementia: PRISM II results. CNS Spectr. 2016;21(6):
450–459. - Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67(4):413–418.
- Chau S, Herrmann N, Ruthirakuhan MT, et al. Latrepirdine for Alzheimer’s disease. Cochrane Database Syst Rev. 2015;2015(4):CD009524.
- Ostrowitzki S, Lasser RA, Dorflinger E, et al. A Phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):95.
- Koh S-H, Kwon HS, Choi SH, et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: a phase II randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res Ther. 2021;13(1):66.
- Hamad AA, Amer BE. Safety of masitinib in patients with neurodegenerative diseases: a meta-analysis of randomized controlled trials. Neurol Sci. 2024;45(7):3503–3507.
- Dubois B, López-Arrieta J, Lipschitz S, et al. Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, Phase III clinical trial. Alzheimers Res Ther. 2023;15(1):39.
- Nirogi R, Ieni J, Goyal VK, et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: A randomized, double-blind, Phase II, proof-of-concept study. Alzheimers Dement (N Y). 2022;8(1):e12307.
- Rosell-Díaz M, Fernández-Real JM. Metformin, cognitive function, and changes in the gut microbiome. Endocr Rev. 2024;45(2):210–226.
- Passmore MJ. The cannabinoid receptor agonist nabilone for the treatment of dementia-related agitation. Int J Geriatr Psychiatry. 2008;23(1):116–117.
- Herrmann N, Ruthirakuhan M, Gallagher D, et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2019;27(7):807–817.
- Turner RS, Hebron ML, Lawler A, et al. Nilotinib effects on safety, tolerability, and biomarkers in Alzheimer’s disease. Ann Neurol. 2020;88(1):183–194.
- Tariot PN, Cummings JL, Soto-Martin ME, et al. Trial of pimavanserin in dementia-related psychosis. N Engl J Med. 2021;385(4):309–319.
- Ye M, Chung HS, An YH, et al. Standardized herbal formula PM012 decreases cognitive impairment and promotes neurogenesis in the 3xTg AD mouse model of Alzheimer’s disease. Mol Neurobiol. 2016;53(8):5401–5412.
- Sohn SH, Kim SJ, Kim Y, et al. Safety and efficacy assessment of standardized herbal formula PM012. BMC Complement Altern Med. 2012;12:24.
- Jin Y. Safety and amyloid plaque reduction effects of remternetug in patients with Alzheimer’s disease: interim analysis from a Phase I study. Presented at: 2023 AD/PD Conference; March 28 to April 1; Gothernburg, Sweden.
- Wang HY, Pei Z, Lee KC, et al. Simufilam suppresses overactive mTOR and restores its sensitivity to insulin in Alzheimer’s disease patient lymphocytes. Front Aging. 2023;4:1175601.
- Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16(3):541–551.
- Mellman TA, Birku K, Sandhu I, et al. Evaluation of suvorexant for trauma-related insomnia. Sleep. 2022;45(5):zsac068.
- Hey JA, Yu JY, Versavel M, et al. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2018;57(3):315–333.
- Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer’s disease. Arch Neurol. 1997;54(4):465–473.
- McDonald JK, van der Westhuizen ET, Pham V, et al. Biased profile of xanomeline at the recombinant human M₄ muscarinic acetylcholine receptor. ACS Chem Neurosci. 2022;13(8):1206–1218.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384:717–726.
- Hafiz R, Alajlani L, Ali A, et al. The latest advances in the diagnosis and treatment of dementia. Cureus. 2023;15(12):e50522
- Selwood A, Johnston K, Katona C, et al. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord. 2007;101(1– 3):75–89.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029.
- National Institute for Health and Care Excellence (NICE). Dementia: Assessment, management and support for people living with dementia and their carers. London: National Institute for Health and Care Excellence; 2018. (NICE Guideline, No. 97.) 11, Cholinesterase inhibitors and memantine for dementia.
- Olivares D, Deshpande VK, Shi Y, et al. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr Alzheimer Res. 2012;9(6):746–758.
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024;20(5):3708–3821.
- Tahami Monfared AA, Phan NTN, Pearson I, et al. A systematic review of clinical practice guidelines for Alzheimer’s disease and strategies for future advancements. Neurol Ther. 2023;12(4):1257–1284.
- Poduval P, Parsekar S, Meena SN. Small molecules vs biologics. In: Meena SN, Nandre V, Kodam K, Meena RS, eds. Progress in Biochemistry and Biotechnology: New Horizons in Natural Compound Research; 2023:179–199.
- Bradshaw AC, Georges J. Anti-amyloid therapies for Alzheimer’s disease: an Alzheimer Europe position paper and call to action. J Prev Alzheimers Dis. 2024;11(2):
265–273. - Waite LM. New and emerging drug therapies for Alzheimer disease. Aust Prescr. 2024;47(3):75–79.
- Dubois B, von Arnim CAF, Burnie N, et al. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res Ther. 2023;15(1):175.
- Ramanan VK, Armstrong MJ, Choudhury P, et al. Antiamyloid monoclonal antibody therapy for Alzheimer disease: emerging issues in neurology. Neurology. 2023;101(19):842–852.
- Zhang J, Zhang Y, Wang J, et al. Recent advances in Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024;9(1):211.
- Carrarini C, Russo M, Dono F, et al. Agitation and dementia: prevention and treatment strategies in acute and chronic conditions. Front Neurol. 2021;12:644317.
- Jones E, Aigbogun MS, Pike J, et al. Agitation in dementia: real-world impact and burden on patients and the healthcare system. J Alzheimers Dis. 2021;83(1):89–101.
- Chen Q, Cao T, Li N, et al. Repurposing of antidiabetic agents as a new opportunity to alleviate cognitive impairment in neurodegenerative and neuropsychiatric disorders. Front Pharmacol. 2021;12:667874.
- Cummings JL, Devanand DP, Stahl SM. Dementiarelated psychosis and the potential role for pimavanserin. CNS Spectr. 2022;27(1):7–15.
- Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019;17(1):64.
