 by Larry Alphs, MD, PhD; Dong-Jing Fu, MD, PhD; David Williamson, PhD; Carol Jamieson, BSc, FIBMS; John Greist, MD; Magdalena Harrington, PhD; Jean-Pierre Lindenmayer, MD; Cheryl McCullumsmith, MD, PhD; David V. Sheehan, MD, MBA; Richard C. Shelton, MD; Paul Wicks, PhD; and Carla M. Canuso, MD, on behalf of the SIBAT Consortium
by Larry Alphs, MD, PhD; Dong-Jing Fu, MD, PhD; David Williamson, PhD; Carol Jamieson, BSc, FIBMS; John Greist, MD; Magdalena Harrington, PhD; Jean-Pierre Lindenmayer, MD; Cheryl McCullumsmith, MD, PhD; David V. Sheehan, MD, MBA; Richard C. Shelton, MD; Paul Wicks, PhD; and Carla M. Canuso, MD, on behalf of the SIBAT Consortium
Dr. Alphs is with Denovo Biopharma in San Diego, California (he was with Janssen Scientific Affairs, LLC in Titusville, New Jersey at the time the work reported herein was performed). Drs. Fu and Canuso are with Janssen Research and Development, LLC in Titusville, New Jersey. Dr. Williamson is with the Departments of Psychiatry and Neurology at University of South Alabama College of Medicine in Mobile, Alabama and Department of Psychiatry and Health Behavior at Augusta University in Augusta, Georgia (he was with Janssen Scientific Affairs, LLC in Titusville, New Jersey at the time the work reported herein was performed). Ms. Jamieson is with Janssen Research and Development, LLC in Milpitas, California. Dr. Greist is a Professor Emeritus–Psychiatry at the University of Wisconsin School of Medicine and Public Health and is with Healthcare Technology Systems, Inc. in Madison, Wisconsin. Dr. Harrington is with Pfizer, Inc. in Cambridge, Massachusetts (she was a Psychometrician/Patient-Reported Outcomes at PatientsLikeMe in Cambridge, Massachusetts at the time the work reported herein was performed). Dr. Lindenmayer is with New York University Grossman School of Medicine, Department of Psychiatry in New York City, New York. Dr. McCullumsmith is with the University of Toledo, Department of Psychiatry in Toledo, Ohio. Dr. Sheehan is a Distinguished University Health Professor Emeritus, University of South Florida College of Medicine in Tampa, Florida. Dr. Shelton is with the University of Alabama at Birmingham School of Medicine, Department of Psychiatry in Birmingham, Alabama. Dr. Wicks is with Wicks Digital Health Ltd. in Lichfield, United Kingdom (he was with PatientsLikeMe in Cambridge, Massachusetts at the time the work reported herein was performed).
FUNDING: Development of the SIBAT was funded by Janssen Research and Development, LLC.
DISCLOSURES: LA was an employee of Janssen Scientific Affairs, LLC at the time of this work and holds Johnson & Johnson stock. He is currently an employee of Denovo Biopharma, San Diego, CA. D-JF, CJ, and CMC are employees of Janssen Research and Development, LLC or Janssen Scientific Affairs, LLC, and are stockholders of Johnson & Johnson. DW was an employee of Janssen Scientific Affairs, LLC at the time of this work and holds Johnson & Johnson stock. He has current affiliations with the Departments of Psychiatry and Neurology at University of South Alabama College of Medicine in Mobile, Alabama and Department of Psychiatry and Health Behavior at Augusta University in Augusta, Georgia. JHG is CEO of Healthcare Technology Systems, which receives royalties for use of the electronic Columbia-Suicide Severity Rating Scale (eC-SSRS). MH was an employee of PatientsLikeMe at the time of this work and is currently employed by Pfizer, Inc. J-PL has received research funding from Roche, Takeda, Neurocrine, Avanir, Jazz, and Lundbeck. CM has been a paid consultant to Janssen and AssureRx, is on the scientific board of Clarigent Health, and is the CEO of WeftHealth LLC. DVS is the author and copyright holder of the Sheehan-Suicidality Tracking Scale Clinically Meaningful Change Measure (S-STS CMCM) mentioned in this manuscript, and he was a paid consultant to Janssen and has received royalties from Janssen for the use of his other scales and structured diagnostic interviews in their research studies. RCS has received grants from Acadia Pharmaceuticals; Alkermes, Inc.; Allergan, PLC; Assurex, Inc.; Avanir Pharmaceuticals, Inc.; Cerecor, Inc.; Intracellular Therapies; Janssen Pharmaceuticals; LivaNova, PLC; Navitor Pharmaceuticals; NeuroRx Inc.; Novartis Pharmaceuticals; Otsuka America; and Takeda Pharmaceuticals and has been a consultant for Acadia Pharmaceuticals; Allergan, PLC; Alfasigma USA, Inc.; Myriad Neuroscience; Novartis International AG; Evecxia Therapeutics; Seelos Therapeutics; Sunovion Pharmaceuticals, Inc.; NeuroRx, Inc.; and Seelos Therapeutics. PW was an employee of PatientsLikeMe at the time of this work and is employed by Wicks Digital Health Ltd., which has received funding from Ada Health, AstraZeneca, Baillie Gifford, Bold Health, Camoni, Compass Pathways, Coronna, EIT, Happify, Health Unlocked, Inbeeo, Kheiron Medical, Sano Genetics, Self Care Catalysts, The Learning Corp, The Welcome Trust, VeraSci, and Woebot.
Innov Clin Neurosci. 2022;19(4–6):36–47.
Abstract
Objective: Most assessments of suicidal ideation and behavior (SIB) are limited by reliance on a single assessor, typically a clinician or patient, with scant detail on patient-related drivers of SIB and inability to detect rapid change in SIB. Furthermore, many techniques do not include a semistructured interview, increasing rater variability. The Suicide Ideation and Behavior Assessment Tool (SIBAT) addresses these limitations.
Design: More than 30 experts in scale development, statistics, and clinical management of suicidal patients collaborated over a greater than four-year period to develop the SIBAT. Input for content and validity was received from patients, clinicians, and regulatory authorities in the United States (US) and Europe. Psychometric properties of the SIBAT were evaluated in validation studies.
Results: The SIBAT is organized into eight independent patient- or clinician-rated modules with branching logic and scoring algorithms, which necessitates computerization. Patient-reported information is first captured in Modules 1 to 5. Thereafter, an experienced clinician reviews the patient’s report, conducts a semistructured interview (Module 6), and assesses the patient’s suicide risk (Module 7) and optimal antisuicide management (Module 8). Input from cognitive interviews of diverse adult, adolescent, and clinician participants was incorporated into the final version of the SIBAT. Psychometric testing demonstrated good inter-rater reliability (intraclass coefficient range: 0.68–0.82), intra-rater reliability (weighted-kappa range: 0.64–0.76), and concurrent validity with other instruments for assessing SIB.
Conclusion: Patient- and clinician-based assessments and the psychometric studies summarized in this report support the validity and reliability of the SIBAT for capturing critical information related to assessment of SIB in adolescents and adults at risk for suicide.
Keywords: SIBAT, suicide ideation, suicide behavior, suicide assessment, psychometric validation, suicidality, suicide attempt, death by suicide
Despite an increasing incidence of suicide deaths (>30% from 1999 to 2017),1 suicide ideation and behavior (SIB) remains under-recognized and undertreated. While causes of this increase are uncertain, identification of SIB offers hope for better interventions, treatments, and prevention of death by suicide. In 2020, the United States (US) Centers for Disease Control and Prevention (CDC) estimated that suicide was the 10th leading cause of death in the US. Among adolescents and young adults aged 10 to 34 years in the US, suicide was second only to unintentional injury as a cause of death.2,3
In 2018, 10.7 million adults in the US reported seriously thinking about death by suicide in the prior year; 3.3 million persons had made suicide plans. Our inability to prevent suicide in those known to be at risk is demonstrated by the finding that many of those who die by suicide have had recent contact with the healthcare system.4 Also, too many individuals who survive their first attempt subsequently die by suicide.5–7
An accurate assessment of SIB and related risk factors is essential for clinicians and researchers, and there is consensus that more reliable and valid suicide assessments are needed.8 In clinical practice, healthcare providers who assess suicide risk and make critical management decisions are often uncomfortable in doing these assessments and tend to bias their questioning toward confirming the absence of suicidality.9,10 Well-designed assessment tools can be valuable for identifying, tracking, and documenting SIB and related risk factors. Such tools can also be used to track responses to investigational medications and other treatments targeting suicidality (e.g., ketamine, esketamine).11,12
Many tools used to assess SIB in clinical trials, such as the International Suicide Prevention Trial (InterSePT) Scale for Suicidal Thinking (ISST),13 ISST-Plus,13,14 and Columbia-Suicide Severity Rating Scale (C-SSRS),15 have several limitations for general clinical use, as follows: they omit certain risk and/or protective factors that are important for clinician-based suicide assessment; they fail to include the perspectives of both patients and clinicians; they conflate measures of suicide risk that are likely to change (i.e., current suicidal ideation) with those that are not (i.e., past suicidal behavior); and they fail to provide either a clinician- or patient-based suicide assessment for SIB management. In addition, data are extremely limited in supporting the utility of these measures in detecting rapid changes in SIB,13,15,16 despite the fact that most suicide attempts are impulsive.17 For these reasons, these SIB scales are not ideal as research tools for assessing the efficacy or safety of potential treatments for suicidality.
To address these shortcomings, a consortium of more than 30 experts in clinical management of suicidal patients, clinical trials, statistics, and scale development developed the Suicide Ideation and Behavior Assessment Tool (SIBAT) (SIBAT Consortium members are listed in the Acknowledgments). Additional input was provided by US and European regulatory authorities.
The SIBAT is a computerized, modular instrument that characterizes SIB as reported by patients and clinician raters. After reviewing the patient-reported information and conducting a semistructured interview with the patient to further clarify information provided by the patient, clinicians provide their impressions of severity of suicidality, imminent suicide risk, and long-term suicide risk, as well as their optimal treatment plans. An algorithm has been developed that can map output from the SIBAT to the Columbia Classification Algorithm of Suicide Assessment (C-CASA)18 to meet US regulatory requirements.
Steps in Establishing the SIBAT Content Validity and Construct Validity
In 2013, the SIBAT Consortium developed an initial draft version of the SIBAT. Two major subdivisions of the SIBAT were created: an extensive patient-reported section (PRO) and a shorter clinician-rated section (ClinRO), which captures a clinician’s outcome assessment of the information provided by the patient. The SIBAT underwent multiple reviews and revisions based on feedback from patients with a history of SIB, clinical experts, and regulators. As final steps in its development, the SIBAT underwent review by adults and adolescents with a history of SIB and clinicians not previously familiar with the instrument to evaluate the content of the instructions, items, and response scales of the SIBAT.
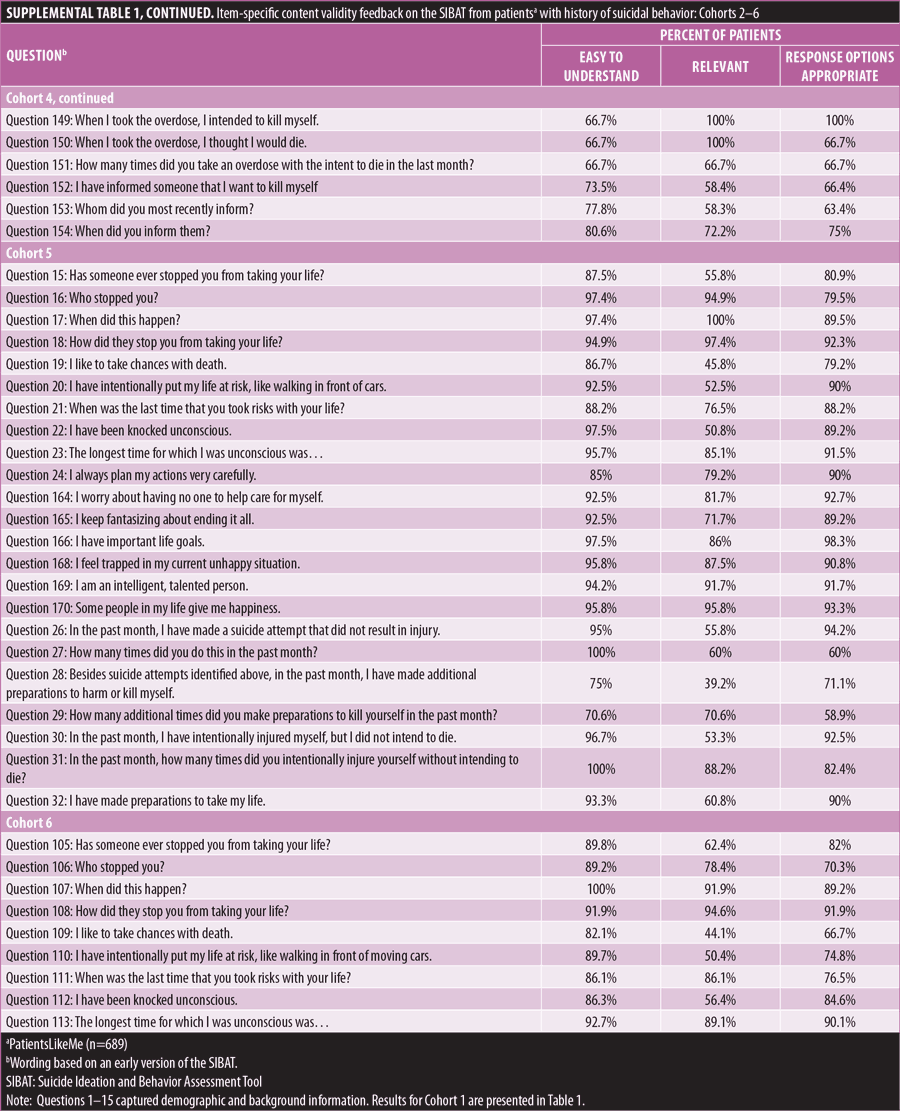
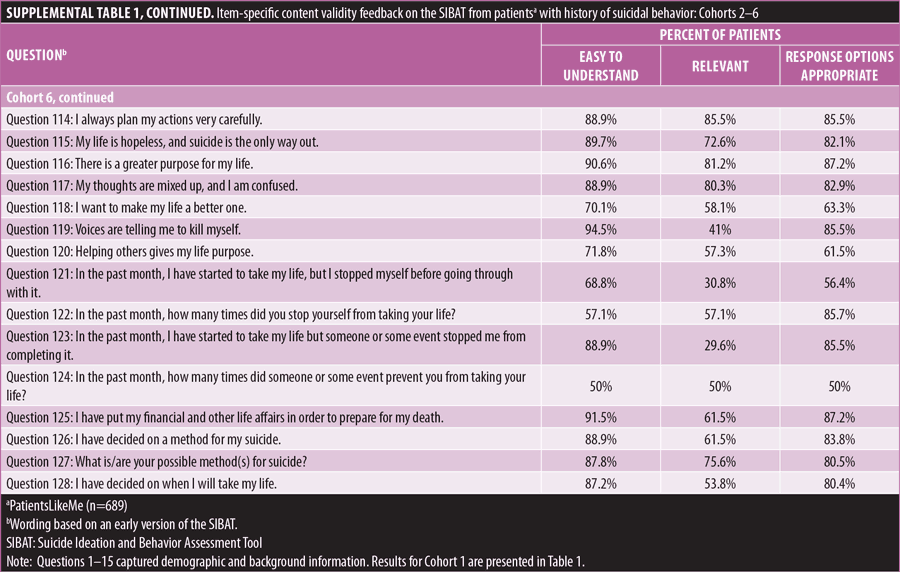
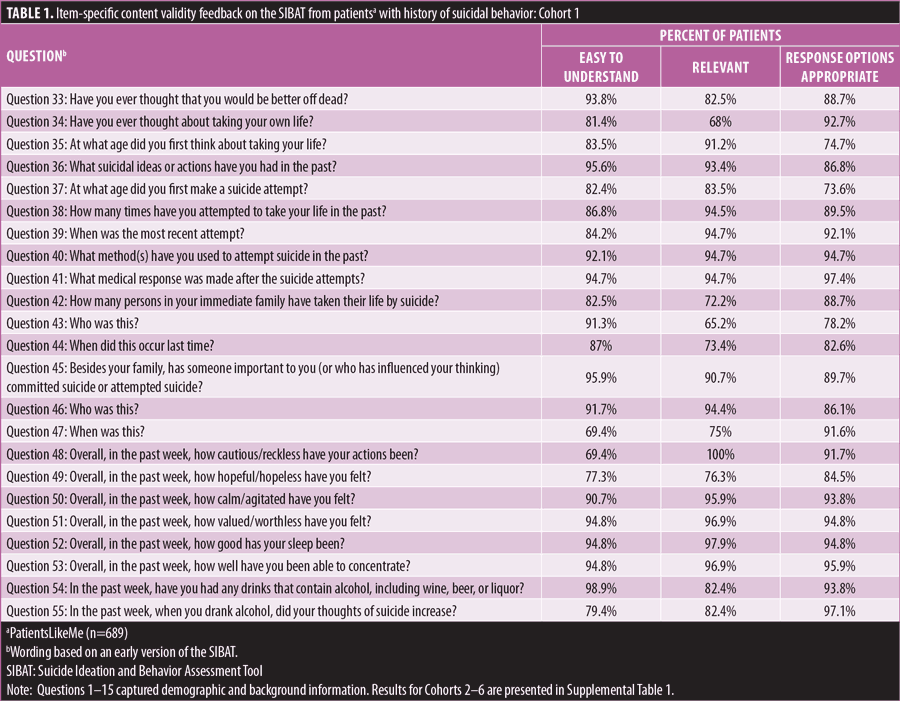
Patient-related content validity. Online cognitive interview study of patients with a history of suicidal behavior and/or ideation. A total of 689 adult members of an online patient community, PatientsLikeMe (PLM),19 with a history of SIB were identified by their affirmative response to the screening question, “Did you have any thoughts about harming yourself or committing suicide in the past 12 months?” This study, using qualitative and quantitative methodology, was conducted on the Open Research Exchange (ORE)20 software platform. Eligible subjects were asked to participate in the study through an invitation sent via private message to their PLM member account. The mean age was 48.3 years (range 18–81 years); 79 percent were female and 90 percent were White. A high proportion reported depression (50%) and/or anxiety disorders (39%).
Due to the large number of items in the SIBAT, the PRO section of the draft instrument was divided into six cohorts consisting of 30 to 33 items each. Items were balanced across different modules of the SIBAT, with some key items included in all cohorts. Study participants were randomly assigned to one of the six cohorts. Each SIBAT question was displayed on the top half of the computer screen, and the four survey feedback questions were presented at the bottom half of the screen.
The majority of participants reported that all of the items were easy to understand (Table 1 [Cohort 1], Supplemental Table 1 [Cohorts 2–6]). Items that were identified as the easiest to understand included how many times have you attempted to take your life in the past (87% rated the question as easy to understand), when was the most recent attempt [to take your life] (84%), and when did the most recent intentional cut or self-injury take place (100%). The items demonstrated a wide range of perceived relevance (21.1–100%). The majority of participants (≥60%) indicated that 107 of the 155 items presented to them were personally highly relevant. Patients’ comments regarding the appropriateness of available response options produced a wide range of positive feedback (42.9–100%). The majority of participants (≥60%) indicated the response options were appropriate for 147 out of 155 items in the version presented to them. Following this review, experts in translation to multiple languages (i.e., Mandarin Chinese, Japanese, Ukrainian, French, German, Spanish, and Swedish) confirmed the translatability and cross-cultural relevance of the language used in the SIBAT.
In summary, the data collected from this study supported the broad ease of understanding, relevance, and appropriateness of the SIBAT PRO module content for individuals with a history of SIB and potential risk factors for SIB. These data and additional changes suggested by SIBAT Consortium members were used to generate a revised version of the SIBAT that underwent psychometric evaluation in studies described below.
Cognitive interview study of adolescents with current history of suicidal behavior and/or ideation. Evaluations of ease of understanding, age-appropriate contextual validity, and burden imposed by the SIBAT were completed in face-to-face cognitive interviews with 15 adolescents (mean age 16.5 years [range 12–19], 9 [60%] female, 10 [67% White]) who had a recent history of suicidal ideation and/or behavior (14 [93%] in the last month) and whose current level of suicidality ranged from none to requiring constant supervision to prevent a suicide attempt (Janssen Protocol 54135419SUI0002).
Eligible adolescent subjects provided responses to the PRO modules of the SIBAT, which were administered and recorded on an electronic tablet. After each module was completed, the subjects completed a semistructured cognitive interview of the content and wording of the instrument conducted by trained clinician raters. The clinician rater then completed the ClinRO modules of the SIBAT, including the clinician interview module, which was videotaped for a subsequent cognitive interview study with clinical experts (described in the next section; Janssen Protocol 54135419SUI0003). These clinician ratings were recorded on the same electronic tablet. The SIBAT version included in this study was rated at a 7.2 grade reading level, as assessed by the Microsoft Word implementation of the Kincaid algorithm,21 and was judged suitable for a target population in this age group.
In general, most of these adolescent subjects found the PRO modules (Modules 1–5) in the instrument to be “very easy to understand” (73–100% of subjects), “not at all offensive or insensitive” (87–100%), and “not at all burdensome” (80–93%). An exception was an exploratory module consisting of a short version of the Suicide Implicit Association Test (S-IAT).22 As a consequence, this module was removed from the final instrument. Wording revisions suggested by these cognitive interviews (e.g., change “unconscious by a blow to the head” to “unconscious by a hit on the head;” add “straight” as an alternative word for “heterosexual;” change the wording from “competent” to “skilled;” change “reduced” to “lowered”) reduced the Flesch-Kincaid reading level of the SIBAT from grade level 7.2 to 5.8. Many subjects (47%) preferred answering these questions with a computer; 13 percent preferred face-to-face interactions, and 33 percent had no preference.
Clinician-related content validity. Cognitive interview study with clinical experts. Sixteen clinicians (7 psychiatrists, 4 clinical raters/social workers, 3 clinical psychologists, 2 other profession) who had worked with suicidal patients for a median time of 16.5 years evaluated the SIBAT’s clinical comprehensiveness, meaningfulness, and scoring guidelines. To be eligible for study participation, the clinicians must have been trained and experienced in working with persons who exhibit suicidal and other forms of self-injurious behavior and not involved in the development of the SIBAT.
After being trained on the use of the SIBAT, the clinician raters reviewed the SIBAT output of patient-rated modules of the SIBAT (Modules 1–5) on the tablet of three previously interviewed adolescent subjects (from study summarized above) and the video recordings of the associated semistructured interview. Clinicians were asked about the value of the overall instrument, the SIBAT training materials, and how they would use information from each of the patient-rated modules to construct their final ratings.
Regarding the SIBAT training materials, 10 clinicians (62%) rated the training materials as extremely or quite helpful. They suggested that guidance on how to rate the clinical global impressions (i.e., Clinical Global Impression of Severity of Suicidality-revised [CGI-SS-r], Clinical Global Impression of Imminent Suicide Risk [CGI-SR-I], and Clinical Global Impression of Long-Term Suicide Risk [CGI-SR-LT]) be added to the SIBAT training materials. They also requested that a video example on how to use SIBAT be provided and that this include some case examples or vignettes.
In general, the clinician reviewers reported that the PRO modules provided information that was highly informative for their assessment of suicide and its management. The information collected was generally regarded as complete and “quite useful” or “extremely useful” for overall suicide risk assessment.
Regarding the SIBAT semistructured interview, 11 (69%) clinician raters described it as either “quite useful” or “extremely useful.” A key finding was the need to expand the semistructured interview, particularly providing additional information on social support.
Taken together, input from these patients and clinicians was incorporated into a final version of the SIBAT, for which full psychometric testing results (i.e., inter-rater and intra-rater reliability, internal consistency, convergent and discriminant validity, and factor analysis) have been reported and summarized below.23
Psychometric testing of the SIBAT. A cross-sectional study was conducted to evaluate the psychometric properties of the SIBAT and to validate its use.23 Inter- and intra-rater reliability were assessed for clinician-rated outcomes, including the CGI-SS-r. Concurrent validity of the SIBAT was evaluated against independent ratings of the C-SSRS, the Sheehan-Suicidality Tracking Scale Clinically Meaningful Change Measure (S-STS CMCM),24,25 and patient-reported modules, with the Patient-reported Outcomes Measurement Information System (PROMIS) depression scale.26,27
The full SIBAT study sample included 130 participants (12–81 years old; 75 [58%] female; 91 [70%] White), with suicidal severity ranging from none to severe, who were identified at psychiatric care settings in the US. Of the 130 participants, 52 consented for multiple interviews, 25 of whom were chosen for rater reliability testing.
The SIBAT demonstrated good intra-rater reliability for the clinician-rated outcomes (i.e., CGI-SS-r, CGI-SR-I, CGI-SR-LT, Frequency of Suicidal Thinking [FoST], and Clinical Judgment of Optimal Suicide Management; weighted-kappa range: 0.64–0.76; CGI-SS-r: 0.75) and good inter-rater reliability (intraclass coefficient range: 0.68–0.82; CGI-SS-r: 0.81). Correlations were strong between SIBAT Module 5 ratings and PROMIS depression scores (Spearman correlations, r=0.64–0.74) and moderate between SIBAT Modules 2, 3, and 5 ratings and S-STS CMCM patient-rated and clinician-rated items (r=0.29-0.72).23
Mapping to C-CASA. One of the aims of the SIBAT is to assign C-CASA categories for safety assessments in clinical trials of central nervous system (CNS) drugs. In the study by Alphs et al,23 three C-CASA versions were evaluated, including the C-CASA 2010, C-CASA 2012, and C-CASA 2012 Plus. The C-CASA categories were scored based on the SIBAT, S-STS CMCM, and C-SSRS, and concordance between these C-CASA scores was evaluated. Agreement (weighted kappa) was moderate between SIBAT C-CASA mappings and corresponding mappings from S-STS CMCM (0.54) and C-SSRS (0.56).23
Taken together, the SIBAT provided a valid assessment of SIB, with reliable ratings, as well as adequate C-CASA categorization.
Description of the SIBAT
The SIBAT was developed to capture patients’ and clinicians’ assessment of imminent and long-term suicide risk, as well as its management based on computer-facilitated patient information. The SIBAT aims for low patient burden, while being comprehensive and efficient in the systematic collection of key information necessary to assess suicide risk. The computerized format provides consistent documentation of information across a broad range of patient types.
The final version of the SIBAT is organized into eight independent modules using branching logic that allows skipping of questions not relevant to individual patients. The eight modules have two major subdivisions: patient-reported (Modules 1–5) and clinician-rated (Modules 6–8) (Figure 1). After a patient completes the modules, their clinician reviews the patient’s report, conducts a semistructured interview, and records observations made during that interview. On the basis of all the information gathered, the clinician rates the patient’s severity of suicidality and perceived suicide risk and determines an optimal suicide management plan.
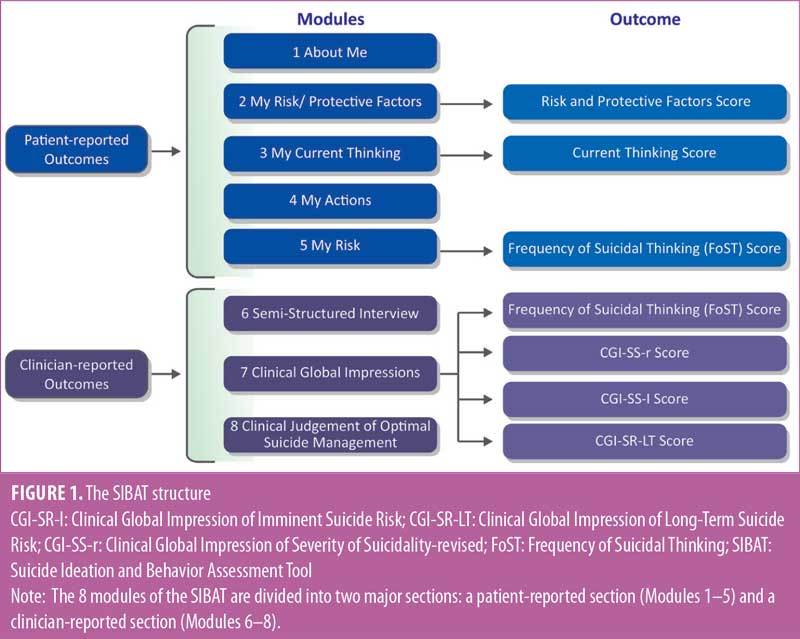
The SIBAT’s patient-reported modules provide a broad, standardized background of information that can be efficiently reviewed by clinician raters as a basis for making clinical judgments regarding imminent and long-term suicide risk. The modules are constructed such that responses less susceptible to change (e.g., demographics) are segregated from responses more likely to fluctuate with changes in the patient’s clinical state (e.g., current SIB). In clinical and research practice, the modules can be selected to meet specific assessment needs. Thus, at an initial visit, all modules would usually be completed. However, at subsequent visits, information from modules capturing more stable information need not be repeated (e.g., Module 1). Computerization makes this prior information readily available to the clinician. Each of the modules is described below.
Introductory question at each scheduled visit. There is one question completed by the clinician at the beginning of each scheduled visit to determine if the patient is present for the visit, and if not, the reason for the missed visit.
Patient-rated section. Module 1: About Me. This module (29 items) captures demographic information and lifetime risk factors for suicide, including the patient’s personal and family history of suicidal behavior. Patient-rated risk factors unlikely to change over short intervals (e.g., “My highest level of education is….”) are included in this module. In contrast, patient-rated factors more likely to change over shorter intervals are included in Modules 2 and 3. Because some information might change over time, it is recommended that this module be updated annually when the patient is receiving long-term follow up.
Module 2: My Risk/Protective Factors. This module (25 items) captures suicide risk and protective factors that the patient has experienced during the previous week. Most responses included in this module require the patient to estimate frequency as “never,” “rarely,” “sometimes,” “often,” “most of the time,” or “all the time.” Questions are anchored for a time frame of “Over the past seven days…” and include items such as “I have felt hopeful” and “I have felt agitated.” This module should be completed at most visits, depending on the patient’s visit frequency.
Module 3: My Current Thinking. This module (48 items) captures information regarding the patient’s in-the-moment severity of suicidal ideation. Responses included in this module require responses that range from “strongly disagree” to “strongly agree” and items such as “I am glad to be alive,” and “I feel worthless.” This module should be completed at every visit.
Module 4: My Actions. This module (14 items) captures information related to the patient’s recent history (“Since I last answered these questions…”) of self-injurious/suicidal ideation or behavior and related preparations, including methods, plans, and/or intent. If patients endorse an item, additional branching questions are asked for some questions. For example, if a patient answers “Yes” to the question “Since I last answered these questions, I tried to end my life,” they are asked subsequent branching questions, which include, “How many times did you try to end your life since you last answered these questions? When did you most recently try to end your life? What was your method?”
This module should be completed at every visit. Modules 1 and 4 are repetitious by design and, therefore, should not be completed at the same visit. Module 1 (About me) is normally completed at the first visit and at least every 12 months thereafter. Module 4 (My actions) is completed at all other visits.
Module 5: My Risk. This module (5 items)captures the patient’s personal assessment of their desire to die and their self-perceived sense of suicide risk. Patients must respond to five questions, including, “Which of the following ratings best describes your intent to end your life in the past seven days?” using a five-point Likert scale (e.g., 0 [I have not wanted to end my life] to 4 [I wanted to end my life all of the time]). This module should be updated at every visit.
Clinician-rated section. Clinician raters of the SIBAT should be clinically trained and experienced in providing care to persons who exhibit suicidal behavior. After the patient completes Modules 1 to 5 of the SIBAT, a summary of the patient’s responses (with critical responses flagged) is immediately provided to the clinician rater who, after thorough review, completes Modules 6 to 8.
Module 6: Semistructured Interview. The semistructured interview includes questions concerning the patient’s chief complaint and assessments of the patient’s mood, suicide and suicide plan status, impulsivity, psychiatric and related conditions, alcohol or drug use, mental status, and support systems. With this interview guide, the clinician gathers additional information and clarifies issues that are necessary for completing the subsequent suicidality rating and patient management modules. These include addressing areas of inconsistency within the patient report. This module should be completed by a clinician at every visit.
Module 7: Clinical Global Impressions. Module 7 captures the clinician’s overall judgment or impression of the patient’s frequency of suicidal thinking, current severity of suicidality (CGI-SS-r), imminent suicide risk (CGI-SR-I), and long-term suicide risk (CGI-SR-LT). These ratings are based on information reported by the patient, information gathered by the clinician during the semistructured interview, and other information that might be available to the clinician (e.g., family or other informants, chart review). The assessments of suicide risk are not measures of actual risk, which is unknowable, but the clinician’s impression of suicide risk to be used in making management decisions. This module should be updated at every visit.
The principal outcomes from the SIBAT are single-item clinician-reported measures yielding item level scores (FoST: 0 [never] to 5 [all the time]; CGI-SS-r: 0 [normal, not at all suicidal] to 6 [among the most extremely suicidal patients]; CGI-SR-I: 0 [no imminent risk of suicide] to 6 [extreme imminent suicide risk]; CGT-SR-LT: 0 [no suicide risk in the long-term] to 6 [extreme risk for suicide in the long-term]).
Assessment of FoST. The clinician answers the question, “Considering all of the information available to you, what is the patient’s frequency of suicidal thinking at this time?” using a rating scale from 0 (never) to 5 (all of the time).
CGI-SS-r. The clinician answers the question, “Considering your total clinical experience with suicidal patients and all information now available to you, how suicidal is this patient at this time?” using a rating scale from 0 (normal, not at all suicidal) to 6 (among the most extremely suicidal patients). A guide to rating was developed to aid clinicians for consistent scoring of this item (Supplemental Table 1).23
CGI-SR-I. The clinician answers the question, “Considering all aspects of this patient’s suicidal thinking, behavior, and related contributory/protective factors, what is your best clinical impression of this patient’s imminent risk for suicide within the next seven days?” using a rating scale from 0 (no imminent suicide risk) to 6 (extreme imminent suicide risk). A guide to rating was developed to aid clinicians for consistent scoring of this item (Supplemental Table 1).23
CGI-SR-LT. The clinician answers the question, “Considering all aspects of this patient’s suicidal thinking, behavior and related contributory/protective factors, what is your best clinical impression of this patient’s long-term risk for suicide (i.e., they will likely end their life by suicide sometime in the future)?” using a rating scale from 0 (no suicide risk in the long term) to 6 (extreme risk for suicide in the long term).
Module 8: Optimal Suicide Management. This module captures the clinician’s assessment of the level of care judged to be optimal for managing the patient, given the patient’s current severity of suicidality. The clinician answers the question, “Considering all of the information available to you, what is your assessment of the best clinical management for this patient at this time (even if the best option is not currently available to you)?” using a rating scale from 0 (no special management needed) to 11 (not ratable). This module should be updated at every visit.
Time for completion of the SIBAT. For most patients, the SIBAT Module 1 takes about 14 minutes to complete, and the rest of the electronic PRO modules each take 2 to 5 minutes to complete. The semistructured interview (Module 6) takes about 15 minutes and the clinician-rated modules (Modules 7 and 8) take less than a minute each to complete. The median time for completion of the SIBAT, when all modules are assessed, is approximately 47 minutes.23
The final version of the SIBAT has been translated into more than 20 languages and was utilized to evaluate rapid change in suicidality in the two Phase III trials of esketamine nasal spray.28,29
Discussion
The SIBAT was developed in collaboration with experts in scale development, statistics, clinical management of suicidal patients, and regulatory experts. The SIBAT includes patient-rated and clinician-rated modules to provide a detailed perspective on the many risks and protective factors related to suicide. With it, patient perspectives and clinician evaluations of information related to suicide can be clarified during the semistructured interview and tracked over time. To facilitate its use, the SIBAT is administered via an electronic device. This allows for significantly faster collection of information through branching logic that individually tailors questions to the patient and electronic documentation of results with minimal transcription errors. Electronic administration also offers the possibility for readily accessible summary reports of prior PRO and ClinRO assessments from the SIBAT, providing a comprehensive overview of current and long-term suicide risk assessment.
Development of the SIBAT involved multiple stages of revision based on patient, clinician, and regulatory input on validation requirements. This iterative approach resulted in the development of a comprehensive instrument that patients and clinicians regard as efficient and well-documented, despite its length. This allows for better tracking of suicidality over time, even if there is a change in primary clinician. In contrast to most other tools for assessing SIB, the SIBAT separates components that fluctuate quickly from those that do not. Suicidal constructs that change over intervals as short as four hours can be quantified.12,28,29 Prior work30–36 and data from patients completing cognitive reviews during the SIBAT’s validation identified many patients’ preferences for providing such sensitive information via an electronic format rather than in-person interview format.
The comprehensive properties of the SIBAT make it an ideal research instrument for SIB. Some patients and clinicians might consider comprehensiveness of the SIBAT formidable. However, most of the data captured by the SIBAT is provided by the patient without physician assistance. Features like electronic administration, branching logic, and modular structure greatly increase the SIBAT’s flexibility and efficiency and have resulted in strong endorsement by patients and clinicians. The time required for patients to complete the SIBAT will vary significantly depending on the patient’s SIB and which modules are being completed. Patients have not reported completion to be burdensome. The time required for physicians to complete the SIBAT will vary depending on the clinician and the patient. In one implementation, the total time required by the clinician was 16 minutes,23 similar to that with other suicide assessment scales.
The results of full psychometric evaluation demonstrated strong evidence supporting concurrent and known-groups validity, good intra-rater and inter-rater reliability, and adequate mapping from the SIBAT to the various C-CASA category systems. These results support use of the SIBAT as a regulatory endpoint in clinical trials. The final SIBAT was used to evaluate suicidality in the Phase III ASPIRE I and ASPIRE II trials of esketamine nasal spray.28,29 An earlier version of the SIBAT was field-tested in a Phase II proof-of-concept clinical trial of esketamine.12 Study of the SIBAT in clinical practice settings is underway and should provide greater insights into how it can be best utilized in such environments.
Limitations. The length and complexity of the SIBAT requires that it be administered electronically. This electronic approach might limit applicability in constrained resource settings. The cohorts used during development of the SIBAT were primarily women and English-speaking with an American/European perspective on the social/cultural definition and experience of SIB. This might require adaptation in other cultural settings. Sample sizes, though respectable, were relatively small. That said, the SIBAT was translated in more than 20 different languages and employed in Phase III pivotal trials including more than 500 patients with major depressive disorder and SIB. Traditional psychometric testing was used to validate the SIBAT. It is unknown how the SIBAT would hold up or be improved upon by other analytical techniques (e.g., Rasch analysis37).
Conclusion
Taken together, the SIBAT represents a comprehensively developed instrument for assessing SIB that incorporates both patient and clinician input. The SIBAT represents a valuable addition to previously developed instruments for generating clinical global ratings of severity of SIB in clinical studies.
Copyright and Access to SIBAT
SIBAT is protected by international copyright held by Janssen Scientific Affairs, LLC. It is readily available for clinical and research use but should not be used without permission. For information on obtaining permission for use of the SIBAT, contact Mapi Research Trust, official distributor of the SIBAT on behalf of Janssen Scientific Affairs, LLC on ePROVIDE™ at https://eprovide.mapi-trust.org/instruments/suicide-ideation-and-behavior-assessment-tool.
Acknowledgments
Sandra Norris, PharmD, of the Norris Communications Group LLC, supported by Janssen Research and Development, LLC, provided medical writing assistance. Ellen Baum, PhD (Janssen Global Services, LLC) provided additional editorial support and was not compensated.
We acknowledge the SIBAT Consortium members Larry Alphs, Frank Wiegand, and David Williamson (Janssen Scientific Affairs, LLC); Steve Ascher, Robin Butler, Carla M. Canuso, Judy Casey, Dong-Jing Fu, Tricia Kolesar, Erin Lee, Lian Mao, Jan Sechler, Bruce Simonson, Jaskaran Singh, Ibrahim Turkoz, and Lynn Yieh (Janssen Research and Development, LLC); Jill M. Harkavy-Friedman (American Foundation for Suicide Prevention); David Miller (Bracket); James Mundt (Center for Psychological Research); Dennis Revicki (Evidera); John Greist (University of Wisconsin School of Medicine and Public Health and Healthcare Technology Systems, Inc.); Janet B.W. Williams (MedAvante and Columbia University); Christopher Gray (Medical Outcome Systems); Jean-Pierre Lindenmayer (New York University); Magdalena Harrington, Shimon Rura, and Paul Wicks (PatientsLikeMe); Phillip Chappell and Michelle Stewart (Pfizer); Emily Hammond, Roberta May, and Richard C. Shelton (University of Alabama at Birmingham); William Coryell (University of Iowa); David V. Sheehan (University of South Florida College of Medicine); and Cheryl McCullumsmith (University of Toledo).
We also acknowledge Dennis Revicki, MD, (deceased) for his contribution to the development and validation of the SIBAT.
Author Contributions
LA and DW led the creation of the SIBAT. All authors were involved in development and testing of the SIBAT. All authors were involved in writing and/or revising the manuscript and had final responsibility for the decision to submit for publication.
References
- Hedegaard H, Curtin SC, Warner M. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330, November 2018. Reviewed 3 Oct 2018. https://www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed 28 Jul 2021.
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS). 2020. Atlanta, GA: National Center for Injury Prevention and Control. https://www.cdc.gov/injury/wisqars/index.html. Accessed 28 Dec 2020.
- Centers for Disease Control and Prevention, National Center for Health Statistics. Suicide and self-harm injury. Reviewed 6 Jan 2022. https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed 28 Jul 2021.
- Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159(6):909–916.
- Bostwick JM, Pabbati C, Geske JR, McKean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;73(11):1094–1100.
- Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189–1194.
- Jenkins GR, Hale R, Papanastassiou M, et al. Suicide rate 22 years after parasuicide: cohort study. BMJ. 2002;325(7373):1155.
- Meyer RE, Salzman C, Youngstrom EA, et al. Suicidality and risk of suicide—definition, drug safety concerns, and a necessary target for drug development: a consensus statement. J Clin Psychiatry. 2010;71(8):e1–e21.
- McCabe R, Sterno I, Priebe S, et al. How do healthcare professionals interview patients to assess suicide risk? BMC Psychiatry. 2017;17(1):122.
- Ryan EP, Oquendo MA. Suicide risk assessment and prevention: challenges and opportunities. Focus (Am Psychiatr Publ). 2020;18(2):88–99.
- Ionescu DF, Swee MB, Pavone KJ, et al. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry. 2016;77(6):e719–e725.
- Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630.
- Lindenmayer JP, Czobor P, Alphs L, et al. The InterSePT scale for suicidal thinking reliability and validity. Schizophr Res. 2003;63(1–2):161–70.
- Ayer DW, Jayathilake K, Meltzer HY. The InterSePT suicide scale for prediction of imminent suicidal behaviors. Psychiatry Res. 2008;161(1):87–96.
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277.
- Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91.
- Mccullumsmith CB, Williamson DJ, May RS, et al. Simple measures of hopelessness and impulsivity are associated with acute suicidal ideation and attempts in patients in psychiatric crisis. Innov Clin Neurosci. 2014;11(9–10):47–53.
- US Food and Drug Administration. Guidance for industry: suicide ideation and behavior: prospective assessment of occurrence in clinical trials–revision 1. Aug 2012. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm315156.htm. Accessed 28 Jul 2021.
- PatientsLikeMe. http://www.patientslikeme.com. Accessed 16 Mar 2022.
- Open Research Exchange. https://www.openresearchexchange.com. Accessed 16 Mar 2022.
- Kincaid JP, Fishburne Jr RP, Rogers RL, Chissom BS. Derivation of new readability formulas (automated readability index, fog count, and flesch reading ease formula) for Navy enlisted personnel. Research branch report 8–75.Naval Technical Training, US Naval Air Station; 1975.
- Nock MK, Banaji MR. Assessment of self-injurious thoughts using a behavioral test. Am J Psychiatry. 2007;164(5):820–823.
- Alphs L, Fu DJ, Williamson D, et al. Suicide Ideation and Behavior Assessment Tool (SIBAT): evaluation of intra- and inter-rater reliability, validity, and mapping to Columbia Classification Algorithm of Suicide Assessment. Psychiatry Res. 2020;294:113495.
- Coric V, Stock EG, Pultz J, et al. Sheehan Suicidality Tracking Scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6(1):26–31.
- Sheehan DV, Giddens JM, Sheehan IS. Status update on the Sheehan-Suicidality Tracking Scale (S-STS) 2014. Innov Clin Neurosci. 2014;11(9–10):93–140.
- Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880.
- Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283.
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191.
- Ionescu DF, Fu DJ, Qiu X, et al Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a Phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31.
- Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173–2175.
- Erdman HP, Greist JH, Gustafson DH, et al. Suicide risk prediction by computer interview: a prospective study. J Clin Psychiatry. 1987;48(12):464–467.
- Greist JH, Gustafson DH, Stauss FF, et al. A computer interview for suicide-risk prediction. Am J Psychiatry. 1973;130(12):1327–1332.
- Hesdorffer DC, French JA, Posner K, et al. Suicidal ideation and behavior screening in intractable focal epilepsy eligible for drug trials. Epilepsia. 2013;54:879–887.
- Levine S, Ancill RJ, Roberts AP. Assessment of suicide risk by computer-delivered self rating questionnaire: preliminary findings. Acta Psychiatr Scand. 1989;80:216–220.
- Trivedi MH, Wisniewski SR, Morris DW, et al. Concise Health Risk Tracking scale. A brief self-report and clinician rating of suicidal risk. J Clin Psychiatry. 2011;72(6):757–764.
- Vitiello B, Silva SG, Rohde P, et al. Suicidal events in the Treatment for Adolescents with Depression Study (TADS). J Clin Psychiatry. 2009;70(5):741–747.
- Boone WJ. Rasch analysis for instrument development: why, when, and how? CBE Life Sci Educ. 2016;15(4):rm4.