 by Søren D. Østergaard, MD, PhD; Mark G. A. Opler, PhD, MPh; and Christoph U. Correll, MD
by Søren D. Østergaard, MD, PhD; Mark G. A. Opler, PhD, MPh; and Christoph U. Correll, MD
Dr. Østergaard is Associate Professor at Aarhus University in Denmark. Dr. Opler is Adjunct Assistant Professor at NYU School of Medicine and Chief Research Officer at MedAvante-ProPhase, Inc. Dr. Correll is Medical Director of the Recognition and Prevention program at the Zucker Hillside Hospital in Glenn Oaks, New York, and Professor at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, and the Charité Universitätsmedizin, Department of Child and Adolescent Psychiatry, Berlin, Germany.
Funding: No funding was provided for this article.
Disclosures: The home institution of SD Østergaard (Aarhus University) holds one-third of the copyright for the Simplified Negative and Positive Symptoms Interview (SNAPSI). Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Bristol-Myers Squibb, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck and Pfizer. He received grant support from Takeda. The home institution of Dr. Correll (The Feinstein Institute for Medical Research, Manhasset, New York, USA) holds one-third of the copyright for the SNAPSI. The home institution of Dr. Opler (MedAvante-ProPhase Inc.) holds one-third of the copyright for the SNAPSI. Dr. Opler also has received grant funding from US NIMH, the Brain and Behavior Foundation (formerly NARSAD), the Stanley Research Foundation, and the Qatar National Research Fund.
Abstract: There is currently a “measurement gap” between research and clinical care in schizophrenia. The main reason behind this gap is that the most widely used rating scale in schizophrenia research, the 30-item Positive and Negative Syndrome Scale (PANSS), takes so long to administer that it is rarely used in clinical practice. This compromises the translation of research findings into clinical care and vice versa. The aim of this paper is to discuss how this measurement gap can be closed. Specifically, the main points of discussion are 1) the practical problems associated with using the full 30-item PANSS in clinical practice; 2) how the brief, six-item version of the Positive and Negative Syndrome Scale (PANSS-6) was derived empirically from the full 30-item PANSS and what the initial results obtained with PANSS-6 entail; and 3) how PANSS-6 ratings, guided by the newly developed, 15–25-minute, stand-alone Simplified Negative and Positive Symptoms Interview (SNAPSI), might help bridge the measurement gap between research and clinical care in schizophrenia. The full 30-item PANSS is often used in research studies, but is too time consuming to allow for routine clinical use. Recent studies suggest that the much briefer PANSS-6 is a psychometrically valid measure of core positive and negative symptoms of schizophrenia and that the scale is sensitive to symptom improvement following pharmacological treatment. SNAPSI is a brief interview that yields the information needed to rate PANSS-6 (and other brief rating scales). We believe that PANSS-6 ratings guided by SNAPSI will help bridge the measurement gap between research and clinical care in schizophrenia.
Keywords: Schizophrenia, psychometrics, rating scale, measurement-based care
Innov Clin Neurosci. 2017;14(11–12):68–72.
The most widely used measure of the symptom severity of schizophrenia in clinical studies is the Positive and Negative Syndrome Scale (PANSS),1 which was published in 1987. PANSS was created by merging 12 items from the Psychopathology Rating Schedule2 with the 18-item Brief Psychiatric Rating Scale (BPRS).3 Due to the large number of items (30 in total), the structured clinical interview for PANSS (SCI-PANSS) often takes an hour or more to administer—and is occasionally impossible to complete in the case of patients in very severe states of illness. For these reasons, the 30-item PANSS is not ideal for routine use in most clinical practice settings and has remained a research tool, despite evidence that supports its use to help characterize, predict, and manage the course of illness.4,5 To help bring the power of rating scales to clinical practice, an abbreviated set of instruments and an accompanying assessment schedule are required that will allow for a quick and valid measurement of symptom severity of core dimensions of schizophrenia across research and clinical practice settings.6 In this commentary, we will make the argument that valid measurements of schizophrenia symptom severity can likely be obtained by rating the six-item subscale of PANSS (PANSS-6)7–10 and other clinician-rated outcomes using the recently developed, stand-alone Simplified Negative and Positive Symptoms Interview (SNAPSI).
From PANSS to PANSS-6
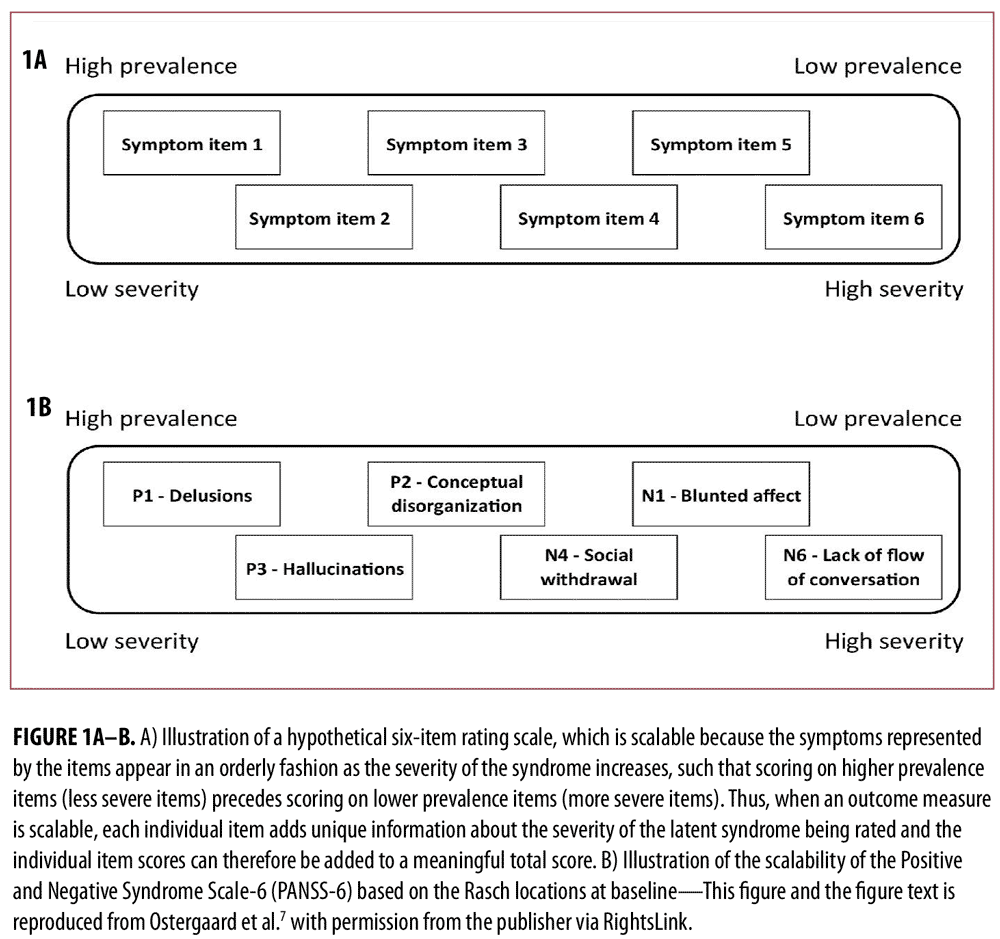
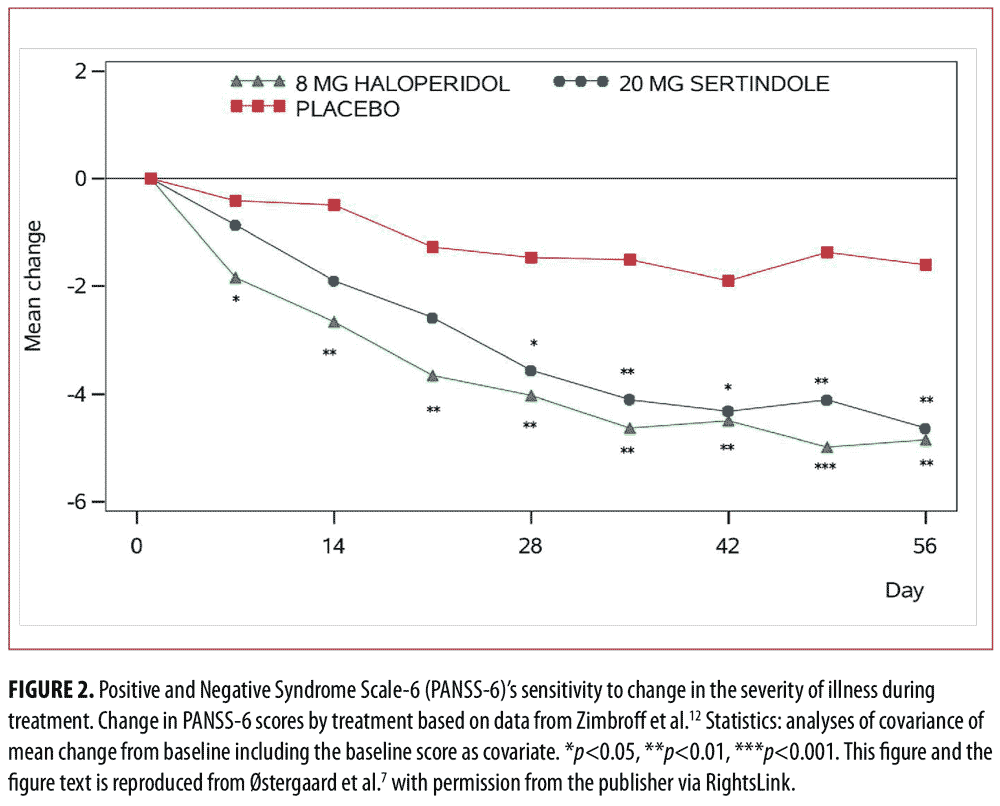
In a first study based on data from two large randomized controlled trials in schizophrenia,11,12 we identified PANSS-6 by means of item response theory analysis.7 PANSS-6 contains the following six items that tap into the core positive and negative symptom dimensions of schizophrenia (and other psychotic disorders): Delusions, Conceptual Disorganization, Hallucinations, Blunted Affect, Passive/Apathetic Social Withdrawal, and Lack of Spontaneity and Flow of Conversation. This study showed that PANSS-6, as opposed to the full 30-item version of PANSS, is “scalable,” which means that each item adds unique information regarding severity and that the total score is therefore a valid measure of severity (Figure 1).13 Furthermore, this study showed that PANSS-6 is sensitive to changes in the severity of schizophrenia and can separate the effects of typical and atypical antipsychotics from that of placebo (Figure 2).7
PANSS-6 and CATIE
Since the initial study on PANSS-67 was based on data from trials in which the participants were acutely ill hospitalized patients with schizophrenia,11,12 we went on to assess the psychometric properties of PANSS-6 in chronic schizophrenia via a reanalysis of data from the Clinical Antipsychotic Trials
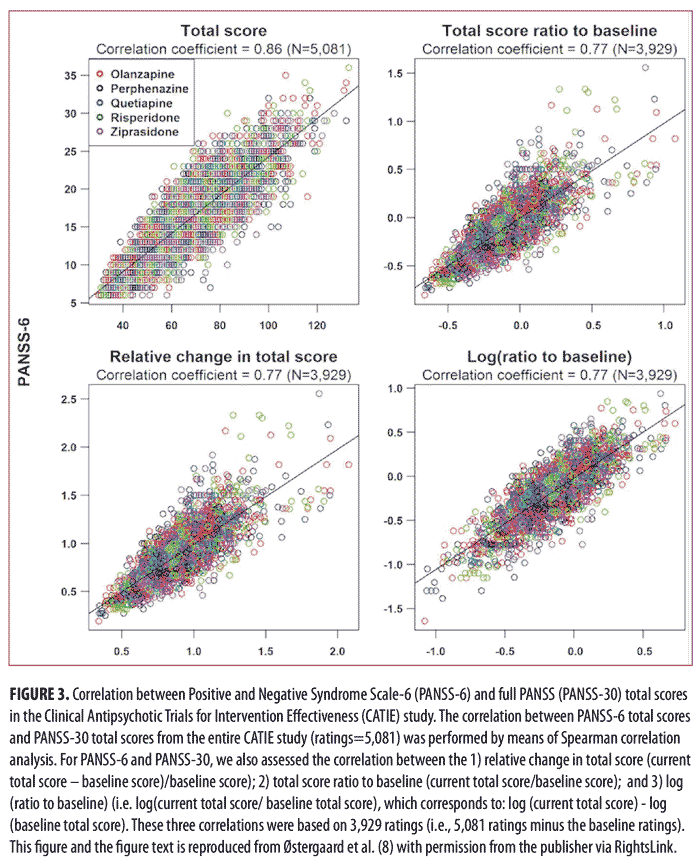
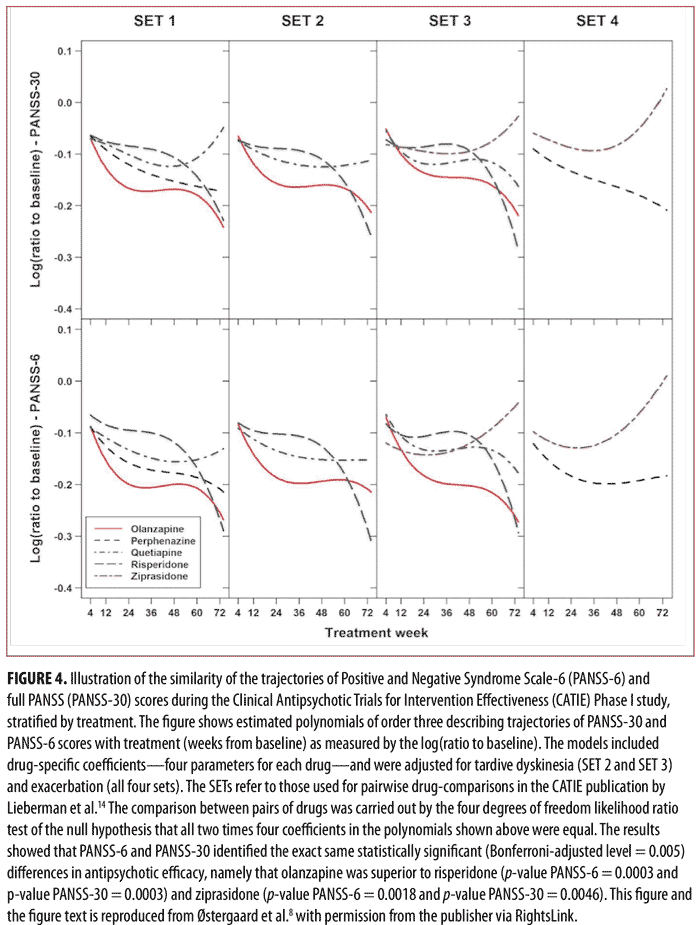
of Intervention Effectiveness (CATIE) study.14 The results of the analysis of the data from CATIE confirmed those from our study in acutely ill patients, namely that PANSS-6 adequately measures symptom severity and antipsychotic efficacy in schizophrenia (Figures 3 and 4).8 Furthermore, this study also established that PANSS-6 can identify symptom remission as defined by the Andreasen et al. expert consensus criteria15 with very high accuracy.8
SNAPSI
Our findings so far suggest that PANSS-6 has the qualities to bridge between research and clinical practice for the following reasons: 1) PANSS-6 has content validity, as it is based upon an expert consensus definition of severity/remission15 and covers core symptoms of schizophrenia;16 2) As opposed to the full 30-item version of PANSS, PANSS-6 is “scalable,” which means that each item adds unique information regarding severity, and the total score is therefore a valid measure of severity; and 3) PANSS-6 is sensitive to changes in the severity of core positive and negative schizophrenia symptoms and can separate the effects of typical and atypical antipsychotics from each other and from that of placebos.7,8 PANSS-6 therefore represents a promising alternative to the full PANSS.
There is, however, a critical limitation to the studies on PANSS-6 that have been conducted so far, namely that PANSS-6 was extracted from the results of studies in which ratings on all 30 PANSS items were obtained.11,12,14 Therefore, it remains an open question as to whether it is possible to obtain sufficient information for PANSS-6 rating via a brief and focused interview, which is a prerequisite for the advantage of PANSS-6 over more comprehensive rating scales, such as the BPRS3 or the full 30-item PANSS.
Until recently, no brief interview with the possibility of extracting the information needed for PANSS-6 rating was available. Additionally, most interview guides are specific to an underlying scale (i.e., built only to gather information necessary to rate a single instrument). There is a need for a highly efficient interview that works in clinical settings, allows clinicians to gather data to support several ratings simultaneously, and is useful across clinical disciplines (e.g., psychiatry, nursing, social work). To meet this need, we have developed the Simplified Negative and Positive Symptoms Interview (SNAPSI). The SNAPSI is an assessment guide that includes probes and structures modeled on both standard semi-structured tools, such as the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders17 and the BPRS, as well as newer tools for the evaluation of negative symptoms, such as the Brief Negative Symptoms Scale (BNSS),18 the Negative Symptoms Assessment (NSA),19 and functional assessments such as the Personal and Social Performance Scale.20 For example, in addition to standard probes for delusional ideas, and hallucinations, SNAPSI contains a section that asks the participant to describe emotional states directly and a section on task sequencing that allows for a direct and more efficient evaluation of thought disorder than passive observation. Additionally, a well-integrated assessment section for caregivers adds an important component, clearly delineating how to evaluate collateral information from third-party sources.
In addition to enabling rating on PANSS-6, SNAPSI can be used to: (1) collect information to rate selected items from the BPRS3; (2) to supplement evaluations of negative symptoms, including those considered in the BNSS18 or the NSA19; and (3) to facilitate standardized rating on global severity rating scales such as the Clinical Global Impression Severity and Improvement Scales.21 In the “feasibility tests” that we have conducted, the final version of the SNAPSI (patient section) has taken approximately 15–25 minutes to administer (by raters who are unfamiliar with the interview and involving patients hearing the questions for the first time). The informal feasibility tests were conducted by seven clinical raters (three medical doctors, one psychologist, and three research assistants) at two hospitals in the United States and one hospital in Denmark, respectively. The raters simply interviewed the patients using the SNAPSI and tested whether they themselves and the patients with schizophrenia or schizoaffective disorder (n = 16 in total) understood the questions—and whether the targeted psychopathology was covered sufficiently to allow for quantitative rating after the interview. The feedback from the feasibility tests led to minor revisions of the SNAPSI. The final version of SNAPSI is freely available for non-commercial clinical and academic use (please email the following address for further information: [email protected]).
Whether valid PANSS-6 ratings can be obtained by means of SNAPSI is an empirical question that we will be addressing in a study to be launched in the fall of 2017. In this study, inpatients with schizophrenia will be interviewed by two independent raters (in a random order). One rater will conduct the SCI-PANSS and subsequently rate the patient on the full 30-item PANSS. The other rater will conduct SNAPSI and rate on PANSS-6. These “sets” of interviews and ratings will be performed two times—as close to admission and as close to discharge, respectively, as possible—to also allow testing of PANSS-6 for sensitivity to change in severity of illness. If the PANSS-6 scores obtained following SNAPSI correspond to the PANSS-6 extracted from the full PANSS, it is safe to assume that the very promising results of the PANSS-6 studies published so far7,8,10 are valid—and that SNAPSI is a valid way of obtaining information on core schizophrenia symptom severity in relation to PANSS-6 rating.
SNAPSI and PANSS-6 May Bridge the Gap Between Research and Clinical Care
One issue that complicates the translation of research findings into clinical care is that in research studies, procedures and outcome measures are very different from those used in clinical care settings.6,22 Because of this, it can be difficult for clinicians to apply research findings to their populations and integrate them into their management of patients. In this context, another potential application of the SNAPSI–PANSS-6 combination is in relation to measurement-based care in real-world settings (i.e., treatment approaches that are guided by quantitative measures of psychopathology). In a recent study, it was demonstrated that measurement-based care significantly improved the treatment of depression as compared to treatment as usual.23 There is no reason to believe that this should be different for the treatment of schizophrenia.6 However, a brief and valid rating scale to measure symptom levels—thereby allowing the treating psychiatrist to make measurement-based treatment choices in collaboration with the patient—has been lacking. As outlined above, we believe that PANSS-6 rating, guided by SNAPSI, is a prime candidate to fill this current void.
References
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276.
- Singh MM, Kay SR. A comparative study of haloperidol and chlorpromazine in terms of clinical effects and therapeutic reversal with benztropine in schizophrenia. Theoretical implications for potency differences among neuroleptics. 1975;43(2):103–113.
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812.
- Best MW, Grossman M, Oyewumi LK, Bowie CR. Examination of the Positive and Negative Syndrome Scale factor structure and longitudinal relationships with functioning in early psychosis. Early Interv Psychiatry. 2016;10(2):165–170.
- Samara MT, Leucht C, Leeflang MM, et al. Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry. 2015;172(7):617–629.
- Correll CU, Kishimoto T, Nielsen J, Kane JM. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther 2011;33(12):B16–B39.
- Østergaard SD, Lemming OM, Mors O, et al. PANSS-6: a brief rating scale for the measurement of severity in schizophrenia. Acta Psychiatr Scand. 2016;133(6):436–444.
- Østergaard SD, Foldager L, Mors O, et al. The validity and sensitivity of PANSS-6 in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Study. Schizophr Bull. 2017 Jun 1.
- Fleischhacker WW. The usefulness of rating scales in patients with schizophrenia. Acta Psychiatr Scand 2016;133(6):435.
- Hochstrasser L, Borgwardt S, Lambert M, et al. Association of Positive and Negative Syndrome Scale short forms with global functioning and quality of life. Acta Psychiatr Scand. 2016;134(6):563–565.
- van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl). 1996;124(1-2):168–175.
- Zimbroff DL, Kane JM, Tamminga CA, et al. Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry. 1997;154(6):782–791.
- Bech P. Clinical Psychometrics. Oxford, UK: Wiley-Blackwell; 2012.
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223.
- Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449.
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629.
- Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305.
- Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptom Assessment. J Psychiatr Res. 1993;27(3):253–258.
- Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329.
- Guy W. Clinical Global Impressions Scale. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare pub no (AMD) 76-338, NIMH, Rockville, MD, USA; 1976.
- Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci. 2011;13(2):155–172.
- Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004–1013.




