by David R. Spiegel, MD; Kathyrn Sommese, MD; Anastasia Turenkov, MD; and Niels Naimon, MD
Drs. Spiegel, Sommese, Turenkov, and Naimon are with the Department of Psychiatry and Behavioral Sciences at Eastern Virginia Medical School in Norfolk, Virginia.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: The perinatal period represents a time of increased vulnerability to psychiatric disorders, including the largely understudied obsessive-compulsive disorder (OCD). In contrast to the gradual onset of typical OCD, postpartum OCD appears to be characterized by the rapid onset of obsessional symptoms after the birth, with onset as early as the second postpartum day with a mean time to onset of 2.2 to 3.7 weeks. We present a case of a patient with prepartum generalized anxiety disorder (GAD) and new-onset postpartum OCD. The patient’s ego-dystonic obsessions were aggressive in nature (“harm to newborn”) with pathological checking compulsions requiring reassurance that she would not engage in this activity. Neurobiologically, there has been speculation that changes in estrogen and progesterone in the puerperium might alter serotoninergic function, placing some women at risk for this subtype of OCD. Some research studies have found evidence to suggest that oxytocin is associated with OCD. We review the growing evidence that suggests oxytocin and gonadal steroids might play a role in the pathogenesis of some forms of OCD.
Keywords: Peripartum, obsessive-compulsive disorder (OCD), gonadal steroids, oxytocin
Innov Clin Neurosci. 2019;16(5–6):41–45
Obsessive-compulsive disorder (OCD) is a psychiatric illness characterized by recurrent, unwanted, intrusive, and anxiety-producing obsessions coupled with neutralizing or anxiety-relieving, repetitive behaviors known as compulsions. The lifetime prevalence in the United States is estimated to be 2.3 percent, with more female individuals affected than male individuals.1 There is evidence suggesting that hormonal variations during reproductive cycle events in a woman’s life, such as menarche, pregnancy, delivery, and menopause, might induce the onset of or cause an exacerbation of OCD.2,3 Specifically, pregnant and postpartum women have a 45- and 138-percent increased risk of developing OCD, respectively, according to a 2013 meta-analysis.4 A history of psychiatric illness is a risk factor for women developing OCD in the peripartum period, particularly during the postpartum period;3,5 however, the reported onset of OCD symptoms during the peripartum period is lower than that of OCD symptom exacerbations.5 Additional factors associated with the onset of OCD symptoms during the peripartum period include lower maternal age and Cesarean section (C-section) delivery.6
OCD symptoms during the peripartum period take on a different focus than the obsessions and compulsions classically associated with OCD. Thoughts usually center around fear of contamination of the infant, infanticide, and general infant harm, while hoarding, perfectionism, and focus on order are less commonly seen.7 Compulsions in peripartum OCD might include repetitive cleaning or washing to prevent contamination and constantly verifying the infant’s safety. Many women develop some form of obsession or compulsion in the peripartum period without meeting criteria for OCD.5,8 Previous work has shown that these symptoms often decline over time during the postpartum period.9 Herein, we describe a case of peripartum-onset OCD and propose a possible pathophysiological mechanism related to the effect of pregnancy-related physiological changes on neurotransmitters.
Case Report
The patient was a 34-year-old Caucasian woman gravida 4, para 1, aborta 2, who presented at 39 weeks and three days gestation for a scheduled elective C-section. She had a history of generalized anxiety disorder (GAD) and posttraumatic stress disorder (PTSD) following military service. Prior to pregnancy, the patient had been taking citalopram but was currently only taking hydroxyzine as needed. On postoperative Day 3, following an uncomplicated C-section, we were consulted for worsening feelings of anxiety. She stated she had always been a “chronic worrier,” but around the beginning of her third trimester, she began to develop distressing thoughts that she would be unable to care for her baby, and, even more so, that she would harm her baby. She started compulsively searching for reassurance from others about these thoughts and looking up her symptoms online, concerned that she might develop postpartum psychosis. The reassurance only briefly relieved her anxiety until these thoughts returned. She was visibly disturbed by these horrific obsessions, which had been present every day since the start of her third trimester. She added that she could never imagine harming her baby. Our patient scored 30 out of 40 possible points on the Yale-Brown Obsessive Compulsive Scale, which was considered severe OCD.10 The patient denied other types of obsessions and compulsions, including contamination obsessions and cleansing compulsions.
Notably, the patient did not develop obsessions or compulsions during her first pregnancy and said that if she had, she “would never have had another child.” Our patient reported that while these thoughts caused her to feel “depressed,” she denied anhedonia, decreased interest, change in concentration, sleep, and appetite. She did state that she felt guilty for having these thoughts. She denied suicidal ideations, homicidal ideations, and psychosis. Our patient denied a history of hypomanic or manic symptoms or episodes. She also denied alcohol, tobacco, or elicit or prescription drug misuse. Both her blood alcohol and urine drug screen were negative. The patient denied any current symptoms of PTSD, including reliving phenomena, avoidance of reminders of the trauma, or changes in cognition. Our patient’s mental status examination was remarkable for an “anxious” mood and constricted, labile, and mood-congruent affect, but was otherwise within normal limits. The patient, who was hospitalized a total of six days, began sertraline 25mg daily and was to follow up with psychiatry as an outpatient one week after discharge as an outpatient. Unfortunately, the patient was lost to follow up.
Discussion
This case featured a patient who experienced relatively typical peripartum OCD characteristics, including a history of prior psychiatric illness, a C-section delivery, symptoms of obsessions and compulsions regarding harming her infant, and onset of symptoms later in the pregnancy, with continuation into the postpartum period. Her case is also atypical in that she was older than might be expected for the onset of these symptoms, and she did not experience these symptoms with her first pregnancy, which also resulted in a C-section.
OCD can be treated with cognitive behavioral therapy (CBT) and/or medication management. The patient preferred medication therapy over CBT. While the patient was taking citalopram prior to pregnancy, we chose to begin treatment with sertraline. The rationale for choosing sertraline over citalopram was multifactorial. The patient was undecided about breastfeeding, and there is more safety data about sertraline than citalopram in breastfeeding women.10,11 Additionally, sertraline has a United States Food and Drug Administration (FDA)-approved indication for the treatment of OCD, while citalopram does not.10,11 Finally, it is not unusual to treat patients with OCD with higher doses of selective serotonin reuptake inhibitors (SSRIs) than are commonly used to treat depression and anxiety-related disorders. These OCD treatment regimens might include SSRI doses of up to 80mg/day.10 This higher dosing could be impacted by the FDA’s warning against prescribing citalopram at doses greater than 40mg/day,11 hence, our decision to treat with sertraline.
Hormonal and neuropeptide changes in peripartum. The commonly accepted pathophysiology of OCD involves low levels of the neurotransmitter serotonin with high levels of dopamine.13–15 There has also been investigation into the role of glutamate dysfunction in OCD, with much focus on increased levels of glutamate and glutaminergic signaling.16
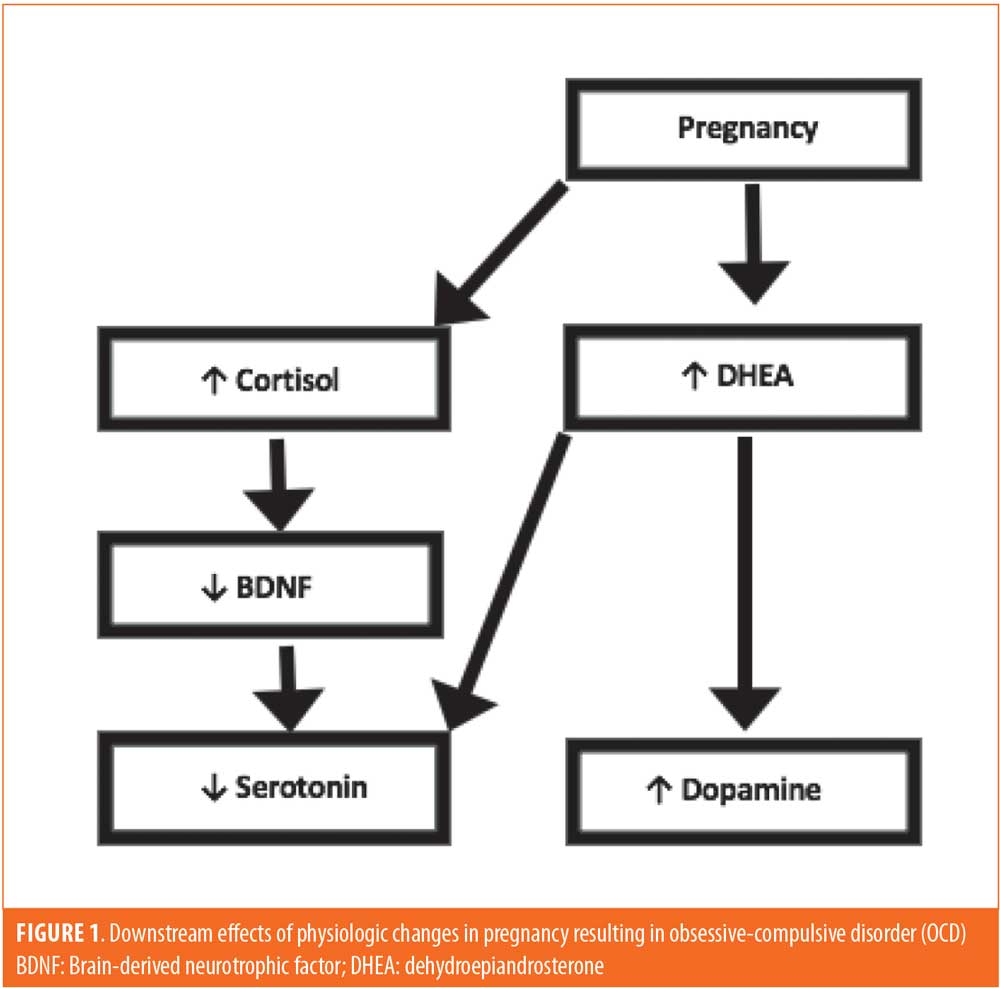
A unique hormonal/neuropeptide environment can occur in the peripartum that might predispose certain women to develop OCD during this time. First, it has been shown that overactivity of the hypothalamic-pituitary-adrenal (HPA) axis occurs in pregnancy, causing the overproduction of cortisol and dehydroepiandrosterone (DHEA).17 We suggest the overproduction of cortisol and DHEA in pregnancy causes downstream effects similar to the dysregulated neurotransmitter states commonly seen in OCD. First, increased cortisol levels have been shown to decrease levels of brain-derived neurotrophic factor (BDNF).18 Normal BDNF levels cause an increase in serotonin and promote serotonergic neuron survival.18 Several studies have implicated low levels of BDNF in patients with OCD.19–21 Thus, increased cortisol from pregnancy decreases BDNF, and, in turn, reduces the levels of serotonin and reduces the survival and function of serotonergic neurons.22 This mechanism can be compounded by the increased production of DHEA. In multiple studies, DHEA has been shown to increase serotonin turnover and have an equivocal or elevating effect on dopamine.23–25 Taken together, the decline of BDNF due to the increase in cortisol and subsequent reduced serotonin combined with the increased dopamine caused by elevated DHEA provide a double hit in pregnancy to provoke the onset of or exacerbation of OCD symptoms (Figure 1). This mechanism is consistent in the greater context of reproductive transitions. During other periods of transition, including menarche and menopause, elevated HPA axis activity is present, explaining the onset of OCD symptoms during these important transitional times as well.26
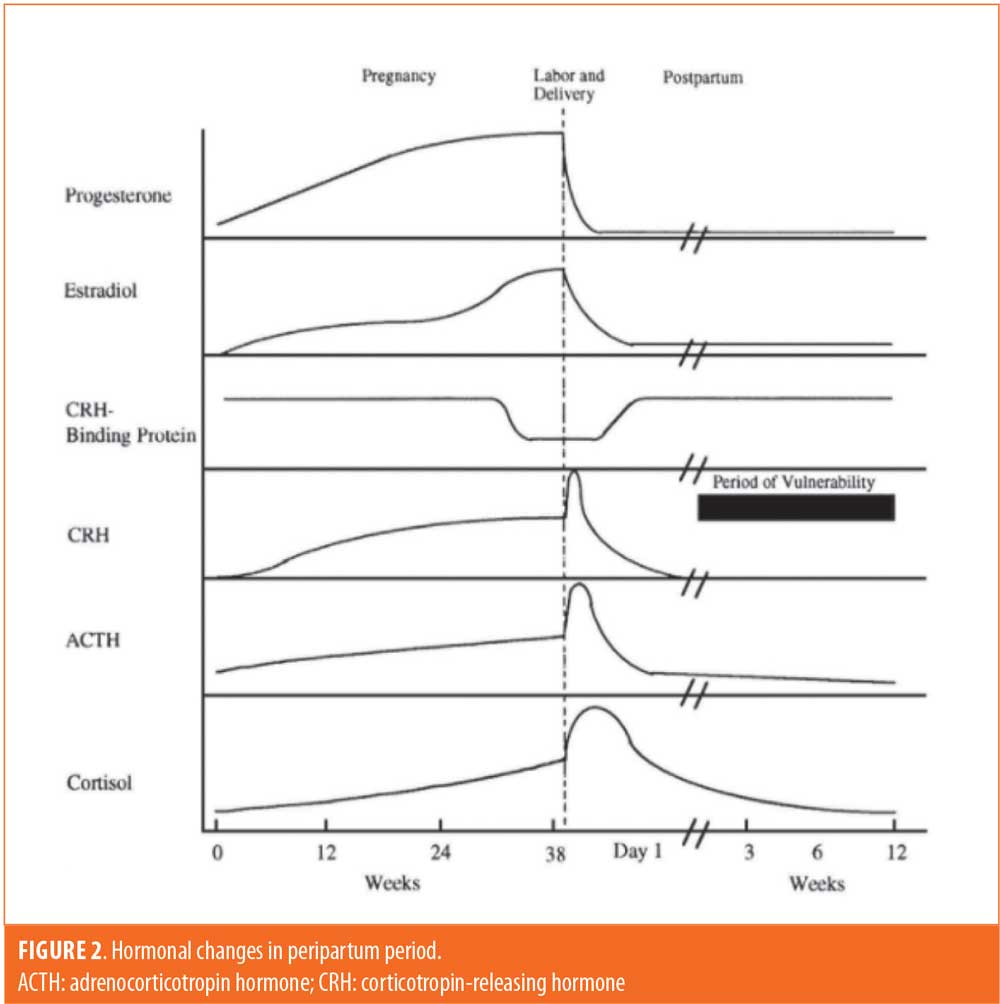
The peripartum period brings about many endocrine alterations, making it important to consider gonadal hormones as well. The plasma concentrations of most hormones during pregnancy follow the same general pattern; they continually increase until parturition, at which point there is a sharp drop (Figure 2).27 While the state of increased cortisol and DHEA might be responsible for OCD symptoms in the antepartum period before hormonal levels attenuate, the lack of estradiol might play a greater role in symptomatology in the postpartum period. One of the ways in which estradiol is thought to influence serotonergic activity is through inhibition of monoamine oxidase A (MAO-A), which promotes serotonin degradation. Following delivery, there is an abrupt decrease in estradiol, possibly causing disinhibition of MAO-A and subsequent serotonergic degeneration.28 This state of serotonergic deficiency might help explain why the early postpartum period, in particular, is a time of increased risk for onset and exacerbation of OCD. The effect of progesterone on serotonin neurotransmission is more unclear but has been shown to increase serotonergic signaling as well, improving OCD symptoms. Thus, both estradiol and progesterone are considered to have an overall neuroprotective effect via serotonin, so the significant decline of these hormones in the postpartum period can put patients at greater risk of developing or exacerbating OCD symptoms.4 While our patient developed symptoms in the third trimester, a study by Kaya et al29 found that the onset of OCD was much more common in the first and second trimesters, at which time there are comparatively lower estradiol levels than the third trimester.29 This suggests that decreased estradiol might play a role in the antepartum period as well. Research has also suggested that both estradiol and progesterone increase dopaminergic signaling and worsening OCD symptoms. This is inconsistent with the current understanding of serotonin-dopamine interactions, and evidence of this relationship is much more limited.4 Regardless, the effect of fluctuating gonadal hormones on neurotransmission more than likely helps to manipulate the course of OCD.
Oxytocin. Another hormone of interest is oxytocin, which is significantly elevated during delivery and breastfeeding in the postpartum period. In brief, oxytocin is produced in neurons of the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus. Oxytocin neurons from both regions project to the posterior pituitary. In addition to this release, the PVN also sends oxytocin projections throughout the brain, including the limbic system.30
Pregnancy and the immediate postpartum period is a time of increased risk for the onset or exacerbation of OCD. Importantly, central oxytocin concentrations peak during the third trimester and puerperium and remain elevated in breastfeeding women. Recent reports indicate that a significant number of women have the onset or exacerbation of OCD during pregnancy or the postpartum period. It might be that a subgroup of women, such as the patient in our case report, is vulnerable to the induction or exacerbation of OCD upon exposure to elevated levels of oxytocin during pregnancy and/or the puerperium.31 Thus, it has been suggested that the pathophysiology of OCD might include an extreme presentation of oxytocin-related innate mechanisms.32
Oxytocin in cerebrospinal fluid. Some,4,33 but not all,34 studies have found elevated cerebrospinal fluid (CSF) oxytocin levels in patients with OCD, but a direct correlation between CSF oxytocin levels and OCD severity has not been established.4,33 In a 1992 study, CSF-oxytocin of 43 children/adolescents correlated positively with depression, but not with OCD symptom severity. In a study two years later, CSF-oxytocin was elevated compared to controls in 22 adult subjects with OCD and without history of tic disorders, and in these patients CSF-oxytocin was also positively related to OCD severity, as measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS).35 This finding supports an oxytocinergic OCD hypothesis, but a study in 1999 found no CSF-oxytocin difference between OCD and control cases and no relation to Y-BOCS ratings; however, only 14 patients with OCD were included.36 More recently, an animal model suggested supporting that oxytocin gives rise to grooming compulsions through links between the PVN and the central nucleus of amygdala.37
Oxytocin levels in patients treated with SSRIs. Only three previous studies have investigated oxytocin changes during SSRI treatment in humans.38–40 In the first of these, 16 children/adolescents with OCD were studied. Clomipramine treatment, ranging between 8.5 and 34 months, caused an overall increase of CSF oxytocin by 11 percent. Intriguingly, however, the individual clinical response was negatively correlated to CSF oxytocin changes (i.e., those with the least increase of CSF-oxytocin were the most improved). Since this study only included treatment responders and no placebo group, conclusion regarding the pharmacological effects of SSRIs on the oxytocin system should be considered with caution.38 In the next study, plasma oxytocin was measured in 40 patients with major depresssive disorder (MDD) before and after successful pharmacological treatment, of which 19 cases were treated with SRIs (venlafaxine or SSRI) in 19 cases. When compared to a control group, the active treatment patients had significantly lower plasma oxytocin at baseline; however, no difference between pretreatment and posttreatment oxytocin levels was found. All included patients were treatment responders, and the time span between samples was not conveyed.39 A third study reported on plasma oxytocin at baseline and after 12 weeks of SSRI treatment in 16 adult patients who were successfully treated for MDD. No difference was found.40 Consequently, placebo-treated patients were not used as control in any of these three studies, nor were responders compared to nonresponders. Two of the studies were for depression, and only one applied a fixed time interval for the second oxytocin sample. Most recently, a study concluded that SSRIs have highly variable effects on plasma oxytocin between individuals. The authors stated that the associations between baseline oxytocin and OCD severity and between oxytocin changes and treatment response support the theories that oxytocin is involved in OCD pathophysiology and that the anti-obsessive effects of SSRIs might be partly exerted through oxytocinergic mechanisms.37
Oxytocin’s relationship with estradiol: animal models and humans. Increased oxytocin affects cognitive, grooming, affiliative, sexual, and reproductive behaviors in animals.31 There are many different theories as to how oxytocin levels might correlate with specific OCD phenotypes. For instance, oxytocin has been reported to attenuate memory consolidation and retrieval. It might be that pathological doubting associated with the need to repeatedly carry out checking compulsions is a clinical manifestation of the cognitive effects of a dysregulated oxytocin system in some forms of OCD. Additionally, violent and horrific thoughts, images, and impulses are also common types of obsessions. Central oxytocin injections in mother hamsters was associated with increased maternal aggression toward intruders. A dose response increase in aggression has also been reported in dominant male squirrel monkeys given intracerebroventricular oxytocin. Blockade of oxytocin receptors reduced aggressive behavior in these same monkeys.31
Oxytocin administered intracerebroventricularly could induce the onset of maternal behavior within one hour in nulliparous, oophorectomized female rats primed with estradiol benzoate.31 Thus, given the putative role of oxytocin in “affiliative/mothering” behavior, with regard to our patient, the pathological doubting she had about being a competent mother with thoughts of possibly harming her baby could be attributed to one manifestation of a pathologically dysregulated oxytocin syndrome.31
Finally, in the nonpathological state, estrogens can act in a synergistic manner with oxytocin, not only by enhancing its anxiolytic effects, but also by increasing oxytocin receptor levels in the mouse brain.30 Interestingly, approximately 85 percent of oxytocin neurons in the rat PVN co-express estrogen receptor (ER)-beta. ER-beta activation increases oxytocin synthesis and reduces anxiety and neuroendocrine responses in animals. In humans, a single dose of estradiol was sufficient to increase plasma oxytocin levels.30 Notably, during peripartum elevated levels of estrogen are present. Thus, this hyperestrogenism could result in a dysregulated oxytocin system.
Conclusion
Increased oxytocin activity has been linked to anxiolytic effects (e.g., in the amygdala or the median raphe nucleus).37 OCD onset is common during the peripartum period, with ranges up to four times the expected rate in the nonperipartum population.41 The acute onset of OCD in the peripartum period might be attributed to the dramatic rise and fall in steroid hormone levels, resulting in serotonergic dysfunction, which is compounded by a predisposition to psychiatric illness. Some research suggests that the rapid increase in oxytocin seen during pregnancy, particularly at nine months, and during the puerperium might trigger the exacerbation or onset of OCD; however, the exact pathophysiology is unclear, and future research could elucidate this relationship.42 Clinical trials that investigated the therapeutic use of oxytocin in OCD found no effect of this molecule over the frequency of repetitive symptoms (neither improvement nor worsening). In contrast, a study reported that levels of oxytocin in ventricular CSF are higher in patients with OCD than in healthy controls and identified a positive correlation between higher CSF levels of oxytocin and higher frequency of repetitive behaviors.34 However, a subsequent study was unable to replicate those findings.43
Ultimately, it is unknown whether oxytocin is critically involved in OCD pathogenesis, and, if so, whether the oxytocinergic activity should be increased, decreased, or changed in other ways to improve the clinical state. In various experiments, elevated oxytocin has been linked to relaxed, affiliative situations, implying anxiolytic and antidepressant effects, but, in other experiments, oxytocin is increased in relation to stress. These disparate findings indicate that different segments of the central oxytocin system might act in different direction.35 Future research directed toward the roles of steroid hormones and oxytocin in the pathogenesis of this “subtype” of OCD (Table 1) could result in expanding the treatment armamentarium for permpartum OCD.
References
- Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol Psychiatry. 2010;15(1):53–63.
- Guglielmi V, Vulink NC, Denys, D, et al. Obsessive-compulsive disorder and female reproductive cycle events: results from the OCD and reproduction collaborative study. Depress Anxiety. 2014;31(12):979–987.
- Laban J, Menchon JM, Alonso P, et al. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry. 2005;66(4):428–435.
- Karpinski M, Mattina GF, Steiner M. Effect of gonadal hormones on neurotransmitters implicated in the pathophysiology of obsessive-compulsive disorder: a critical review. Neuroendocrinology. 2017;105:1–16.
- Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503–509.
- House SJ, Tripathi SP, Knight BT, et al. Obsessive-compulsive disorder in pregnancy and the postpartum period: course of illness and obstetrical outcome. Arch Womens Ment Health. 2015;19(1):3–10.
- Abramowitz JS, Schwartz SA, Moore KM, et al. Obsessive-compulsive symptoms in pregnan-cy and the puerperium. J Anxiety Disord. 2003;17(4): 461–478.
- Lord C, Rieder A, Hall GB, et al. Perinatal obsessive-compulsive Scale (POCS): development and validation. J Anxiety Disord. 2011;25(8):1079–1084.
- Uguz F, Gezginc K, Zeytinci IE, et al. Course of obsessive-compulsive disorder during early postpartum period: a prospective analysis of 16 cases. Compr Psychiatry. 2007;48(6):558–561.
- Zoloft (sertraline hydrochloride) [package insert]. New York, NY: Roerig; 2016
- Celexa (citalopram hydrobromide) [package insert]. Irvine, CA: Allergan USA Inc; 2016
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown obsessive compulsive scale. Arch Gen Psychiatry. 1989;46(11):1006–1011.
- Koo MS, Kim EJ, Roh D, et al. Role of dopamine in the pathophysiology and treatment of ob-sessive-compulsive disorder. Expert Rev Neurother. 2010;10(2):275–290.
- Goddard AW, Shekhar A, Whiteman AF, et al. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov Today. 2008;13(7–8):325–332.
- Ahmari SE. Using mice to model obsessive compulsive disorder: from genes to circuits. Neuroscience. 2016;321:121–137.
- Wu K, Hanna GL, Rosenberg DR, et al. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav. 2012;100(4):726–735.
- Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98(2):106–115.
- Issa G, Wilson C, Terry AV Jr, et al. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010;39(3):327–333.
- Suliman S, Hemmings SMJ, Seedat S. Brain-derived neurotrophic factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. 2013;7:55. eCollection 2013.
- Oliveira-Maia AJ, Castro-Rodrigues P. Brain-derived neurotrophic factor: a biomarker for obsessive-compulsive disorder? Front Neurosci. 2015;9.
- Hemmings SMJ, Kinnear CJ, Van Der Merwe L, et al. Investigating the role of the brain-derived neurotrophic factor (BDNF) val66met variant in obsessive-compulsive disorder (OCD). World J Biol Psychiatry. 2008;9(2):126–134.
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2007;33(1):73–83.
- Zajda ME, Krzascik P, Hill M, et al. Psychomotor and rewarding properties of the neurosteroids dehydroepiandrosterone sulphate and androsterone: effects on monoamine and steroid metabolism. Acta Neurobiol Exp (Wars). 2012;72(1):65–79.
- Pérez-Neri I, Méndez-Sánchez I, Montes S, et al. Acute dehydroepiandrosterone treatment exerts different effects on dopamine and serotonin turnover ratios in the rat corpus striatum and nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1584–1589.
- Muneoka K, Iwata M, Shirayama Y. Altered levels of synapsin I, dopamine transporter, dynorphin A, and neuropeptide Y in the nucleus accumbens and striatum at post-puberty in rats treated neonatally with pregnenolone or DHEA. Int J Dev Neurosci. 2009;27(6):575–581.
- Hoyt LT, Falconi AM. Puberty and perimenopause: reproductive transitions and their implications for women’s health. Soc Sci Med. 2015;132:103–112.
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240.
- Sekiyama T, Nakatani Y, Yu X, et al. Increased blood serotonin concentrations are correlated with reduced tension/anxiety in healthy postpartum lactating women. Psychiatry Res. 2013;209(3):560–565.
- Kaya V, Uguz F, Sahingoz M, et al. Pregnancy-onset obsessive-compulsive disorder: clinical features, comorbidity, and associated factors. Klinik Psikofarmakol Bulteni. 2016;25(3):248–258.
- Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and estrogen receptor beta in the brain: an overview. Front Endocrinol (Lausanne). 2015;6:160.
- McDougle CJ, Barr LC, Goodman WK, et al. Possible role of neuropeptides in obsessive compulsive disorder. Psychoneuroendocrinology. 1999;24(1):1–24.
- Leckman JF, Goodman WK, North WG, et al. The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology. 1994;19:723–749.
- Marazziti D, Baroni S, Giannaccini G, et al. Plasma oxytocin levels in untreated adult obsessive-compulsive disorder patients. Neuropsychobiology. 2015;72:74–80.
- Leckman JF, Goodman WK, North WG, et al. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder: comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry. 1994;51(10):782–792.
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011.
- Altemus M, Jacobson KR, Debellis M, et al. Normal CSF oxytocin and NPY levels in OCD. Biol Psychiatry. 1999;45(7):931–933.
- Humble MB, Uvnäs-Moberg K, Engström I, et al. Plasma oxytocin changes and anti-obsessive response during serotonin reuptake inhibitor treatment: a placebo controlled study. BMC Psychiatry. 2013;13:344.
- Altemus M, Swedo SE, Leonard HL, et al. Changes in cerebrospinal fluid neurochemistry during treatment of obsessive-compulsive disorder with clomipramine. Arch Gen Psychiatry. 1994;13:794–803.
- Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009;13:249–252.
- Keating C, Dawood T, Barton DA, et al. Effects of selective serotonin reuptake inhibitor treatment on plasma oxytocin and cortisol in major depressive disorder. BMC Psychiatry. 2013;13:124.
- Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health (Larchmnt). 2015;24(10):825–830.
- Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110. Epub 2004 Jan 8.
- Cappi C, Diniz JB, Requena GL, et al. Epigenetic evidence for involvement of the oxytocin receptor gene in obsessive-compulsive disorder. BMC Neuroscience. 2016;17:79.





