 by Zeinab Ebrahimian, MSc; Zeinab Karimi, MSc; Mohammad Javad Khoshnoud, Pharm D, PhD; Mohammad Reza Namavar, PhD; Bahram Daraei, PharmD, PhD; and Moshen Raza Haidari, MD, PhD
by Zeinab Ebrahimian, MSc; Zeinab Karimi, MSc; Mohammad Javad Khoshnoud, Pharm D, PhD; Mohammad Reza Namavar, PhD; Bahram Daraei, PharmD, PhD; and Moshen Raza Haidari, MD, PhD
Drs. Ebrahimian and Khoshnoud are with the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Shiraz University of Medical Sciences in Shiraz, IR Iran; Drs. Karimi and Daraei are with the Department of Toxicology, Faculty of Medical Sciences, Tarbiat Modares University in Tehran, IR Iran; Dr. Namavar is with the Section of Histomorphometry and Stereology, Department of Anatomical Sciences, School of Medicine, Shiraz University of Medical Sciences in Shiraz, IR Iran; and Dr. Haidari is with the Section of Neurosciences, Department of Neurology, Faculty of Medicine, Baqiyatallah University of Medical Sciences in Tehran, IR Iran.
Innov Clin Neurosci. 2017;14(1–2):40–52.
Funding: Funding was provided by the Student Training Grant (Z.E.) from the Faculty of Pharmacy, Shiraz University of Medical Sciences, Iran.
Financial disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: MDMA, intermittent feeding, Elevated plus-maze, open field, neuronal numerical density, hippocampus
Abstract: Background—3,4-methylenedioxy-methamphetamine or MDMA (also known as “ecstasy” or “molly”) is a commonly abused drug that affects behavior and can lead to neuronal damage. Intermittent feeding is an effective dietary protocol that promotes neuroprotection and improves behavioral outcomes in animal models of neurotoxicity and neurodegenerative diseases. In this study, we investigated the behavioral and histological outcomes of the effect of intermittent feeding on the orally administered MDMA in mice.
Methods—The animals (male albino mice) were divided into four groups: ad libitum (AL), intermittent feeding (IF) (food given every other day), and AL and IF control groups. After five weeks, AL and IF groups were given a single oral dose of 20 or 60mg/kg MDMA. Behavior was assessed with the elevated plus-maze and the open field tests. Each of the treatment groups were then divided in to two groups: AL-AL (AL diet throughout), AL-IF (IF after MDMA administration), IF-IF (IF diet throughout), IF-AL (AL after MDMA administration). The second behavioral assessment was performed on ninth and 12th day after MDMA administration. The brains were then prepared with cresyl fast violet stain for stereology of the CA1 area of hippocampus.
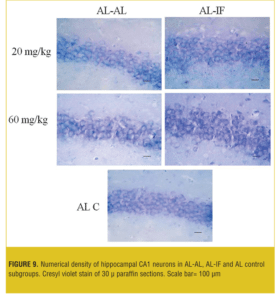
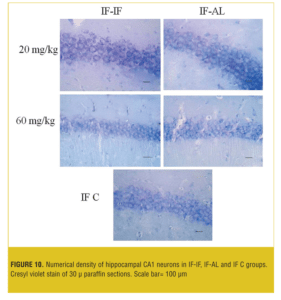
Results—The AL groups showed enhanced locomotion and anxiety compared to the IF (p<0.001); however, IF groups showed significantly (p<0.05) more locomotor activity and less anxiety recovery at ninth and 12th days compared to the AL animals. The neuronal numerical density was significantly (p<0.05) higher in the hippocampus in the AL-IF groups compared to the AL-AL.
Conclusion—IF regimen can significantly modify various behavioral characteristics induced by MDMA and promotes faster recovery from MDMA’s anxiogenic effects. Additionally, IF regimen had neuroprotective effects on the neurons of the CA1 area of the hippocampus after a single oral dose of MDMA. We believe the results of our study support the need for further research examining the behavior modifying and neuroprotective potential of the IF?regminen for the treatment of drug addiction in humans.
Introduction
MDMA, or 3,4-methylenedioxy-methamphetamine, is a chemical compound that is commonly used as a psychoactive recreational drug of abuse. Its reported effects are enhanced energy, endurance, sociability, psychodelic hallucinations, and sexual arousal.[1,2] “Ecstasy” (also known as “XTC,” “E,” or “X”) and “molly” are popular “street” names for MDMA, though it’s important to note that some formulations of ecstacy/molly may be contaminated with other amphetamines or may not actually contain 3,4-methylenedioxymeth-amphetamine at all.[2,3] MDMA is usually consumed orally in 80 to 250mg tablet form (rarely capsules). Often 2 to 3 tablets are taken together.[4] MDMA is listed as a Schedule 1 drug by the United States Drug Enforcement Agency, meaning that currently there are no accepted medical uses for MDMA in the United States, there is a lack of accepted safety for use under medical supervision, and there is a high potential for abuse.[5] However, MDMA is currently being studied for its potential use in the treatment of certain psychiatric disorders, particularly posttraumatic stress disorder.[6]
MDMA facilitates serotonin (5-HT) neurotransmission and, to a lesser extent, the release of dopamine (DA) and noradrenaline (NE) from their respective axon terminals. MDMA acts by indirectly increasing serotonin at the synaptic cleft by binding to, and thus inhibiting, the 5-HT transporter involved in its reuptake.[2,4] In humans, even when taken in moderate doses for recreational purposes, MDMA may produce undesirable effects including anxiety, depression, impulsiveness, aggression, and deficits of memory and awareness. These effects are thought to be due to the known neurotoxic properties of MDMA, especially on the serotonin (5-HT) system in the forebrain.[2,7] The target areas of MDMA in the brain include the cerebral cortex and the hippocampus, both important for learning and memory functions.[4]
MDMA neurotoxicity and its various behavioral effects have been investigated in several studies using different mice strains, including C57BL/6J, BALBC, Swiss Webster and NIH/Swiss mice.[8–11] Animal studies from different species have shown that MDMA induces anxiogenic effects.[12] These effects are evaluated by various tests, including functional observational battery, models of anxiety, locomotor activity, and the elevated plus maze test.[3] The animals typically show decreased open-arm entries and increased enclosed entries that are indicators of anxiogenic effects.[12] Acute MDMA effects also include a dose-dependent hyperlocomotor response.[4]
At the cellular level, MDMA can lead to toxic effects on neurons associated with various neurotransmitter systems, including nigrostriatal and mesolimbic dopaminergic pathways.[11,13–15] In mice, neurons of the CA1 area of hippocampus are more vulnerable than those in the adjacent regions and cortex.[16] Oxidative stress plays a major role in MDMA-induced neurotoxicity, and in various experimental studies mice have shown that MDMA increases free radicals and oxidative stress.[9,17,18] These findings indicate a higher susceptibility of the hippocampus to oxidative stress and are a primary target of MDMA in mice.[8,19–21] Additionally, MDMA has a suppressive effect on neurogenesis in the hippocampus in mice.[22]
Calorie restriction (CR) is defined as the reduction in caloric intake without malnutrition. Intermittent feeding (IF) is a form of CR where meals are not limited in calories but decreased in frequency, such as alternate days of feeding. IF has been shown to increase the lifespan in humans, mice and other animal species and may ward off many neurodegenerative disorders.[23–26,49] It has been shown that IF is more effective than limited daily feeding in protecting the hippocampal neurons against excitotoxic injury in mammals, including humans and mice.[27–29] IF protects neurons against various toxic insults through various cellular, molecular, and genetic mechanisms and increases the ability of the brain to restore its function after injury.[30–32] In animal models of aging and neurotoxic studies, CR protected hippocampal, striatal, and cortical neurons and ameliorated functional decline.[33] Short-term CR has been reported to increase neurogenesis in the dentate gyrus of young mice and rats as well as increase the thickness of the CA1 pyramidal cell layer in the hippocampus of mice.[33,34]
Mechanisms of neuroprotection of IF diet on the CA1 area of the hippocampus in mice include reduction in oxidative stress and increased hippocampal neurogenesis.[33–40] Dietary restriction (DR) also increased thickness of the CA1 pyramidal cell layer and induced expression of heat-shock protein 70 and glucose-regulated protein 78 in dopaminergic neurons, suggesting the involvement of these cytoprotective proteins in its neuroprotective actions.[33,41]
Additionally, CR has also been reported to modify pharmacological effects and induction of mood-elevating and analgesic effects in humans, demonstrated antidepressant and anxiolytic effects in various animal models, and increase incentive motivational responses in humans and rodents.[42]
In this study, we investigated the behaviorial effects via the CA1 hippocampal region of an IF diet versus an ad libitum (AL) diet on albino mice who were acutely administered a neurotoxic dose of MDMA. Since MDMA is commonly used as a recreational drug, especially amound young populations, this study provides insight into the potential use of CR diet as a tool for drug addiction treatment. Additional research is warranted.
Methods
Animals. All the experiments were carried out on male albino mice (N=140). The animals were purchased from Tehran Pasteur Institute. They were housed in groups of eight, and all were maintained under constant temperatures of 22 to 24 ºC and a 12 hours of light/12 hours of dark cycle (7am–7pm or 0700–1900 hours). The animals were maintained under these conditions for about one week before the starting the experiments. Animal treatment and experiments were carried out according to the policies of the Institutional Animal Care and Use Committee for the Society for Neuroscience (Washington, DC). The research project was approved by the Medical Research Ethics Committee of the Shiraz University of Medical Sciences in Tehran, Iran.
Drug. MDMA tablets were provided by the Antinarcotics Police (Applied Research Unit, Tehran, Iran). Purity of the compound, (±)-MDMA hydrochloride, (DL -3,4-methylenedioxymethamphetamine) was verified by LC-mass (quantitative analysis) and GC-mass (qualitative analysis) by the same organization as per our request, and no contaminants were detected (see Khajeamiri et al42).
(±)-MDMA hydrochloride was dissolved in 0.9 percent NaCl to the concentrations of 20 and 60mg/kg. These doses were chosen according to the reports published previously.[22,44] Since small mammals eliminate drugs at a faster rate than large mammals, to achieve a similar effect seen in humans, higher doses of drugs were required as estimated according to the relationship Dhuman=Danimal (Whuman/Wanimal)0.7 where D=dose of drug in milligrams and W=body weight in kilograms. Ecstasy tablets have been reported to generally contain between 80 and 250mg of MDMA, and 2 to 3 tablets are typically taken together.[3] Based on this knowledge, the doses selected for the mice were approximately equivalent to an acute dose of 391mg and 1,174mg in a 70kg human or 5.6?10-3mg/kg and 1.6?10-2mg/kg. Due to the much slower elimination of MDMA in humans, our scientific rationale was to administer higher doses the mice.[45] We selected the oral route for our study because MDMA is typically taken by mouth in humans. Vehicle (0.9% NaCl) was administered alone to the mice in the control group.
Diet. For the induction of dietary caloric restriction, we used the IF protocol in this study.[46,50] Mice were weighed and divided in four groups; AL treatment, AL control, IF treatment, and IF control. The mice were put on either an AL or IF diet at eight weeks of age with a weight of about 20 to 25g. The AL group mice had free access to food, while the IF group mice were maintained under an alternate day feeding schedule (allowed access to food AL every other day) for five weeks.[47] Both groups were fed with a standard laboratory mouse chow. For the IF groups, food was added or removed at 8am, and the animals were weighed every week. Previous studies have shown that rodents fed on IF protocol will consume 30 to 40 percent less calories over time compared to AL rodents, and have about 20-percent reduction of body weight.[48,49]
We begain our experiments after about five weeks of maintaining the mice on their regimens, with a weight difference of about 25 to 30 percent less in the IF (26.1±2.3g) group compared to the AL group (36.4±4.1g). Food was withdrawn from mice in AL and IF groups six hours prior to experimental treatment.[50,51]
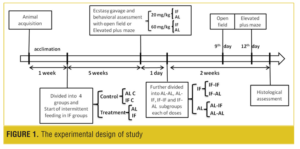
Experimental groups. The animals were divided into four groups (for open field and plus maze separately): AL, IF (every other day diet), and two control groups: AL C and IF C. After five weeks, the food was withdrawn from the mice in AL (n=56) and IF (n=64) groups, and six hours later MDMA was administrated by gavage.[48,50,51] The AL and IF animals were divided into two groups; each group (AL=28, IF=32) was given a single dose of 20 or 60mg/kg MDMA orally. The control mice (both AL C and IF C [n = 12]) were given saline orally. The animals of AL and IF groups were further divided into two groups for behavior assessment: elevated plus maze (AL=14, IF=16) and open field test (AL=14, IF=16). These two behavioral tests were performed on separate days. All animals (AL and IF) were then further divided into four groups each of 20 and 60mg/kg: AL-AL (AL diet throughout, n=7) and AL-IF (IF after MDMA administration, n=7), IF-IF (IF diet throughout, n=8), and IF-AL (AL after MDMA administration, n=8). The second behavioral assessment was performed on ninth day (open field) and 12th day (elevated plus-maze) after MDMA administration. The brains of the animals (n=2 for each group) were then prepared for histological study of the hippocampus with cresyl fast violet stain. The experimental design of the study is illustrated in Figure 1.
Elevated plus maze. Anxiety-like behavior was also examined with the elevated plus maze test. The elevated plus-maze (EPM) was made of black Plexiglas and consisted of two opposing open arms (30×5cm, surrounded by a 0.25cm high border) and two enclosed arms (30×5cm, surrounded by 15cm high walls) that joined at a central square area (5×5cm) and raised 50cm above the room floor by means of a stand. Three white 47-watt fluorescent lamps provided the only source of light in the testing room. Thirty minutes after gavage of MDMA, a single mouse was placed onto the central area of the maze facing an open arm and allowed to freely explore the maze. The animal was recorded for five minutes by the video camera. After removal of each animal, the maze was cleaned with a wet cloth and wiped dry. The behaviors of the mice recorded on videotapes were later scored by a trained observer blinded to the treatments. The number of entries and the amount of time spent in each zone was recorded. Behavioral measures included percent time spent in open arms (% open time) expressed as a percentage of total time on both open and enclosed arms. Arm entry was defined as all four paws having crossed the dividing line between an arm and the central area. This test was repeated on 12th day after MDMA gavage for recording its effects during recovery period.[52,53]
Open field. Locomotor activity was assessed by the open field test (OFT). The recording area was limited by gray Plexiglas (40cm×40cm×40cm). A painted grid divided the Plexiglas floor into 16 identical squares each measuring 10cm×10cm. The Plexiglas floor was located on a table 50cm above the floor, and was illuminated by three white 40-watt fluorescent indirect and homogenous lamps. Black curtains surrounded the recording area, and behavior was monitored using an overhead video camera. Following gavage of MDMA, the mice were immediately placed in the open field and behaviors were monitored for 60 minutes by the experimenter.[54] Animals were re-tested on ninth day post-gavage to assess effects on locomotor activity during recovery period.[55] Mice were monitored for frequency of line-crossing and rearing behaviors. Frequency of these behaviors was assessed in the peripheral zone (defined as the 12 squares directly adjacent to the walls) and central zone (all remaining squares).[2]
Tissue fixation and Nissl staining. After the second behavioral assessment, the mice were anesthetized with ketamine 100mg/kg and xylesin 10mg/kg i.p and perfused transcardially with 0.9% saline, followed by 10% buffered formalin. Brains were immediately removed and post-fixed in the same fixative overnight at 40ºC. The brains were then embedded in paraffin. They were coronally sectioned 30?m thick and stained with Nissl stain (Cresyl fast violet). The sections containing the hippocampus from the anterior to posterior parts were taken according to the mouse brain atlas of Paxinos.[56] Cell count of the hippocampal CA1 neurons was then undertaken using stereology.
Stereological analysis. The numerical density of the hippocampal CA1 neurons was estimated using the optical dissector technique.[57,58] The optical dissector setting included an Eclipse microscope (E200, Nikon, Tokyo, Japan) with a high-numerical-aperture (NA=1.25)×100 oil-immersion objective with a video camera that was connected to a monitor and an electronic microcator with digital readout (MT12, Heidnehain, Traunreut, Germany) for measuring the movements in the Z-direction with 0.5-?m precision. A computer-generated counting frame was superimposed on the screen using a stereology software system (Stereolite, SUMS, Shiraz, Iran). The neuronal density was defined with following formula: NV=?Q/[?P×a(f)×h], where ?Q is the number of neurons counted within the sampling volume, ?P is number of dissector, a(f)=0.0033mm2 is the area of the sampling frame, and h=0.01mm is the height of the dissector.[59] Neuronal nuclei were counted in approximately 600 (±1) of the sections evaluated during the entire experiment, counting all the groups and 20 (± 5) optical dissectors per hippocampus in each animal, providing the mean the coefficient of error (CE) on the estimates of total number of eight percent.
Cells were identified as neurons if they had a nucleolus, dendritic processes, euchromatin material within the nucleus, and nuclei surrounded by cytoplasm.[60,61] This study was based on a systematic, uniform, random sampling that allowed unbiased and efficient estimates of all parameters under study to be obtained. Cell counts were performed in the CA1 region of the hippocampus on both sides.
Chemicals. All the chemicals were purchased from Sigma Chemical Company unless otherwise mentioned.
Statistical analysis. Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by pairwise comparison with one-way ANOVA followed by the post-hoc Tukey test and a probability of less than 0.05 was considered to indicate significant difference between the groups’ means. Statistical analysis was performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
results
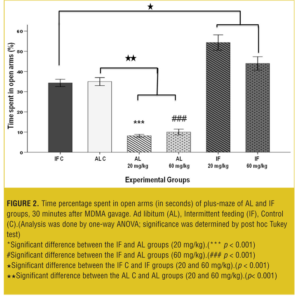
EPM test-acute test. MDMA affected acute EPM behavior in a dose-dependent manner: In AL groups, both of 20 and 60mg/kg, open arm time percentage was significantly decreased (p< 0.001) compared to vehicle and IF groups. However, open arm time percentage in IF groups was significantly increased (p< 0.001) compared to the IF control and AL control groups (Figure 2).
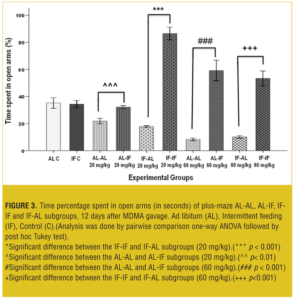
EPM test 12 days after treatment. At both doses, AL-IF subgroups compared to AL-AL subgroups (with 20mg/kg, p<0.01 and with 60mg/kg, p<0.001) and IF-IF subgroups compared to IF-AL subgroups (p<0.001) spent significantly more time in open arm (Figure 3).
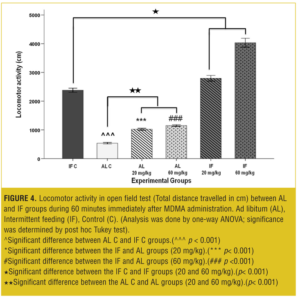
OFT—acute test. Total traveled distance and rearing behavior in the IF control group was increased significantly (p<0.001) compared to the AL control group.
The MDMA affected locomotor activity in a dose-dependent manner: In AL groups at both 20 and 60mg/kg, the total traveled distances were significantly increased (p<0.001) compared to the vehicle. Also, total traveled distances in IF groups were significantly increased (p< 0.001) compared to the IF control and AL control groups (Figure 4).
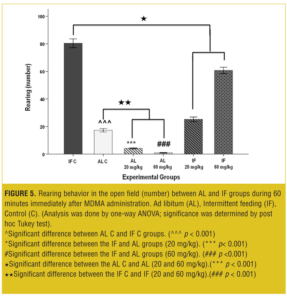
At both the doses, the rearing behavior was significantly decreased (p<0.001) in AL test groups compared to the AL control group. Also the IF test groups showed significant decrease (p<0.001) in rearing behavior compared to the IF control group; however, rearing behavior increased significantly (p<0.001) when compared to the AL groups (Figure 5).
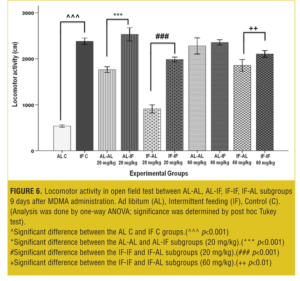
OFT—Nine days after treatment. At both the doses, AL-IF subgroups compared to AL-AL subgroups (with 20mg/kg, p<0.001) and IF-IF subgroups compared to IF-AL subgroups (with 20mg/kg, p<0.001 and with 60mg/kg, p<0.01) had significant increases in total traveled distances; however, total traveled distances was not significant at the 60mg/kg between AL-AL and AL-IF subgroups (Figure 6).
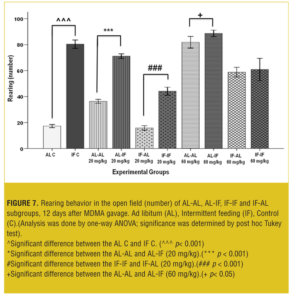
Figure 7 shows that at both doses, AL-IF subgroups compared to the AL-AL subgroups (20mg/kg, p<0.01 and 60mg/kg, p<0.001) and IF-IF subgroups compared to IF-AL subgroups (p<0.001) had significant increases in rearing behavior.
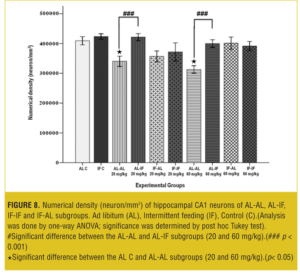
Stereological analysis. In each group, the brains (both sides) of two mice were examined. Numerical density (neuron/mm3) of hippocampal CA1 neurons in the AL-AL subgroups was significantly less than the AL control group (p< 0.05). At both doses, the numerical density of neurons in CA1 region of the hippocampus was significantly less (p<0.05) in the AL-AL subgroup compared to the AL-IF subgroups (Figure 8). Additionally, the higher dose of MDMA (60mg/kg) produced a greater reduction in the numerical density of hippocampal CA1 neurons; however, it was not significant. Figure 9 and Figure 10 show that the AL-IF subgroups had an increased numerical density of neurons in CA1 region of the hippocampus; however, in AL-AL subgroups, there was a decrease two weeks after the administration of MDMA.
Discussion
In this study, we sought to determine if an IF diet can protect albino mice from MDMA-induced neurotoxicity in the CA1 hippocampal region by examining any behavioral changes. For this purpose, we compared MDMA-induced neurotoxicity and behavioral effects between AL and IF animal groups. Behavioral changes (locomotor activity and anxiety) were recorded by an open field test and elevated plus maze, and neuronal numerical density in the hippocampus was evaluated by stereology after single MDMA administration.
MDMA neurotoxicity and its behavioral effects have been previously studied in different strains of mice. These include effects on tau phosphorylation; monoamine assay; motor activity; electrocardiogram (ECG) abnormalities; neuronal degeneration in C57BL/6J; locomotor activity; exploratory behavior; anxiety; defensive behavior; neuronal damage in BALBC, DA, and 5-HT concentrations; hyperthermia; locomotor response in Swiss Webster; hyperactivity; social interaction; passive avoidance; anxiety and brain monoamines concentrations in OF1 mice; and free radical formation, lipid peroxidation, and neurodegeneration of DA nerve endings, locomotor activity, and hyperthermia in NIH/Swiss mice.[8–11,19,21,52,59,62,63]
The IF diet is an effective method for neuroprotection and revival of the neuronal system and recovery of behavioral function in animal models of neurotoxicity and neurodegeneration.[28,39,41,49,64]
Effect of IF on anxiety behavior in the EPM model. Anxiety in?uences animal behavior in different ways and causes mice to avoid places that entail risk.[65] The elevated plus-maze test is based on the assumption that in a state of anxiety the mice will have an aversion to heights and open spaces, preferring to spend more time in the closed arms rather than in the open arms of the maze. This model has been validated in mice, and it is useful for evaluation of anxiogenic-like and anxiolytic-like properties of drugs.[66] In the past decade, a large number of pharmacological studies on anxiety have been done with this model. There is good evidence for the neuropharmacological and neuroanatomical parallels between rodent emotionality and human anxiety.[12]
Typical exploratory behavior in this test was in favor of the open arms; forced or voluntary entry into the close arms was associated with hormonal and behavioral changes that are indicative of anxiety.[12,53] Animals were re-tested at 12 days post-gavage to assess long-term anxiety levels.[55]
In our study, when EPM test was carried out 30 minutes after the MDMA gavage, AL treatment groups showed a decrease in time spent in open arms compared to the AL control and IF groups, which indicated anxiety-like behavior. On the other hand, IF treatment groups showedan increase in time spent in open arms compared to the IF control and the AL groups, indicating anxiolytic-like behavior. When the EPM test was carried out 12 days after of MDMA gavage, subgroups that were under IF protocol showed less anxiety-like behavior than the AL subgroups. It is noteworthy that the anxiety-like behavior persisted in AL groups after 12 days of MDMA administration while animals in IF groups showed negligible signs of such behavior.This suggests that the IF diet had a reparative effect, leading to faster recovery from the MDMA toxicity and a quicker return to normal behavior.
In previous studies, MDMA caused anxiety-like behavior in drug users. The consistency of human and animal observations confirms the notion that MDMA has anxiogenic-like effects.[12] Studies in mice also report similar effects of MDMA.[53] MDMA is an active releaser of neurotransmitters responsible for diverse behavioral effects. It has moderate affinities for a variety of receptor subtypes of neurotransmitter systems, including serotonin, dopamine, and noradrenaline, that play important roles in the neural processes that control anxiety.[12]
One of the leading behavioral effects of the CR diet is a decrease in anxiety-like behavior, which was also observed in the mice.[67] The anxiolytic effects of IF are not due to a general decrease in calories or reductions in specific caloric elements, such as fat, protein, and carbohydrates, or vitamins and minerals. For example, a study of rats showed an anxiolytic-like effect that continued for 10 days after the normalization of feeding following 10 days of CR.[47]
In various strains of mice, studies have shown that animals maintained on a CR diet display alterations in expression of receptors, neuropeptides, and hormonal release. For example, a CR diet has been shown to increase neuropeptide orexin levels, which mediate locomotor activity and have anti- depressive effects. Additionally, food restriction has been shown to cause a significant increase in prepro-orexin gene expression in obese mice in comparison to AL-fed animals.[32,68,69] It is well known that corticotropin-releasing factor (CRF) is also involved in the expression of stress and anxiety-like behavior via multiple neurotransmitter systems. The IF diet appears to down-regulate CRF receptors in several areas of brain, resulting in a decrease of anxiety-like behavior in rats.[47] Mice lacking CRF receptor subtype 1 displayed reduction in anxiety-like behavior compared to the wild type animals.[63] However, the exact neurochemical mechanisms related to the anxiolytic-like properties of IF have yet to be determined and require further investigation.
Effect of IF on locomotor activity and explorative behavior in the OFT model. In our study, total traveled distance in OFT was used to measure locomotor activity, and rearing frequency was used to measure explorative behavior of the animals during OFT.[10,47,64,65] In comparison with control groups, MDMA induced hyperactivity in AL groups, and, additionally, in OFT the rearing activity was almost absent during 60 minutes of observation. This finding suggests that the dosages of MDMA used in this study produced a significant increase in locomotor activity and a decrease in exploratory activity, in concordance with the anxiogenic-like profile of the drug that confirms the findings of previous studies.[65–75]
Several studies have established that the alteration in MDMA-induced locomotor behavior is due to its interaction with 5-HT1B receptor. In knockout mice lacking this receptor, locomotor response was abolished after MDMA administration.[3,75]
Release of serotonin is known to produce hyperactivity in rodents, and studies have shown that this effect is produced through catecholaminergic mechanisms. MDMA also binds with low affinity to dopaminergic receptors and releases dopamine.[65]
Moreover, it has been suggested that hyperactivity following psychostimulant administration is a result of activation of dopamine-releasing neurons placed mainly in structures known communally as the motive circuit. However, hyperactivity induced by MDMA is different from that induced by other psychostimulants, such as amphetamine. MDMA is thought to act on 5-HT pathways and specifically co-activate dopaminergic pathways in mice.[13] Increases in extracellular dopamine have been reported following the first few hours of MDMA administration in mice.[9,11]
In our study, MDMA administration caused a decrease of exploratory behaviors (especially rearing) during the OFT without reducing locomotion. This behavioral profile is an indicator of anxiogenic-like activity of the drug in mice and is completely independent of the 5-HT1B receptor.[73] Additionally, after the administration of MDMA, the IF test groups showed more hyperactivity in the OFT compared to the IF control group and both AL groups; however, the IF test group had lower rearing frequency compared to the IF control. There was an increase in exploratory behaviors and locomotor activity in the IF animals compared to the AL animals after MDMA administration. Also, when OFT was carried out on ninth day after MDMA gavage, the experimental animals that were under the IF protocol subgroups still showed more hyperactivity than the AL subgroups. Therefore, in this study, IF caused persistent increase in hyperactivity and exploratory responses to MDMA administration. This could be due to behavioral and neurobiological relationship between ingestive and drug seeking behaviors, as it has been previously shown that chronic food restriction enhances the rewarding locomotor- and cellular-activating effects of abused drugs.[76]
Increased behavioral activity by MDMA and IF could be due to elevated serum corticosterone, which is a classic indicator of stress in rodents and leads to an increase of locomotor activity.[77,78] MDMA also increases serum corticosterone levels, and it has been reported that elevated corticostrone levels increase psychostimulants-induced locomotor activity.[4,10,79] Additionally, high serum corticosterone levels in IF mice and rats have been observed in several studies.[32,47,80–83] Chronic food restriction (FR) generally acts as a stressor and has been shown to increase the locomotor and neurochemical effects of psychostimulants in mice and rats.[84–87]
Effect of IF on neuronal density of hippocampus. Our data showed that numerical densities of CA1 neurons in the hippocampus of AL-AL subgroups were less than other groups. The data also showed that in the AL-IF group and the other subgroups that were maintained on IF diet before or after of administration of MDMA, numerical densities of CA1 hippocampal neurons were higher compared to the AL-AL subgroups. These results are supported by previous studies.[3,16,75] In animal models of neurodegenerative disorders, an IF diet is known to enhance resistance of neurons to age-related and disease-specific stresses, as well have protective effects after toxic insults.[24,49–51,88]
Thickness of pyramidal cell layer of CA1 region has been previously used as a marker to evaluate the alteration of gross brain structures caused by IF. Mice on an IF diet have thicker CA1 pyramidal cell layers than their control counterparts.[33] Additionally, an IF diet is known to promote neurogenesis and has been shown to have a neuroprotective effect in different models. One mechanism of these effects is the expression of neurotrophic factors.[37] More specifically, there is increase in BDNF levels in hippocampus in IF animals, and BDNF signaling is known to regulate adult hippocampal neurogenesis during an IF diet.[37,89] During food restriction, less glucose is available in the mitochondria, leading to less ROS production and to less damage to proteins; DNA and membrane lipids are lessened as well, leading to neuroprotection.[41,90] MDMA-induced damage to neurons involves ROS generation caused by massive release of 5-HT and other catecholamine transmitters and their metabolic intermediates that eventually leads to neuronal degeneration. Therefore, the role that the IF diet plays in protecting CA1 neurons after MDMA administration is important in our study and requires further investigation to determine how ROS is involved in IF diet-induced neuroprotection.[9,17]
The mechanisms whereby caloric restriction enhances cognitive functions and memory and resistance to aging and neurodegenerative disease are studied extensively.[24,39,49,64] Animal studies suggest that CR and specifically IF diets may benefit the brain by reducing levels of oxidative stress and enhancing cellular stress resistance mechanisms. Mild metabolic stress associated with CR stimulates secretion of neuroprotective factors, such as BDNF, by cells to produce proteins that increase cellular resistance to disease processes.[28,41,64] Interestingly, it has been reported that the mouse brain exhibits a greater increase in the number of injured neurons in some brain regions, especially in cerebellum, cortex, and hippocampus after MDMA treatment compared to that in rats.[21] This finding highlights the importance of interspecies variation in neuroprotective mechanisms after MDMA administration and requires further investigation into the role the IF diet plays.
Some neurotransmitters, including 5-HT and dopamine, play roles in the regulation of hippocampal neurogenesis. 5-HT depletion decreases hippocampal neurogenesis, and, similarly, dopamine depletion affects neuronal proliferative activity, although the specific effects of dopamine on neurogenesis are still arguable.[22] It will be interesting to study the effects of the IF diet on various aspects of these neurotransmitter systems in MDMA-treated mice.
Conclusion
In conclusion, the results of this study show that an IF diet leads to significantly less anxiety-like behavior and promotes faster recovery in mice when compared to an AL diet. Additionally, the IF diet exerted neuroprotective effects on neurons of the CA1 area of hippocampus. We suggest that the behavioral and histological effects of the IF diet be further explored in rodent experimental models for its possible role in the treatment of MDMA-induced neurotoxicity in humans.
Acknowledgment
The authors wish to thank Dr. Firuzi for providing access to lab facilities for our behavioral work; the Section of Applied Research and Tehran Antinarcotics Police for providing the study drug; Dr. Ali Reza Khajeamiri, Mrs. Keshavarzi, and Mrs. Ghalavandi for their assistance with stereological work; and Mrs. Aghazadeh and Ms. Khalilian for their editorial assistance. This work was part of a MSc thesis of Z. Ebrahimian, supervised jointly by MJ Khoshnoud and MR Haidari.
References
- Freye E, Levy J. Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs. 1st ed. New York: Springer Netherlands; 2009:143–172.
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917–928.
- Capela JP, Carmo H, Remiao F, et al. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39(3):210–271.
- Green AR, Mechan AO, Elliott JM, et al. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev. 2003;55(3):463–508.
- Controlled Substance Schedules. United States Department of Justice Drug Enforcement Agency [website]. Diversion Control Division. December 2016. https://www.deadiversion.usdoj.gov/schedules/#define. Accessed February 2017.
- Yazar-Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194–196.
- Song BJ, Moon KH, Upreti VV, et al. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol. 2010;11(5):434–443.
- Busceti CL, Biagioni F, Riozzi B, et al. Enhanced tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (“Ecstasy”). J Neurosci. 2008;28(12):3234–3245.
- Colado MI, Camarero J, Mechan AO, et al. A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) on dopamine neurones in mouse brain. Br J Pharmacol. 2001;134(8):1711–1723.
- Ferraz-de-Paula V, Stankevicius D, Ribeiro A, et al. Differential behavioral outcomes of 3,4-methylenedioxymethamphetamine (MDMA-ecstasy) in anxiety-like responses in mice. Braz J Med Biol Res. 2011;44(5):428–437.
- O’Shea E, Esteban B, Camarero J, et al. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2001;40(1):65–74.
- Lin HQ, Burden PM, Christie MJ, Johnston GA. The anxiogenic-like and anxiolytic-like effects of MDMA on mice in the elevated plus-maze: a comparison with amphetamine. Pharmacol Biochem Behav. 1999;62(3):403–408.
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42(2):155–168.
- Sprague JE, Nichols DE. Neurotoxicity of MDMA (ecstasy): beyond metabolism. Trends Pharmacol Sci. 2005;26(2):59–60.
- Stone DM, Hanson GR, Gibb JW. Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology. 1987;26(11):1657–1661.
- Liang X, Nagai A, Sheikh AM, et al. Increased vulnerability of hippocampal CA1 neurons to hypoperfusion in ataxia and male sterility (AMS) mouse. Brain Res. 2013;1494:109–117.
- Cadet JL, Thiriet N, Jayanthi S. Involvement of free radicals in MDMA-induced neurotoxicity in mice. Ann Med Interne (Paris). 2001;152 Suppl 3:IS57–59.
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25(10):505–508.
- Fornai F, Gesi M, Lenzi P, et al. Effects of repeated low doses of MDMA on EEG activity and fluoro-jade B histochemistry. Ann N Y Acad Sci. 2004;1025:181–188.
- Frenzilli G, Ferrucci M, Giorgi FS, et al. DNA fragmentation and oxidative stress in the hippocampal formation: a bridge between 3,4-methylenedioxymethamphetamine (ecstasy) intake and long-lasting behavioral alterations. Behav Pharmacol. 2007;18(5-6):471–481.
- Sharma HS, Ali SF. Acute administration of 3,4-methylenedioxymethamphetamine induces profound hyperthermia, blood-brain barrier disruption, brain edema formation, and cell injury. Ann N Y Acad Sci. 2008;1139:242–258.
- Cho KO, Rhee GS, Kwack SJ, et al. Developmental exposure to 3,4-methylenedioxymethamphetamine results in downregulation of neurogenesis in the adult mouse hippocampus. Neuroscience. 2008;154(3):1034–1041.
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994.
- Valter DL, and Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19(2):181–192.
- Ingram DK, Zhu M, Mamczarz J, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108.
- Minor RK, Allard JS, Younts CM, et al. Dietary Interventions to Extend Life Span and Health Span Based on Calorie Restriction. J Gerontol A Biol Sci Med Sci. 2010;65(7):695–703.
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–722.
- Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003;84(3):417–431.
- Prolla TA, Mattson MP. Molecular mechanisms of brain aging and neurodegenerative disorders: lessons from dietary restriction. Trends Neurosci. 2001;24(11 Suppl):S21–31.
- Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63.
- Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228–240.
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221.
- Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069.
- Bondolfi L, Ermini F, Long JM, et al. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25(3):333–340.
- Halagappa VK, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26(1):212–220.
- Hsieh EA, Chai CM, Hellerstein MK. Effects of caloric restriction on cell proliferation in several tissues in mice: role of intermittent feeding. Am J Physiol Endocrinol Metab. 2005;288(5):E965–972.
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002a;82(6):1367–1375.
- Stranahan AM, Lee K, Martin B, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–961.
- Wang J, Ho L, Qin W, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19(6):659–661.
- Zhu H, Guo Q, Mattson MP. Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res. 1999;842(1):224–229.
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res.1999;57(2):195–206.
- Carr KD. Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav. 2011;104(1):162–167.
- Khajeamiri AR, Kobarfard F, Ahmadkhaniha R, Mostashari G. Profiling of ecstasy tablets seized in Iran. Iran J Pharm Res. 2011;10(2):211–220.
- Mueller M, Maldonado-Adrian C, Yuan J, et al. Studies of (+/-)-3,4-methylenedioxymethamphetamine (MDMA) metabolism and disposition in rats and mice: relationship to neuroprotection and neurotoxicity profile. J Pharmacol Exp Ther. 2013;344(2):479–488.
- Meyer JS, Piper BJ, Vancollie VE. Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann N Y Acad Sci. 2008;1139:151–163.
- Mager DE, Wan R, Brown M, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20(6):631-7
- Levay EA, Govic A, Penman J, et al. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav. 2007;92(5):889–896.
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001a;76(2):619–626.
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16(3):129–137.
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45(1):8–15.
- Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci.
- Daza-Losada M, Rodriguez-Arias M, Maldonado C, et al. Acute behavioural and neurotoxic effects of MDMA plus cocaine in adolescent mice. Neurotoxicol Teratol. 2009;31(1):49–59.
- Navarro JF, Maldonado E. Acute and subchronic effects of MDMA (“ecstasy”) on anxiety in male mice tested in the elevated plus-maze. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1151–1154.
- Ludwig V, Mihov Y, Schwarting RK. Behavioral and neurochemical consequences of multiple MDMA administrations in the rat: role of individual differences in anxiety-related behavior. Behav Brain Res. 2008;189(1):52–64.
- Ho YJ, Pawlak CR, Guo L, Schwarting RK. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res. 2004;149(2):135–144.
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Waltham, MA: Elsevier Academic Press; 2004.
- Howard C, Reed M. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. 2nd ed. Abingdon, Oxon: Garland Science; 2005:89–90.
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. 1st ed. Baltimore, MD: Johns Hopkins University Press; 2002.
- Namavar MR, Raminfard S, Jahromi ZV, Azari H. Effects of high-fat diet on the numerical density and number of neuronal cells and the volume of the mouse hypothalamus: a stereological study. Anat Cell Biol. 2012;45(3):178–184.
- Cotter D, Mackay D, Landau S, et al. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58(6):545–553.
- Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Brain Res Dev Brain Res. 2000;125(1–2):83–88.
- Fantegrossi WE, Godlewski T, Karabenick RL, et al. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl). 2003;166(3):202–211.
- Itzhak Y, Ali SF, Achat CN, Anderson KL. Relevance of MDMA (“ecstasy”)-induced neurotoxicity to long-lasting psychomotor stimulation in mice. Psychopharmacology (Berl). 2003;166(3):241–248.
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5(3):332–353.
- Wahlsten D. Mouse Behavioral Testing: How to Use Mice in Behavioral Neuroscience. 1st ed. Waltham, MA: Elsevier Academic Press; 2010.
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl). 1987;92(2):180–185.
- Yamamoto Y, Tanahashi T, Kawai T, et al. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics. 2009;39(3):227–235.
- Lutter M, Krishnan V, Russo SJ, et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075.
- Yamamoto Y, Ueta Y, Serino R, et al. Effects of food restriction on the hypothalamic prepro-orexin gene expression in genetically obese mice. Brain Res Bull. 2000;51(6):515–521.
- Contarino A, Dellu F, Koob GF, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835(1):1–9.
- Mechan AO, Moran M, Elliott M, et al. A study of the effect of a single neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) on the subsequent long-term behaviour of rats in the plus maze and open field. Psychopharmacology (Berl). 2002;159(2):167–175.
- Risbrough VB, Masten VL, Caldwell S, et al. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31(11):2349–2358.
- Maldonado E, Navarro JF. Effects of 3,4-methylenedioxy-methamphetamine (MDMA) on anxiety in mice tested in the light-dark box. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):463–472.
- Powell SB, Lehmann-Masten VD, Paulus MP, et al. MDMA “ecstasy” alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl). 2004;173(3-4):310–317.
- Scearce-Levie K, Viswanathan SS, Hen R. Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology (Berl). 1999;141(2):154–161.
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76(3):353–364.
- Heiderstadt KM, McLaughlin RM, Wright DC, et al. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab Anim. 2000;34(1):20–28.
- Keeney AJ, Hogg S, Marsden CA. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav. 2001;74(1-2):177–184.
- Pauly JR, Robinson SF, Collins AC. Chronic corticosterone administration enhances behavioral sensitization to amphetamine in mice. Brain Res. 1993;620(2):195–202.
- Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156(5):1781–1787.
- Makimura H, Mizuno TM, Isoda F, et al. Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol. 2003;3:5.
- Stewart JW, Koehler K, Jackson W, et al. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26(6):1077–1084.
- Woodward CJ, Hervey GR, Oakey RE, Whitaker EM. The effects of fasting on plasma corticosterone kinetics in rats. Br J Nutr. 1991;66(1):117–127.
- Dietrich MO, Mantese CE, Dos Anjos GM, et al. Increased locomotor response to amphetamine, but not other psychostimulants, in adult mice submitted to a low-protein diet. Physiol Behav. 2004;83(1):129–133.
- Itoh T, Murai S, Nagahama H, et al. Effects of 24-hr fasting on methamphetamine- and apomorphine-induced locomotor activities, and on monoamine metabolism in mouse corpus striatum and nucleus accumbens. Pharmacol Biochem Behav. 1990;35(2):391–396.
- Mamczarz J, Bowker JL, Duffy K, et al. Enhancement of amphetamine-induced locomotor response in rats on different regimens of diet restriction and 2-deoxy-D-glucose treatment. Neuroscience. 2005;131(2):451–464.
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101.
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293-315.
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002b;80(3):539–547.
- Sohal RS, Ku HH, Agarwal S, et al. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74(1–2):121–133.