 by Philip D. Harvey, PhD; Anzalee Khan, PhD; and Richard S. E. Keefe, PhD
by Philip D. Harvey, PhD; Anzalee Khan, PhD; and Richard S. E. Keefe, PhD
Dr. Harvey is Professor of Psychiatry and Behavioral Sciences at University of Miami School of Medicine in Miami, Florida. Dr. Khan is Senior Biostatistician at NeuroCog Trials and holds an appointment in the Psychopharmacology Research Program at the Nathan S. Kline Institute for Psychiatric Research in Orangeburg, New York. Dr. Keefe is an employee of NeuroCog Trials and Professor of Psychiatry and Behavioral Sciences at Duke University Institute for Brain Sciences in Chapel Hill, North Carolina.
Funding: The collection of the data in this study was supported by NIMH grants MH63116, MH78775, and MH93432.
Disclosures: Dr. Harvey has served as a consultant to AbbVie, Allergan, Akili, Boehringer Ingelheim, Forum Pharmaceuticals, Genentech, Lundbeck Pharmaceuticals, Minerva Pharma, Otsuka Digital Health, Roche Pharma, Sanofi, Sunovion, and Takeda Pharmaceutical during the past three years. He has a research grant from Takeda. Dr. Khan currently or in the past three years has received funding support and honoraria from, served as consultant for, and served as speaker for the National Institute of Mental Health, Research Foundation for Mental Hygiene, Inc., the New York State Office of Mental Health, Qatar Foundation Weill Cornell Medicine and/or eResearch Technologies. She also holds positions at NeuroCog Trials, Nathan S. Kline Institute for Psychiatric Research, and Manhattan Psychiatric Center. Dr. Keefe currently or in the past three years has received investigator-initiated research funding support from the Department of Veteran’s Affairs, the National Institute of Mental Health, and the Singapore National Medical Research Council. He has received honoraria and served as a consultant, speaker, or advisory board member for Abbvie, Acadia, Akebia, Akili, Alkermes, Astellas, Asubio, Avanir, AviNeuro/ChemRar, Axovant, Biogen, Boehringer-Ingelehim, Cerecor, CoMentis, FORUM, Global Medical Education (GME), GW Pharmaceuticals, Intracellular Therapeutics, Janssen Pharmaceutica, Lundbeck, MedScape, Merck, Minerva Neurosciences Inc., Mitsubishi, the Moscow Research Institute of Psychiatry, Neuralstem, Neuronix, Novartis, the New York State Office of Mental Health, Otsuka, Pfizer, Reviva, Roche, Sanofi, Sunovion, Takeda, Targacept, the University of Moscow, the University of Texas Southwest Medical Center, and WebMD. Dr. Keefe also receives royalties from the BACS testing battery, the MATRICS Battery (BACS Symbol Coding), and the Virtual Reality Functional Capacity Assessment Tool (VRFCAT), and is a shareholder in NeuroCog Trials, Inc. and Sengenix.
Abstract: Background: Reduced emotional experience and expression are two domains of negative symptoms. The authors assessed these two domains of negative symptoms using previously developed Positive and Negative Syndrome Scale (PANSS) factors. Using an existing dataset, the authors predicted three different elements of everyday functioning (social, vocational, and everyday activities) with these two factors, as well as with performance on measures of functional capacity. Methods: A large (n=630) sample of people with schizophrenia was used as the data source of this study. Using regression analyses, the authors predicted the three different aspects of everyday functioning, first with just the two Positive and Negative Syndrome Scale factors and then with a global negative symptom factor. Finally, we added neurocognitive performance and functional capacity as predictors. Results: The Positive and Negative Syndrome Scale reduced emotional experience factor accounted for 21 percent of the variance in everyday social functioning, while reduced emotional expression accounted for no variance. The total Positive and Negative Syndrome Scale negative symptom factor accounted for less variance (19%) than the reduced experience factor alone. The Positive and Negative Syndrome Scale expression factor accounted for, at most, one percent of the variance in any of the functional outcomes, with or without the addition of other predictors. Implications: Reduced emotional experience measured with the Positive and Negative Syndrome Scale, often referred to as “avolition and anhedonia,” specifically predicted impairments in social outcomes. Further, reduced experience predicted social impairments better than emotional expression or the total Positive and Negative Syndrome Scale negative symptom factor. In this cross-sectional study, reduced emotional experience was specifically related with social outcomes, accounting for essentially no variance in work or everyday activities, and being the sole meaningful predictor of impairment in social outcomes.
Keywords: Schizophrenia, negative symptoms, factor analysis
Innov Clin Neurosci. 2017;14(11–12):18–22
Negative symptoms in schizophrenia have been described since the early days of psychiatry. These symptoms reflect the absence of putatively normal processes, such as motivation, emotion, communication, and experience. Despite several scientific advances in the fields of genetics, biology, and psychopharmacology in relation to the epidemiology, diagnosis, and treatment of schizophrenia, individuals with enduring negative symptoms have significantly poor everyday functioning and overall poor quality of life.1–4 Along with cognitive impairments and related impairments in the ability to perform everyday functional skills, negative symptoms are among the most important contributors to disability. A lack of agreement and consistency in the construct of negative symptomology has impeded research efforts in the field. Primarily, 55 years after the Brief Psychiatric Rating Scale (BPRS)5 was developed, 33 years after the Scale for the Assessment of Negative Symptoms (SANS)6 defined negative symptoms, and 30 years after the Positive and Negative Syndrome Scale (PANSS)7 identified a negative symptom subscale and yielded multiple factor structures, uncertainties about the symptoms that comprise the negative symptom dimension and their optimal assessment8-9 still exist.
Recent research has suggested that negative symptoms can be considered in terms of two different dimensions: diminished expression (expressive deficit) and reduced experience (experiential deficit).10–13 Expression includes displays of facial affect (referred to as blunted affect), reduced vocal inflection, and reduced vocal output. Experience includes the motivation to engage in potentially pleasurable activities and the subjective experience of enjoyment when engaging in reinforcing activities. This second dimension has also been referred to as reflecting avolition and apathy, as a descriptor of the observable consequences of deficits in experience. These two domains have been reported to be quite separable, with factor analyses suggesting that experience and expression are separate factors.14 Importantly, different clusters of patients can be identified whose primary presentations reflect diminished expression, reduced experience, or low levels of negative symptoms.15 Thus, these domains of negative symptoms appear quite robust and disinct.
Studies examining the prediction of functional outcomes in schizophrenia have suggested that social functioning, everyday activities, and vocational and other productive outcomes might have different determinants. Specifically, deficits in cognition and functional capacity appear to predict residential and vocational outcomes better than social outcomes.16 Cross-sectionally, negative symptoms appear to be more strongly related with social outcomes, with additional contributions from social cognition and social competence.17 However, some of the research on domains of negative symptoms has suggested that reduced experience (i.e., avolition-asociality) has a more potent impact on social outcomes than reduced expression.18 Thus, studies in which overall scores on negative symptoms are found to predict social outcomes might not capture the specifics of prediction as precisely as possible. In fact, in our research, overall scores on negative symptoms accounted for less variance in social outcomes than did two of the symptoms on the PANSS typically seen to reflect reduced experience: active and passive social avoidance.
We present the results of a study using PANSS-derived factors reflecting reduced expression and experience from the study of Khan et al.19a We took these factors and then used them to predict three different aspects of functional outcome in a dataset on which we had previously published.16 Our primary aim is to assess the relative prediction potential of PANSS expression deficits and PANSS experience deficits in comparison with each other and as compared with the combined negative symptom factor (PANSS NSF) for the prediction of social, vocational, and everyday activities in a large and well-assessed sample of people with schizophrenia. In that previous study,16 the best fitting predictor model was that overall negative symptoms predicted social outcomes, but not vocational or residential outcomes, and that cognition and functional capacity predicted vocational and residential outcomes, but not social functioning.
Methods
The sample of patients and their assessments was previously reported by Strassnig et al.16 We will review the details of the previous study briefly.
Participants. The data are part of four study cohorts collected in five different geographical areas within the United States, aimed at identifying the course and correlates of change in functional status as well as the optimal method for rating everyday functioning among schizophrenia outpatients.
The study participants were patients (n=821) with schizophrenia or schizoaffective disorder receiving treatment at one of several different outpatient service delivery systems in Atlanta, Dallas, Miami, San Diego, and the city of New York. All research participants provided signed informed consent per standards approved by the responsible local Institutional Review Boards. These data were collected between March 2003 and May 2014.
All enrollees completed a structured diagnostic interview, administered by a trained interviewer. The Structured Clinical Interview for the DSM (SCID19) was used at the Atlanta sites, the Mini International Neuropsychiatric Interview, 6th Edition20 was used in Dallas, San Diego, and Miami, and the Comprehensive Assessment of Symptoms and History (CASH21) was used in New York; all diagnoses were verified in local consensus procedures. Patients were excluded if they had a history of traumatic brain injury, brain disease such as seizure disorder or neurodegenerative condition, a reading score below the sixth grade in all samples, or the presence of another Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis that would exclude the diagnosis of schizophrenia. These procedures were described in previous publications.1,22–24
Assessment strategy. Real world functioning. Real world functioning was rated with the SLOF25 across all study cohorts. Across the studies, ratings were generated by a high-contact clinician, either a case manager, a residential facility manager, or a psychotherapist who stated that they knew patient “very well.” The original SLOF was abbreviated to assess five functional domains from which we selected the following domains to be examined in all studies: interpersonal functioning (e.g., initiating, accepting, and maintaining social contacts; effectively communicating), independent participation in everyday activities (e.g., shopping, using telephone, paying bills, use of leisure time, use of public transportation), and vocational functioning (e.g., employable skills, level of supervision required to complete tasks, ability to stay on task, completes tasks, punctuality). The dependent variables for the statistical analyses were the scores on these three different subscales.
Negative Symptoms Assessment. Severity of negative symptoms was assessed using PANSS,7 which was administered in its entirety by trained raters who did not perform the functional outcomes ratings or performance-based assessments.
Cognition. As described in the previous paper,16 slightly different cognitive batteries were used, reflecting the development of cognitive assessment in schizophrenia culminating in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus cognitive battery (MCCB26). As this is only a secondary focus in this study, we provide some minimal details and refer the reader to the previous publication.16 We developed a cognitive performance latent trait, using the common tests across the samples. These included overlapping tests of processing speed, verbal fluency, working memory, verbal learning, and memory. We chose to model a single latent trait because of the limited set of cognition measures and the previous findings that these measures had previously been found to be the major contributors to a unifactorial factor structure in a large sample of patients with schizophrenia.27 We used that previously modeled latent trait as our indicator of cognitive performance.
Functional capacity. The brief version of the UCSD Performance-based Skills Assessment (UPSA-B) was used to assess functional capacity. Participants performed everyday tasks related to communication and finances.28 During the Communication Roleplay subtest, participants perform tasks using a telephone (e.g., making an emergency call; dialing a number from memory; calling to reschedule a doctor’s appointment). For the Finance subtest, participants count change, read a utility bill, and write and record a check for the bill. The UPSA-B requires approximately 10 minutes to complete, and raw scores are converted into a total score ranging from 0 to 100. Higher scores indicate better functional capacity.
Negative symptom models. As described in Khan et al,19a a two-factor model of expression and experience was developed and replicated in multiple samples. The items in each of the factors were as follows:
PANSS expression: PANSS Blunted Affect (N1), Poor Rapport (N3), Lack of Spontaneity (N6), and Motor Retardation (G7),
PANSS experience: Emotional Withdrawal (N2), Passive Social Withdrawal (N4) and active social avoidance (G16).
In order to create factors for regression modeling, we took the items in each factor and used unrotated principal components analysis to create a single principal component for each of the two factor domains.
Data analyses. We adopted a regression based approach. We predicted each of the three SLOF functional domains with a stepwise entry approach, entering both negative symptom subfactor scores. We then repeated the analysis with the single PANSS NSF “Marder factor” for comparison purposes. Our goal was to examine shared variance between negative symptoms and functional outcomes. After those analyses, we added functional capacity and the cognition as the latent trait to the best fitting regression model predicting each of the everyday functional domains in order to determine iin order to determine if a more homogenous approach to the assessment of negative symptoms broadened the power of negative symptoms for the prediction of nonsocial functioning.
Results
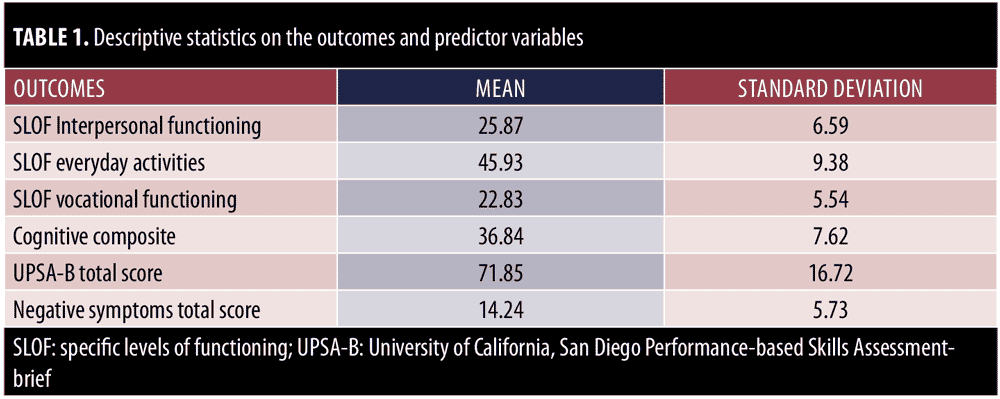
Severity scores on the three functional domains are presented in Table 1, along with the scores for the predictor variables. The negative symptoms factor scores were derived with principal component analysis, so these scores have no direct interpretability. In this study, as we did not use full information methods, we excluded cases that were missing information on any of the variables; the resulting sample size was 630 cases. We compared the demographic information for these cases to the 191 cases with at least one missing observation. There were no significant differences between the current sample and the previous sample. The PANSS reduced emotional experience factor and the PANSS reduced expression factor were significantly correlated with each other in this dataset (r=0.30, p<0.001).
In Table 2, we present the results of the stepwise regression analyses for the two PANSS factor scores and regression analysis for the overall PANSS negative symptom factor. For interpersonal functioning, the overall regression analysis was significant when the two PANSS negative symptoms factors were entered into the analysis [F (2,627)=75.99, p<0.001]. As can be seen in Table 2, the PANSS experience factor accounted for 21 percent of the variance, with the PANSS expression factor not entering the equation. When predicting everyday activities, the overall regression analysis was not significant [F (2,627)=1.03, p=0.36]. For the prediction of vocational activities, the overall analysis was significant, [F (2,627)=4.44, p<0.05]. The PANSS expression factor entered the equation and predicted one percent of the variance in vocational outcomes.

When examining the overall negative symptoms factor, the pattern of significant and nonsignificant overall results was the same. As can be seen in Table 2, total negative symptoms predicted 19 percent of the variance in interpersonal functioning and three percent of the variance in vocational outcomes.
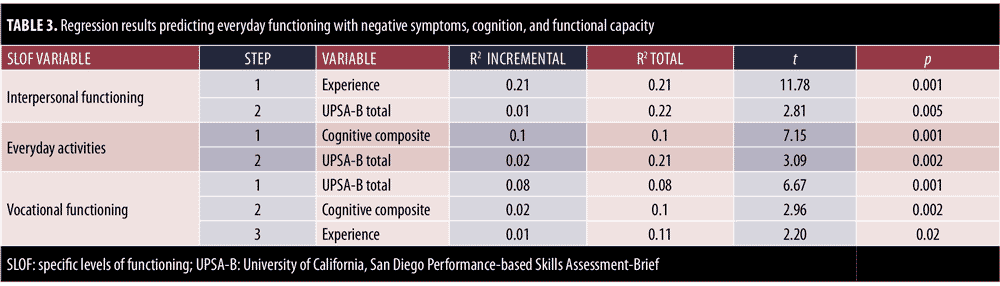
In the next set of analyses, we added two other predictors, composite cognitive performance, and UPSA-B total scores, to the two negative symptom factors to predict the three SLOF subscales. For the prediction of interpersonal functioning, the overall analysis was significant [F (4,625)=37.45, p<0.001]. In the stepwise results, the PANSS experience factor again accounted for 21 percent of the variance in social outcomes, with UPSA-B scores adding one percent of the variance. When predicting everyday activities, the overall analysis was significant [F (4,625)=30.86, p<0.001]. As noted in Table 3, neither of the PANSS negative symptoms factors entered the equation, and the cognitive performance composite and UPSA-B scores combined to account for 12 percent of the variance in everyday activities. Finally, when predicting work functioning, the overall regression was again significant [F (4,625)=19.72, p<0.001]. UPSA-B scores and cognitive composite scores accounted for 10 percent of the variance in work outcomes, while the PANSS experience negative factor added an incremental one percent of variance.
Discussion
A benefit of our approach in this study was the use of the two-factor negative symptom solution, rather than relying on single PANSS NSF items in the model. Reduced experience and reduced expression as negative symptom factors had clearly different patterns of prediction of everyday functioning. Social functioning appears to be related to experience-related negative symptoms, while expression-related symptoms showed no correlation. PANSS experience-related negative symptoms predicted social outcomes slightly more efficiently than total negative symptoms (PANSS NSF). In only one analysis was expression-related symptoms associated with functional outcomes, accounting for one percent of the variance in vocational outcomes. However, separation of negative symptoms into experience-related and expression-related components did not increase the cross-sectional predictability of activities and functioning by negative symptoms measures.
There are implications with the use of the PANSS in the assessment of negative symptoms. Although the PANSS was not designed to separate reduced experience and reduced expression symptoms, these two factors seem to have clear discriminant validity in this study. This finding suggests that the PANSS can adequately serve as an assessment measure in trials with an eventual goal of improving social outcomes when separating the PANSS expressive-related and experience-related subfactors.
Expression-related symptoms, although more apparently pathological and obvious, do not contribute to indices of disability in this study. Much like flagrant psychotic symptoms, obvious affective flattening appears to be less functionally significant than symptoms more related to motivational factors. Treatment efforts seem better directed toward experience-related symptoms, targeting increases in motivation.
Limitations. The limitations of the study include its cross-sectional design and lack of attempt to stratify on the basis of negative symptom severity. Negative symptoms predict other outcomes longitudinally, particularly when the studies begin early in the course of illness.29 As the goal of these analyses was to test the ability of the PANSS to define separable dimensions of negative symptoms, we made no attempt to perform additional negative symptoms assessments. Positive symptoms had already been shown to fail to predict any aspects of outcome in this large database.
Conclusion
In conclusion, a factor from the PANSS defined by three items was at least as efficient as the total negative symptom score in predicting everyday social outcomes and showed clear separation from symptoms of reduced expression in predicting impairment in social outcomes in people with schizophrenia. This delineation did not improve the prediction of other aspects of outcome, suggesting a reasonably specific correlation between social impairment and symptoms of reduced emotional experience as measured by the PANSS and associated motivational deficits in people with schizophrenia.
Acknowledgments
The authors would like to acknowledge the following authors of the previous study that served as the database for these analyses: Martin T. Strassnig, Tenko Raykov, Cedric O’Gorman, Christopher R. Bowie, Samir Sabbag, Dante Durand, Thomas L. Patterson, Amy Pinkham, and David L. Penn.
References
- Bowie C., Leung WW, Reichenberg, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–511.
- Strauss GP, Sandt AR, Catalano LT, Allen DN. Negative symptoms and depression predict psychological well-being in individuals with schizophrenia. Compr Psychiatry. 2012;53(8):1137–1144.
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr., Marder S R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219.
- Quinlan T, Roesch S, Granholm E. The role of dysfunctional attitudes in models of negative symptoms and functioning in schizophrenia. Schizophr Res. 2014;157(1-3):182–189.
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):149–165.
- Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132(2-3):140–145.
- Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305.
- Blanchard JJ, Cohen A S. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245.
- Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369.
- Jang JK, Choi HI, Park S. et al. A two-factor model better explains heterogeneity in negative symptoms: evidence from the positive and negative syndrome scale. Front Psychol. 2016;7:707.
- Kring AM, Gur R E, Blanchard JJ, et al. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165–172.
- Strauss GP, Hong LE, Keller WR, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1-3):96–98.
- Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatric Res. 2013;47(6):783–789.
- Strassnig, MT, Raykov T, O’Gorman C, et al. Determinants of different aspects of everyday outcome in schizophrenia: the roles of negative symptoms, cognition, and functional capacity. Schizophr Res. 2015;165(1):76–82.
- Kalin M, Kaplan S, Gould F, et al. Social cognition, social competence, negative symptoms and social outcomes: inter-relationships in people with schizophrenia. J Psychiatric Res. 2015;68:254–260.
- Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr Bull. 2006;32(2):246–249.
- Khan A, Liharska L, Harvey PD, et al. Positive and Negative Syndrome Scale (PANSS) negative symptom dimensions across geographical regions: implications for social, Llnguistic, and cultural consistency. Innov Clin Neurosci. 2017;14(11–12)xx–xx
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). New York, NY: New York Psychiatric Institute, Biometrics Research; 1997.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1999;59 Suppl 20:22–33;quiz 34–57.
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing psychopathology and diagnosis. Arch Gen Psychiatry. 1992;49(8):615–623.
- Harvey PD, Raykov T, Twamley EW, et al. Validating the measurement of real-world functional outcome: Phase I results of the VALERO study. Am J Psychiatry. 2011;168(11):1195–2001.
- Durand D, Strassnig M, Sabbag S, et al. Factors influencing self-assessment of cognition and functioning in schizophrenia: implications For treatment studies. Eur Neuropsychopharmacol. 2015;25(2):185–191.
- Pinkham A, Penn DL, Green MF, et al. The Social Cognition Psychometric Evaluation study: results of the expert survey and RAND panel. Schizophr Bull. 2014;40(4):813–823.
- Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983;19(3):9–21.
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213.
- Keefe RSE, Blder RM, Harvey, PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. 2006;31(9):2033–2046.
- Mausbach BT, Harvey PD, Goldman SR, et al. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33(6):1364–1372.
- Ventura J, Subotnik KL, Gitlin MJ, et al. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr Res. 2015;161(2–3):407-411.




