 by Philip D. Harvey, PhD; Cynthia O. Siu, PhD; and Antony D. Loebel, MD
by Philip D. Harvey, PhD; Cynthia O. Siu, PhD; and Antony D. Loebel, MD
Dr. Harvey is with the University of Miami Miller School of Medicine in Miami, Florida, and the Research Service of the Bruce W. Carter VA Medical Center in Miami, Florida. Dr. Siu is with COS and Associates Ltd. in Central District, Hong Kong. Dr. Loebel is with Sunovion Pharmaceuticals Inc., in Fort Lee, New Jersey.
Funding: This study was sponsored by Sunovion Pharmaceuticals Inc. The sponsor was involved in the study design and collection of data.
Disclosures: Dr. Harvey serves as a consultant/advisory board member for Allergan, Akili, Boehringer-Ingelheim, Lundbeck, Otsuka Digital Health, Sanofi, Sunovion, and Takeda. Dr. Siu has received funding and consulting fees from Sunovion that support research and the use of lurasidone clinical trial databases for analyses and preparation of this manuscript. She has also received funding and consulting fees from Schizophrenia Program, Centre for Addiction and Mental Health, University of Toronto, Pfizer, Hong Kong Health and Medical Research Grant, and the Chinese University of Hong Kong that support research and the use of clinical trial and genetic databases for analyses, preparation of manuscripts, and data science activities. Dr. Loebel is an employee of Sunovion Pharmaceuticals.
Abstract: Objective: The objective of this post-hoc analysis was to evaluate the effect of lurasidone and quetiapine extended-release (XR) on insight and judgment and assess the longitudinal relationships between improvement in insight and cognitive performance, functional capacity, quality of well-being, and depressive symptoms in patients with schizophrenia. Design: Clinically unstable patients with schizophrenia (N=488) were randomized to once-daily, fixed-dose treatment with lurasidone 80mg, lurasidone 160mg, quetiapine XR 600mg, or placebo, followed by a long-term, double-blind, flexible-dose continuation study involving these agents. Results: Significantly greater improvement in insight and judgment (assessed by the Positive and Negative Syndrome Scale G12 item) for the lurasidone and quetiapine XR groups, compared to the placebo group, was observed at Week 6. Over a subsequent six-month continuation period, the flexible dose lurasidone group showed significantly greater improvement in insight from acute phase baseline compared to the flexible-dose quetiapine XR group (QXR-QXR) (p=0.032). Improvement in insight was significantly correlated with improvement in cognition (p=0.014), functional capacity (p=0.006, UPSA-B), quality of well-being (p=0.033, QWB), and depressive symptoms (p=0.05, Montgomery–Åsberg Depression Rating Scale [MADRS] score) across treatment groups and study periods. Conclusion: In this post-hoc analysis, flexibly dosed lurasidone 40 to 160mg/d was found to be associated with significantly greater improvement in insight compared to flexibly dosed quetiapine XR 200 to 800mg/d over long-term treatment in patients with schizophrenia. Across treatment groups, improvement in insight and judgment was significantly associated with improvement in cognition, functional capacity, quality of well-being, and depressive symptoms over time.
Keywords: Insight, schizophrenia, cognition, functional capacity, quality of well-being, depressive symptoms, lurasidone, quetiapine XR
Innov Clin Neurosci. 2017;14(11–12):23–29
Poor insight, including impairments in awareness of illness, commonly occurs in patients with schizophrenia and represents a major risk factor for poor treatment outcomes.1–8 Reduced insight has been found to be associated with poor treatment adherence;8–11 more severe symptoms of illness;3 and various deficits in cognition, social cognition, and functional performance.4,6,8 Improving insight is therefore a key therapeutic goal for patients with schizophrenia.12
Poor insight in schizophrenia has been linked to reduced awareness of the presence and significance of psychotic symptoms,13,14 impaired self-assessment of cognitive and functional performance,15 deficits in appraising and responding to effort-based tasks,16 and reduced subjective quality of well-being.5,12,17–20 A recent study suggested that the inability to make an accurate self-assessment of cognition and everyday functioning skills was the most influential predictor of real-world functioning compared to clinical symptoms, cognitive performance, or functional capacity.15 Insight impairment can bias assessment of capability in either direction, leading to the possibility of over- or under-estimation of abilities and likely outcomes. In addition, poor insight constitutes a significant barrier to accepting and adhering to treatment.4,6,7–10,12,21 Post-hoc analysis of data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)study in schizophrenia showed that patients with marked psychotic symptoms and insight deficits (as assessed by the Positive and Negative Syndrome Scale [PANSS] item G12), yet who reported being “pleased” or “delighted” with their everyday lives, were more likely to discontinue treatment and have a poorer attitude toward treatment.8
Although lack of insight regarding illness is considered a core feature of schizophrenia, there is a paucity of information, particularly treatment-related data, on the longitudinal relationships between improved insight and neurocognitive performance, functional capacity, quality of well-being, and depressive symptoms over time.
The objective of this post-hoc analysis was to evaluate the short-term and long-term effects of lurasidone and quetiapine extended release (XR) on clinically rated insight and judgment in a six-month, double-blind, continuation period that followed an acute six-week trial. We also evaluated the extent to which treatment-related changes in insight were associated with improvements in cognition, functional capacity, quality of well-being, and depressive symptoms in patients followed for up to six months in the continuation study.
Methods
We conducted a post-hoc analysis based on data from a previously reported randomized, double-blind, six-week, placebo- and active-controlled acute study,22 followed by a one-year, double-blind continuation study.23 Study conduct was consistent with the Declaration of Helsinki and in accordance with Good Clinical Practices as required by the International Conference on Harmonization guidelines. All patients provided written informed consent prior to study enrollment.
Subjects and study treatment. This multiregional study, conducted in the United States and five other countries, enrolled patients with a primary diagnosis of schizophrenia who had recently been hospitalized for an acute exacerbation of psychotic symptoms. Patients who met entry criteria were randomized to receive six weeks of double-blind treatment with once-daily doses of lurasidone (80mg/d or 160mg/d), quetiapine XR (600mg/d), or placebo. Upon completion of the initial six-week study, patients were eligible to receive continued treatment with either flexible-dose lurasidone 40 to 160mg/d (the lurasidone-to-lurasidone cohort) (LUR-LUR) or flexible dose quetiapine XR 200-800mg/d (the quetiapine XR-to-quetiapine XR cohort) (QXR-QXR) in the one-year, double-blind continuation study. Subjects who had been treated with placebo in the initial six-week study were switched in blinded fashion to flexible-dose lurasidone 40 to 160mg/d treatment (the placebo-to-lurasidone cohort) (PBO-LUR). All study medications were taken once daily, in the evening, with food.
Statistical methods. This post-hoc analysis was based on the intent-to-treat sample, which consisted of all patients who received at least one dose of study medication, and had at least one postbaseline PANSS assessment during the 6 weeks in the core study followed by the 6 months in the extension study. The effect of treatment on change in outcome measure from baseline (week 0 in acute phase) was evaluated using Mixed Model for Repeated Measures (MMRM) (33), with fixed effects terms for treatment, baseline score, visit, treatment-by-visit, and study site. Longitudinal relationships between improvement in insight and changes in outcome measures from acute baseline (week 0) to week 6 (end of acute phase) and week 32 (month 6 of extension phase) were assessed based on the regression coefficient (slope) of change score in insight in a mixed-effects longitudinal data analysis (LDA) model.(33) The sign of change in PANSS-item G12 (where higher score indicates greater symptom severity) was reversed so that positive values of change scores represented improvement from baseline, when examining its longitudinal association with improvement of both cognition, UPSA-B and QWB-SA scores (where higher scores indicate better functioning and quality of well-being). For purposes of categorical analysis, an item score of > 4 (moderate severity) was set as the threshold for impaired insight.
Results
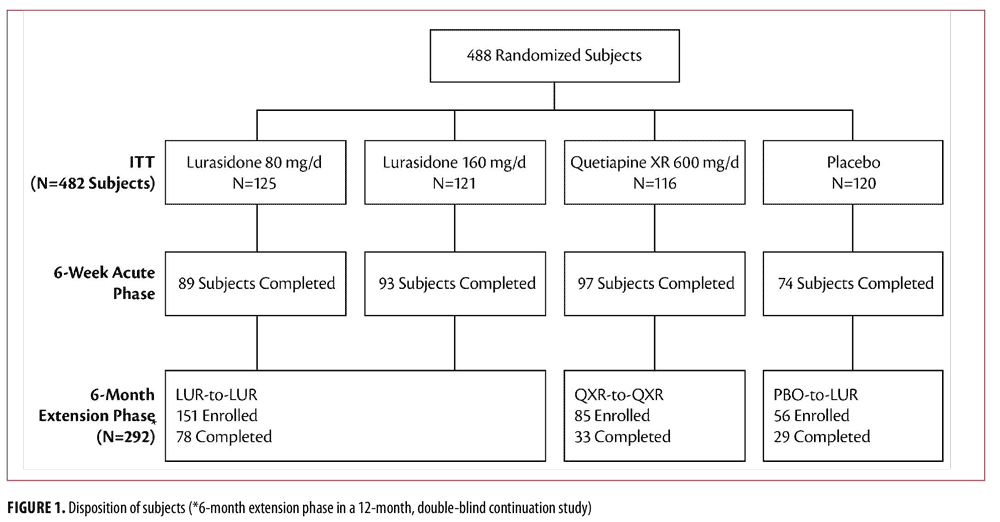
Figure 1 depicts the disposition of subjects who were randomized and completed both treatment periods. Among the 482 subjects with a primary diagnosis of schizophrenia who had recently been hospitalized for an acute exacerbation of psychotic symptoms, impaired insight was found in 287 (59.5%) subjects (PANSS-item G12 item score of at least 4 at acute study baseline). Most patients were male (68.3%) and Caucasian (57.1%), with mean age 37.2 years. Similar clinical characteristics were observed for patients randomized to lurasidone or quetiapine XR at baseline of the randomized, double-blind, acute phase (N=482, overall baseline PANSS score 97.4, G12 item score 3.7, cognitive composite z-score 2.86) and for the cohort who continued throughout the six-month, double-blind extension phase (N=292, baseline PANSS score 97.6, G12 item score 3.8, cognitive composite z-score -2.97). Symptom severity at acute study baseline was similar for the LUR-LUR group (PANSS 97.7, G12 item score 3.78) and the QXR-QXR group (baseline PANSS score 97.9, G12 item score 3.89). In addition, symptom severity at Week 6 (end of acute phase) was comparable for the LUR-LUR group (PANSS 66.7, G12 item score 2.85) and the QXR-QXR group (PANSS 67.8, G12 item score 2.96).
Cross-sectional analysis of acute phase baseline data. In acute phase baseline analyses of 482 patients, more severe insight and judgment impairment (higher PANSS G12 item score) was associated with lower cognitive performance (p<0.001, regression slope= -0.54, standard error [SE]=0.16, t= -3.42, df=420), lower functional capacity (as assessed by the University of California, San Diego Performance-Based Skills Assessment-brief [UPSA-B] score) (regression slope= -2.85, SE= 0.93, p=0.0023, t= -3.06, df=433) and greater uncooperativeness (as assessed by PANSS G8 item) (regression slope=0.29, SE=0.05, p<0.001, t= 6.17, df=435). Higher scores on item G12 were significantly associated with higher probability of failure for completing cognitive testing and/or obtaining valid scores at acute baseline visit (odds ratio [OR]=1.34, p=0.002, chi-square [÷2]=9.385).
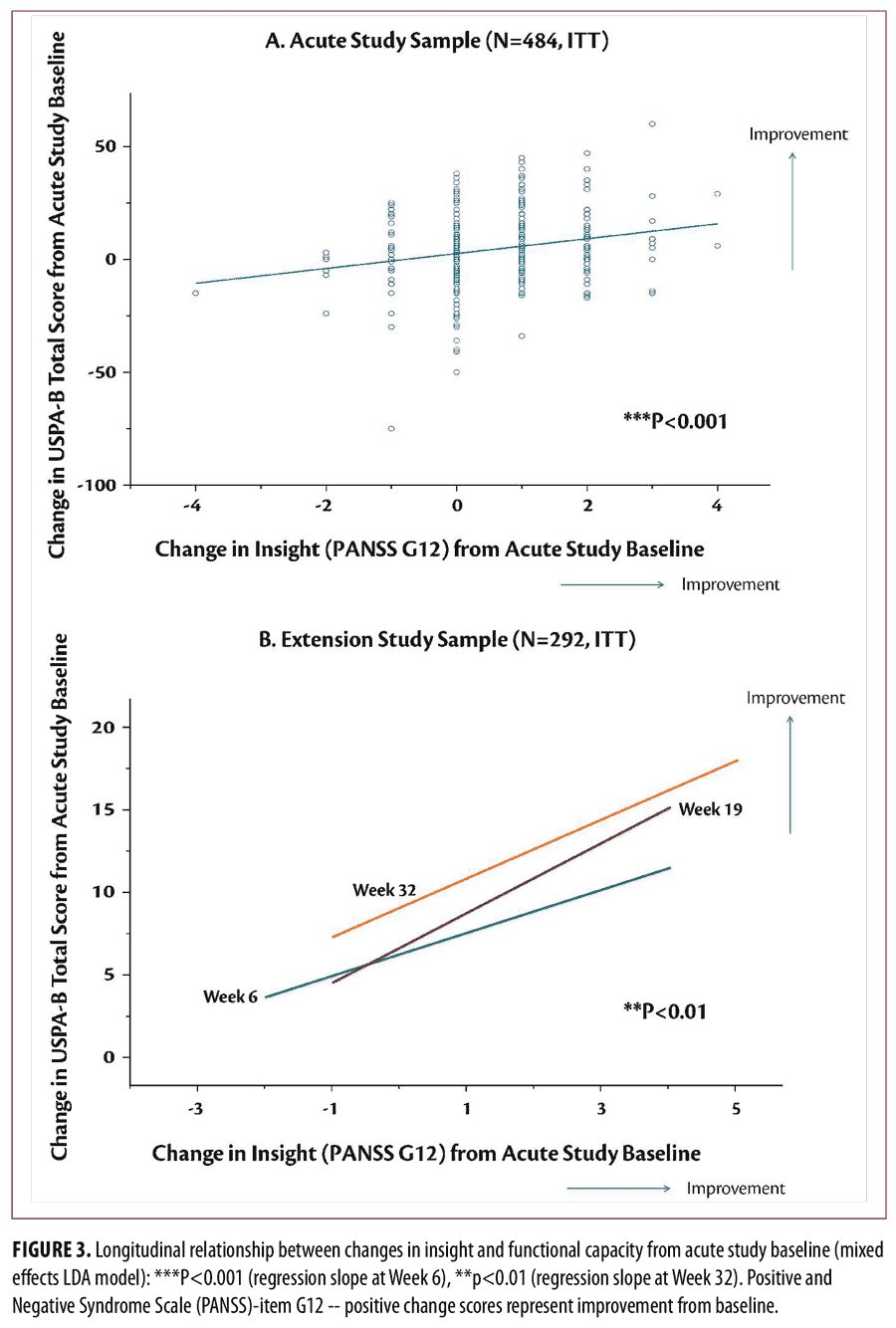
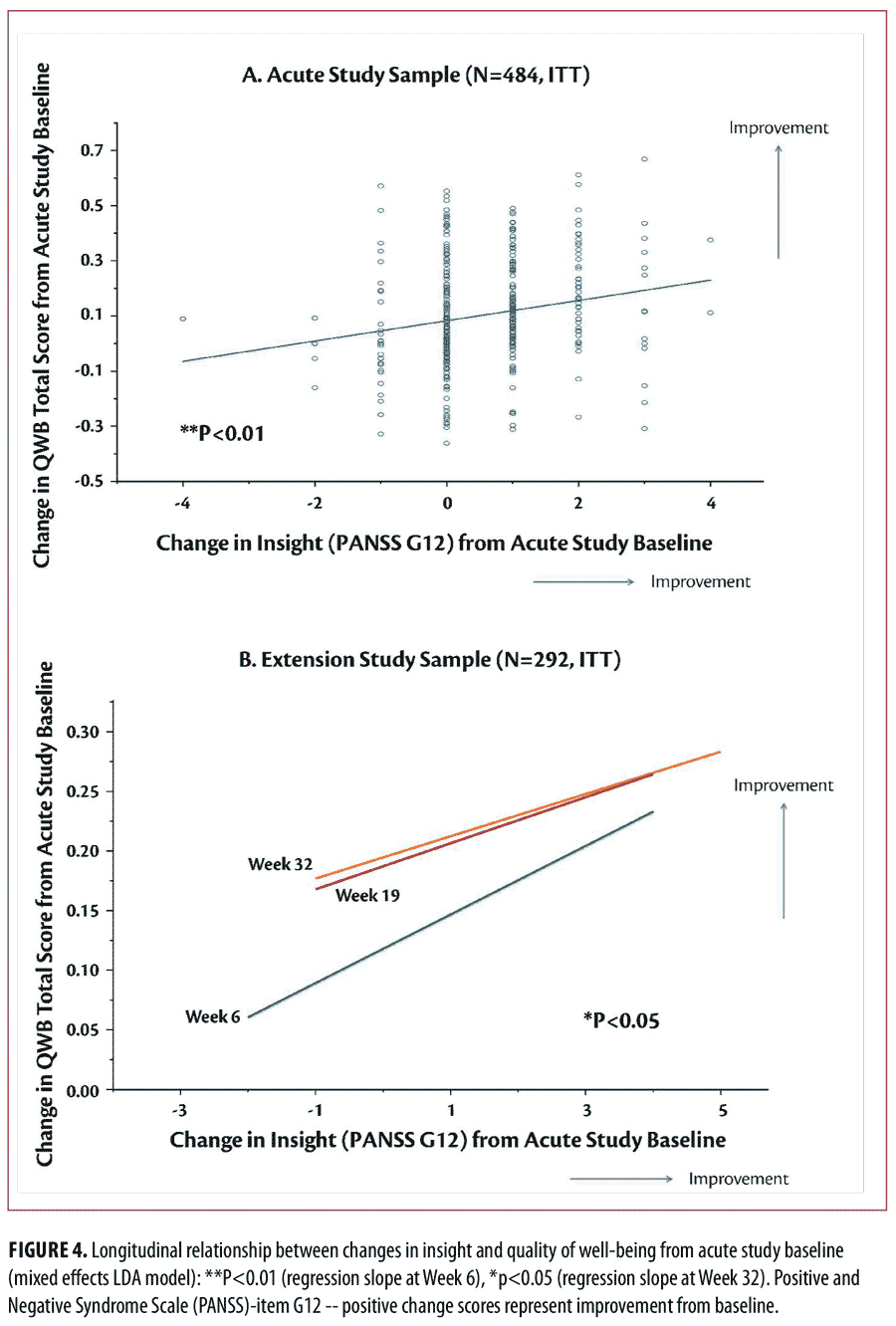
Longitudinal analysis of outcomes. Improvement in “insight and judgment” from acute phase baseline to Week 6 was significantly greater for the lurasidone groups (effect size=0.61 for 160mg/d vs. placebo, p<0.001, t= -4.02, df=434; effect size=0.58 for 80mg/d vs. placebo, p<0.001, t= -3.71, df=434) and the quetiapine XR 600mg/d group (effect size=0.67 vs. placebo, p<0.001, t= -4.44, df=434), compared to the placebo group (Figure 2, top). Treatment-related improvement in “insight and judgment” from baseline to Week 6 was significantly associated with improvement in depressive symptoms (regression slope=1.41, SE=0.28, p<0.001, t=5.00), neurocognitive performance (regression slope=0.42 , SE=0.12, p<0.001, t= 3.49), functional capacity (regression slope=3.31, SE=0.67, p<0.001, t=4.96) (Figure 3, top), and the rater-administered quality of well-being (regression slope=0.024, SE=0.008, p=0.004, t=2.93) in the acute study (Figure 4, top).
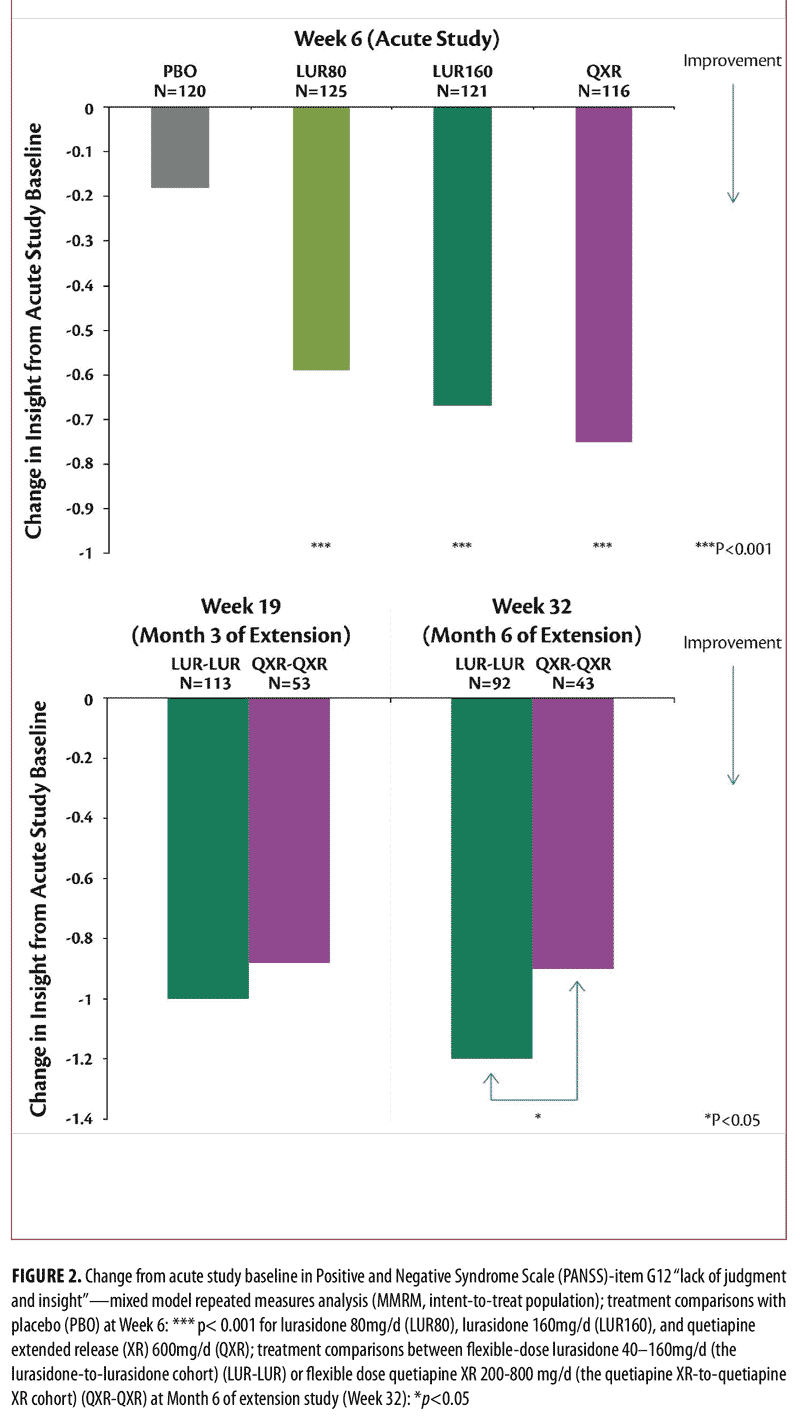
At Month 6 of the double-blind, continuation study (Week 32), the flexible dose lurasidone 40 to 160mg/d group (LUR-LUR) showed significantly greater improvement on PANSS-item G12 “insight and judgment” from acute phase baseline, compared to the flexible-dose quetiapine XR 200 to 800mg/d group (QXR-QXR) (effect size=0.36, p=0.032, t=2.16, df=226) (Figure 2, bottom). At Week 32 (Month 6 of the continuation study), improvement in PANSS total score (effect size=0.55, p=0.001, t=3.32, df=226), PANSS positive subscale score (effect size=0.43, p=0.010, t=2.60, df=226), and PANSS negative subscale score (effect size=0.41, p=0.014, t=2.47, df=226) was significantly greater in the lurasidone 40 to 160mg/d group (LUR-LUR) compared to the quetiapine XR 200 to 800mg/d group (QXR-QXR). Improvement in “insight and judgment” from acute phase baseline significantly mediated reduction in PANSS total score (p<0.001), PANSS positive (p<0.001) and negative (p<0.001) subscale scores with lurasidone 40 to 160mg/d (LUR-LUR) and quetiapine XR 200 to 800mg/d (QXR-QXR) treatment.
Treatment-related improvement in “insight and judgment” (PANSS-item G12 score) from acute phase baseline to Week 32 was significantly associated with improvement in depressive symptoms (regression slope= 0.40, SE= 0.20, p=0.05, t=1.95), neurocognitive performance (regression slope=0.29, SE=0.12, p=0.014, t=2.47), functional capacity (regression slope=2.05, SE=0.73, p=0.006, t=2.79, Figure 3, bottom), and the rater-administered quality of well-being (regression slope=0.02, SE=0.008, p=0.033, t=2.15, Figure 4, bottom) across treatment groups and study periods.
These relationships between change in insight and change in functional capacity and depressive symptoms were not statistically significant when both PANSS-G12 item score and psychopathology (as assessed by PANSS total and subscale measures) were included in the same mixed linear model. The difference in change in “insight and judgment” for the LUR-LUR and QXR-QXR groups was not independent of overall change in psychopathology (as assessed by PANSS total and subscales).
Discussion
In this post-hoc analysis involving patients with schizophrenia, lurasidone and quetiapine XR treatment groups demonstrated significant improvement in insight and judgment compared to placebo at the six-week study endpoint (assessed using the PANSS G12 item). Lack of insight and judgment was significantly associated with inability to validly complete neurocognitive testing at acute study baseline. Furthermore, significant associations were found across all treatment groups for improvement in insight with cognitive performance (assessed by the CogState composite score), functional capacity (assessed by UPSA-B), depressive symptoms (assessed by MADRS), and quality of well-being (assessed by rater-administered Quality of Well-Being Scale Self-Administered [QWB-SA] scale) over the double-blind, six-week acute study period.
Long-term improvement in insight and judgment, as well as schizophrenia symptom severity, was significantly greater for lurasidone 40 to 160mg/d compared to quetiapine XR 200 to 800mg/d assessed over a double-blind, six-month continuation treatment period that followed the six-week, acute treatment study. In the current analysis, significant longitudinal associations between long-term improvement in insight and functional outcomes, as well as reduction in depressive symptoms, were found across treatment groups. These results are consistent with analyses showing significant association between change in insight, medication attitudes, symptoms, and functioning in previous studies,5,34,40 including our reports that lurasidone 40 to 160mg/d improved cognition from baseline more than quetiapine XR 200 to 800mg/d in the six-month continuation study, independently of treatment related improvement in symptoms.34,35 These results raise the possibility that lurasidone might enhance everyday functioning (vs. placebo) through a set of related outcomes, including symptom reduction, increased cognitive performance, increased ability to perform tests of functional capacity, and increased awareness of current level of functioning, mediated in part by improvements in insight and judgment.
Insight impairment (as assessed by PANSS-item G12 or other multidimensional measures) is a core feature of schizophrenia and other psychotic disorders, and hence an integral part of clinical status. Several published factor analyses have shown that insight regarding illness is not an independent psychopathological factor41,42 and is closely related to disorganized symptoms.40–42 The PANSS-item G12 “insight and judgment” is a component of PANSS and contributed to the overall PANSS score. Consistent with previous studies,5,12,40 the improvement in “insight and judgment” observed with lurasidone and quetiapine XR treatment over the acute and continuation studies in this analysis was not independent of the general change in psychopathology (as assessed by changes in PANSS total score and PANSS positive and negative subscale scores).
The causality and directionality of changes in insight and overall improvement in clinical status, however, are not clear. It seems evident that patients lacking insight and awareness of illness might not see the need for treatment, might not attribute symptoms properly to their illness, and might have difficulty assessing the effects of symptoms on subjective measures of quality of life (QoL) and functioning.8,12,40 A post-hoc analysis of the large-scale CATIE trial found that lack of insight and judgment at pre-treatment baseline moderated the relationships among subjective QoL, symptom severity, everyday functioning, and treatment dropout in schizophrenia.8 These CATIE study findings supported the presence of a subgroup of patients with marked psychiatric symptoms, poor insight, low self-reported depressive symptoms, and impairment in cognitive functioning. Patients in this subgroup were also less cooperative and more likely to discontinue treatment.8 In our analysis of acute phase baseline data, we identified a similar trend showing that patients with an acute exacerbation of schizophrenia and impairment in insight tended to be uncooperative, have greater impairment in cognition, and perform poorly on objective measures of cognition and functional capacity. This post-hoc analysis represents the first longitudinal study to demonstrate that treatment-related improvement in insight is significantly associated with better performance on objective measures of cognition, functional outcomes, and health-related quality of life and reduction in depressive symptoms in patients with schizophrenia.
A recent meta-analysis12 confirmed that insight “is a potential therapeutic target and that it is amenable to improvement.” The meta-analysis also made the striking observation that there were almost no randomized trials of psychosis, except one two-year study,43 that reported separately the effects of antipsychotics on change in insight. Given that reduced insight has been found to be associated with poor treatment adherence and poor outcomes,5,12,15,17–20 there is an important need to assess and report insight as a separate, targeted outcome in controlled treatment studies.
Limitations. The use of the single PANSS-item G12 for measuring insight and judgment is a limitation of this study. This one-item measure of insight and judgment has, however, demonstrated a robust psychometric relationship with the more global Insight and Treatment Attitudes Questionnaire (ITAQ) measure as assessed in the CATIE trial (Spearman rank correlation r=0.49, p<0.001, N=1232).8 Sanz et al also reported that PANSS insight and judgment item (G12) had concurrent validity with three other common measures of illness insight in schizophrenia,25 including ITAQ (r=0.904),2 Schedule for the Assessment of Insight ([SAI], r=0.884; SAI-expanded version [E], r=0.895),13 and Berrios and Markova’s scale.28 The validity of PANSS-item G12 for for the assessment of insight and judgment in patients with schizophrenia was supported in this study by the item’s significant cross-sectional (at baseline) and longitudinal (both six weeks and six months) associations with objective assessments of cognitive performance, function and quality of well-being outcomes, that were observed in the current analysis.8 The statistically significant separation from placebo on improvement in PANSS-item G12 score in the treatment groups (lurasidone 80mg/d, lurasidone 160mg/d, and quetiapine XR 600mg/d) in the acute phase, as well as significant separation between LUR-LUR and QXR-QXR at Week 32 in the extension phase, demonstrated the ability of this single PANSS-item G12 to detect score change associated with treatment effect. This analysis presented here confirms the results of previous studies that the single PANSS-item G12 (which has been shown to have robust psychometric relationships with global measures of illness insight in patients with schizophrenia) can detect clinically meaningful score changes associated with treatment effect.
It should also be noted that the evaluations of long-term effects of lurasidone and quetiapine XR on change from acute phase baseline (Week 0) in “insight and judgment” and functional outcomes were based on subjects who had completed the six-week acute phase and participated in the six-month, double-blind continuation study. Our findings showed that the demographic and clinical characteristics for randomized subjects were similar between treatment groups and comparable to the completers of the acute phase, suggesting minimal impact of possible selection bias due to dropout over the acute or continuation study phases.
In summary, in this post-hoc analysis of a placebo-controlled schizophrenia trial followed by a double-blind, continuation study, patients treated with flexibly dosed lurasidone 40 to 160mg/d demonstrated significant improvement in insight and judgment compared to those treated with quetiapine-XR 200 to 800mg/d at Week 32 (six months) (end of the double-blind, continuation study). In addition, our findings suggest that treatment-related improvement in insight and judgment from acute baseline were associated with better performance on objective measures of cognition, functional outcomes, health-related quality of life, and reduction in depressive symptoms across the treatment groups over the six-week acute phase and a six-month, double-blind continuation treatment period. Further research is warranted to examine the extent to which impaired illness awareness among patients with schizophrenia can be ameliorated by treatment intervention and the effect of improvement in insight on long-term patient outcomes.
References
- Amador XF, Strauss DH, Yale SA, et al. Awareness of illness in schizophrenia. Arch Gen Psychiatry. 1994;51:826–836.
- McEvoy JP, Joy-Apperson L, Appelbaum P, et al. Insight in schizophrenia: its relationship to acute psychopathology. J Nerv Ment Dis. 1989;177:43–47.
- Schwartz RC. Insight and illness in chronic schizophrenia. Compr Psychiatry. 1998;39:249–254.
- Keshavan M, Rabinowitz J, DeSmedt G, et al. Correlates of insight in first episode psychosis. Schizophr Res. 2004;70:187–94.
- Mohamed S, Rosenheck R, McEvoy J, et al. Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes. Schizophr Bull. 2009;35:336-346.
- Harvey PD, Helldin L, Bowie CR, et al. Performance-based measurement of functional disability in schizophrenia, a cross-national study in the United States and Sweden. Am J Psychiatry. 2009;166:821–827
- Cavelti M, Beck EM, Kvrgic S, et al. The role of subjective illness beliefs and attitude toward recovery within the relationship of insight and depressive symptoms among people with schizophrenia spectrum disorders. J Clin Psychol. 2012;68:462–476.
- Siu C, Harvey PD, Agid O, et al. Insight and subjective quality of life in chronic schizophrenia. Schizophr Res Cogn. 2015;2:127–132.
- Kemp R, David A. Insight and compliance. In: Blackwell B (ed.). Treatment Compliance and the Treatment Alliance in Serious Mental Illness. The Netherlands: Harwood Academic;1997:61–86.
- Olfson M, Marcus SC, Wilk J, et al. Awareness of illness and non-adherence to antipsychotic medications among persons with schizophrenia. Psychiatr Serv. 2006;57:205–211.
- Arango C, Amador X. Lessons learned about poor insight. Schizophr Bull. 2011;37:27–28.
- Pijnenborg GHM, Donkersgoed RJM, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophrenia Research. 2013;144: 109–117.
- David AS. Insight and psychosis. Br J Psychiatr. 1990;156:798–808.
- Parellada M, Boada L, Fraguas D, et al. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull. 2011;37:38–51.
- Gould F, McGuire LS, Durand D, et al. Self-assessment in schizophrenia: accuracy of evaluation of cognition and everyday functioning. Neuropsychology. 2015;29:675–682.
- Reddy LF, Horan WP, Barch DM, et al. Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 1-psychomatric characteristics of 5 paradigms. Schizophr Bull. 2015;44:1045–1054.
- Sabbag S, Twamley EM, Vella L, et al. Assessing everyday functioning in schizophrenia: Not all informants seem equally informative. Schizophr Res. 2011; 131:250–255.
- Kurtz MM, Tolman A. Neurocognition, insight into illness and subjective quality-of-life in schizophrenia, what is their relationship? Schizophr Res. 2011;127:157–162.
- Tolman A, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38:304–315.
- Sellwood W, Morrison AP, Beck R, et al. Subjective cognitive complaints in schizophrenia, relation to antipsychotic medication dose, actual cognitive performance, insight and symptoms. PLoS One. 2013;8: https://doi.org/10.1371/journal.pone.0083774. Accessed June 11, 2017.
- Cuffel BJ, Alford J, Fischer EP, et al. Awareness of illness in schizophrenia and out-patient treatment compliance. J Nerv Ment Dis. 1996;184:653–659.
- Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101–109.
- Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, continuation study. Schizophr Res. 2013;147:95–102.
- Lysaker P, Bell M. Insight and cognitive impairment in schizophrenia: performance on repeated administrations of the Wisconsin Card Sorting Test. J Nerv Ment Dis. 1994;182:656–660.
- Sanz M, Constable G, Lopez-Ibor I, et al. A comparative study of insight scales and their relationship to psychopathological and clinical variables. Psychol Med. 1998;28:437–466.
- Roseman AS, Kasckow J, Fellows I, et al. Insight, quality of life, and functional capacity in middle-aged and older adults with schizophrenia. Int J Geriatr Psychiatry. 2008;23:760–765.
- Misiak B, Frydecka D, Besz?ej JA, et al. Effects of antipsychotics on insight in schizophrenia: results from independent samples of first-episode and acutely relapsed patients. Int Clin Psychopharmacol. 2016;31:185–91.
- Markova IS, Berrios GE. The assessment of insight in clinical psychiatry: a new scale. Acta Psychiatrica Scandinavica. 1992;86:159–164.
- Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState schizophrenia battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31:848–859.
- Mausbach BT, Harvey PD, Goldman SR, et al. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007; 33:1364–1372.
- Kaplan RM, Sieber WJ, Ganiats TG. The quality of well-being scale: comparison of the interviewer-administered version with a self-administered questionnaire. Psychol Health. 1997;12;783–791.
- Seiber WJ, Groessl EJ, David KM, et al. Quality of Well Being Self-administered (QWB-SA) scale: user’s manual https://hoapucsdedu/qwb-info/QWB-Manual.pdf. 2008. Accessed June 11, 2017.
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New Jersey: John Wiley and Sons, Inc; 2004.
- Harvey PD, Siu CO, Hsu J, et al. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;3:1373–1382.
- Harvey PD, Siu CO, Ogasa M, Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: post-hoc analysis of a long-term, double-blind continuation study. Schizophr Res. 2015;166:334–338.
- Wilson WH, Ban TA, Guy W. Flexible system criteria in chronic schizophrenia. Compr Psychiatry J. 1986;27:259–265.
- Amador XF, Strauss DH. Poor insight in schizophrenia. Psychiatr. 1993;Q64:305–318.
- Lysaker PH, Buck KD, Salvatore G, et al. Lack of awareness of illness in schizophrenia, conceptualizations, correlates and treatment approaches. Expert Rev Neurother. 2009; 9:1035–1043.
- Durand D, Strassnig M, Sabbag S, et al. Factors influencing self-assessment of cognition and functioning in schizophrenia, implications for treatment studies. Eur Neuropsychopharmacol. 2015;25:185–191.
- Lincoln TM, Lullmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia: a systematic review. Schizophr Bull. 2007;33: 1324–1342.
- Xavier RM, Pan W, Dungan JR, et al. Unraveling interrelationships among psychopathology symptoms cognitive domains and insight dimensions in chronic schizophrenia. Schizophr Res. 2017; in press.
- Hopkins SC, Ogirala A, Loebel A, Koblan KS. Transformed PANSS factors intended to reduce pseudospecificity among symptom domains and enhance understanding of symptom change in antipsychotic-treated patients with schizophrenia. Schizophr Bull. 2017; in press.
- McEvoy JP, Johnson J, Perkins D, et al. Insight in first-episode psychosis. Psychol Med. 2006;36: 1385–1393.




