Innov Clin Neurosci. 2025;22(7–9):18–23.
by Robert G. Bashuk, MD; Stephen L. Scranton, MD, FACP; Robert S. Allen, AuD, and Marco Cecchi, PhD
Drs. Bashuk and Allen are with the Concussion Center in Atlanta, Georgia. Dr. Scranton is with Brain Fitness Centers of Florida in Clearwater, Florida. Dr. Cecchi is with Cognision in Louisville, Kentucky.
FUNDING: No funding was provided for this article.
DISCLOSURES: Marco Cecchi is an employee of Cognision. No writing assistance was utilized in the production of this manuscript. The other authors declare no conflicts of interest relevant to the content of this article.
Patients with concussion often receive insufficient and/or ineffective diagnostic workups. A limiting factor for prompt and accurate diagnosis is the scarcity of practical and effective concussion diagnostics that can be used by private-practice physicians. This gap in the diagnostic process can delay the implementation of crucial interventions. The absence of an affirmative concussion diagnosis leaves patients with few options when follow-up treatment would otherwise be indicated, potentially leading to worsening symptoms, prolonged recovery, and a higher likelihood of developing long-term complications. Neurophysiological assessments such as electroencephalography (EEG) and event-related potentials (ERPs) offer an opportunity for an objective evaluation of brain deficits that can occur after a concussion. By detecting abnormalities in brain patterns that are often associated with concussive injuries, these tests can help provide timely diagnosis and treatment of the disorder, thus reducing the likelihood of chronification of post-concussive symptoms. We reviewed a battery of neurophysiological assessments that have been scientifically validated to detect the pathophysiological effects of concussion and can be performed in office settings by nonspecialist technicians. These assessments are designed to help with diagnosis and prognosis of concussion, while also being accessible and practical to administer for private-practice physicians who can incorporate them into routine evaluations of patients with traumatic brain injury (TBI). The implementation of neurophysiological tools in primary care and outpatient settings has the potential to bridge the gap between symptom presentation and definitive diagnosis, thus mitigating the risk for long-term adverse outcomes. Keywords: Concussion, TBI, event-related potentials, EEG, diagnosis, prognosis, office settings
The Centers for Disease Control and Prevention (CDC) defines a concussion as: “A type of traumatic brain injury—or TBI—caused by a bump, blow, or jolt to the head or by a hit to the body that causes the head and brain to move rapidly back and forth. This sudden movement can cause the brain to bounce around or twist in the skull, creating chemical changes in the brain and sometimes stretching and damaging brain cells.”1 The severity of a TBI can range from “mild” (ie, a transient change in mental status or consciousness) to “severe” (ie, an extended period of unconsciousness or memory loss after the injury). Mild TBIs, commonly referred to as concussions, are the most common2 and challenging for clinicians to evaluate.
Current guidelines for the diagnosis of concussion have recently been delineated by the American Congress of Rehabilitative Medicine Diagnostic Criteria for Mild Traumatic Brain Injury.3 It is worth noting that the primary clinical role of neuroimaging is to “rule out head and brain injuries that might require neurosurgical or other medical intervention in an acute care setting.”3 Indeed, at present, there are no radiological investigations that can diagnose a concussion.4–8 A recent review article in the Cleveland Clinical Journal of Medicine titled “Concussion: Evaluation and Management” states that “Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that might cause similar symptoms but require different management.”9 There is also little evidence that a standard neurological examination or traditional cognitive testing can reliably detect the neuropathological effects of brain injury after a mild concussion.10 Finally, there are currently no blood biomarkers to diagnose a concussion. While blood biomarkers are available that can determine the need to perform a computed tomography (CT) scan, there are significant time restraints in performing these tests, as they are approved to be administered within 24 hours of the injury.11
The use of inadequate protocols for concussion diagnosis can lead to a failure to recognize the condition and/or provide poor diagnostic accuracy in a significant portion of patients. According to a 2023 University of Pittsburgh Medical Center study, 50 percent of concussions go undetected or unreported.12
While the majority of concussions improve within one month, 15 to 30 percent of patients develop persistent postconcussion symptoms (PPCS), previously known as postconcussion syndrome.13 PPCS is a nociplastic disorder with a time-dependent process that can potentially be interrupted. Multiple studies have demonstrated that early, proactive care significantly improves concussion outcomes.13 Preventing PPCS is a major concern in concussion management. Thus, there needs to be urgency in the diagnosis of concussion, so that tailored interventions can be applied promptly to prevent the neuroplastic changes that are responsible for pain chronification.
The purpose of this article is to review neurophysiological assessments that have been scientifically validated to detect the pathophysiological effects of concussion and can be performed in office settings.
In 2017, the American College of Occupational and Environmental Medicine (ACOEM) published a 1,027-page consensus guideline that recommends (or explicitly does not recommend) specific diagnostic modalities for concussion based on scientifically reported evidence.14
Audiometry testing. Studies have shown that some form of hearing loss is common after a minor head injury.15 There are two primary causes of postconcussive hearing loss: mechanical damage and/or neurological damage.16
If an injury affects the delicate hearing structures, the ear might not effectively transmit sound to the brain. This is the most common cause of hearing loss after head injury and can often be detected by performing a pure-tone audiometry test and reviewing the resultant audiogram (Figure 1). However, if there is synaptic damage to the auditory cortex, the brain might no longer be able to properly process sounds. Therefore, even if the ear’s anatomical structures function normally, a patient can still experience hearing problems. This presentation can often be identified in patients reporting hearing issues while having a normal audiogram.
The AECOM guideline recommendations for audiometry testing are as follows:
[T]here is a high incidence of audiological deficits in head-injured subjects. Peripheral and central auditory areas are affected as revealed by the subjective as well as electrophysiologic auditory investigation.14
An accurate audiogram result is also essential in performing a valid event-related potential (ERP) test (see ERP discussion below).
Electroencephalography (EEG) testing. Common head injuries not only result in macroscopic damage, such as tissue injury, bone fractures, and bleeding; mild injuries can also induce microscopic damage to cellular and synaptic structures in the brain. These cellular-level injuries can disrupt electrolyte homeostasis, trigger the release of excitatory neurotransmitters and other molecules with cytotoxic effects, and elevate the metabolic demand for neuronal repair and equilibrium restoration.17 The pathologic consequences can manifest as diverse concussion symptoms that cannot be detected by a CT or other imaging modalities.
A well-studied modality to monitor diffuse brain function is electroencephalography (EEG). EEG is a recording of cortical function measured as voltage potentials on the surface of the scalp. This modality can be an important tool in examining and evaluating a wide range of brain networks and processes that might be impaired in a concussion.
A recent review on the application of EEG technology to concussion diagnosis in clinical practice states:
Electroencephalography (EEG) is a well-suited technology for the evaluation of [mild TBI (mTBI)]. This low-cost technology is rapid, portable, and easily deployed in multiple clinical settings. The development of computerized quantitative analysis (qEEG) has made this technology sensitive and specific to mTBI both in the acute and convalescent setting.18
Among the most useful qEEG analytical approaches, spectral analysis is of particular interest in the study of concussion and postconcussive symptoms.19 In this analysis, the raw EEG is transformed into frequency versus power and plotted as a frequency spectrum (Figure 2). The power and frequency of the electrical energy generated by groups of cortical neurons varies with the level of synaptic/neuronal damage and with the integrity of the thalamocortical circuits in which they participate (ie, injury to and/or dysfunction of those circuits results in a shift to slower frequencies and lower power recorded at scalp electrodes).
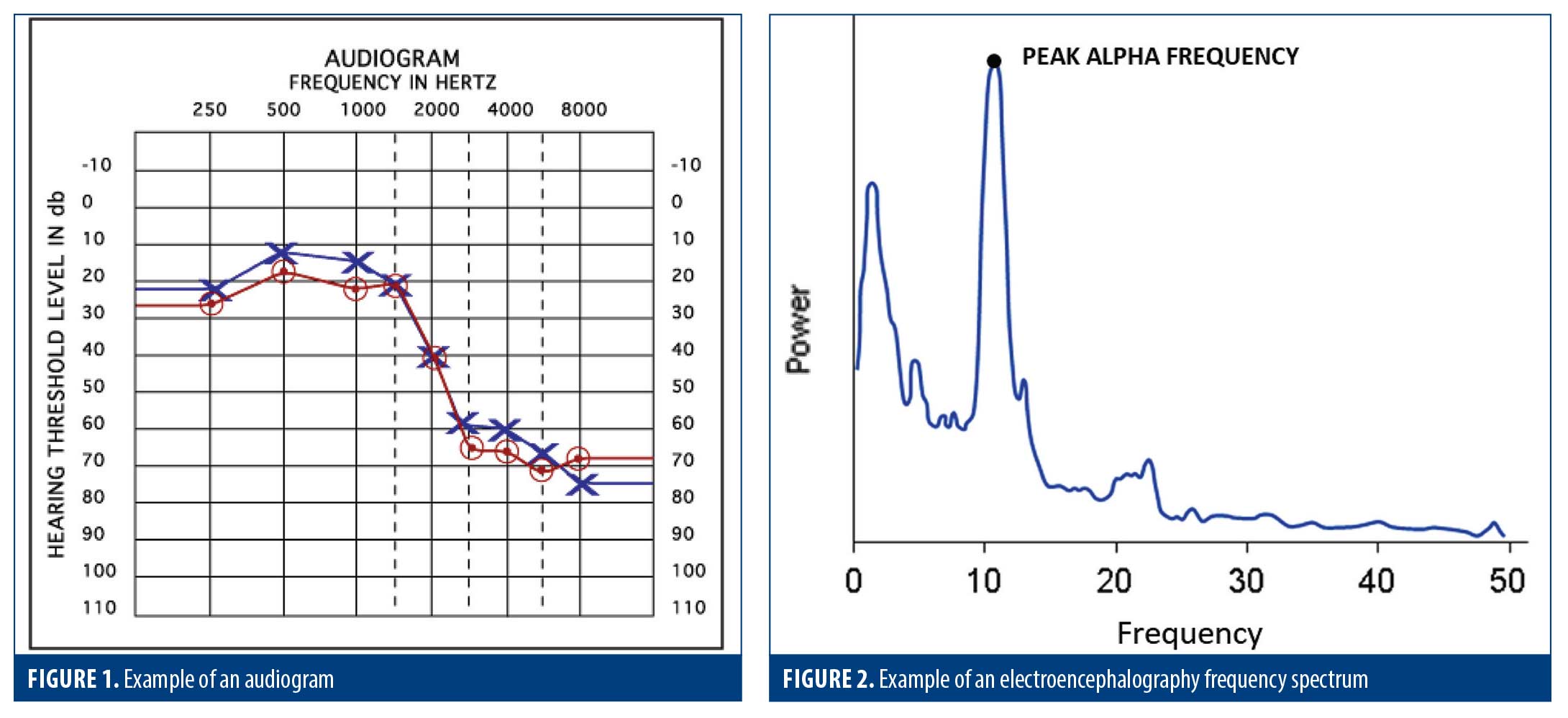
The same qEEG spectral analysis can also be a useful prognostic tool in predicting recovery from concussion:
[A]lteration of the EEG alpha power dynamics in conjunction with balance data in the acute phase of injury with respect to baseline measures may predict the rate of recovery from a single concussive blow.20
Cognitive ERP testing. ERPs are the part of the EEG generated by sensory and cognitive processing of external stimuli (for an overview of the ERP technique, see Luck21). Most often, these external stimuli are auditory, in which cases a pure-tone audiometry test, as described above, is necessary to ensure that the patient has sufficient hearing acuity to perform the ERP procedure.
At the end of the ERP test, the time-locked EEG recordings are averaged according to stimulus type, and all brain activity not related to the specific stimulus group is filtered out. What is left are the ERP waves that represent the neurophysiologic responses evoked by each stimulus type played during the test (Figure 3).
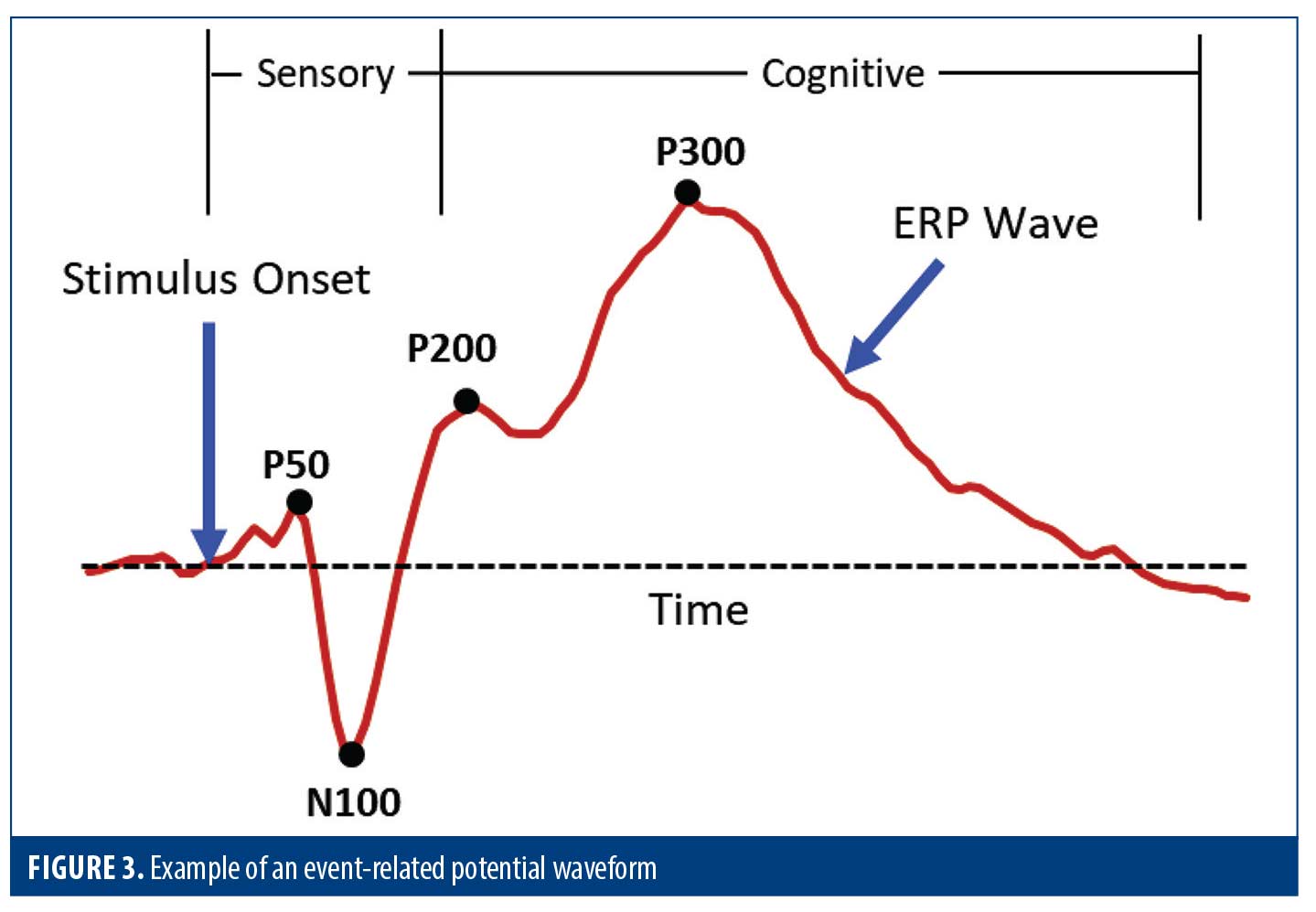
The ERP waveforms contain a series of positive and negative peaks (such as P50, N100, P200, and P300) that have been extensively characterized in the scientific literature.22 The early peaks are primarily “sensory” responses that depend largely on the physical parameters of the stimulus. The sensory responses are followed by “cognitive” peaks, which reflect information processing.10
ERPs have been used to elucidate and characterize sensory and cognitive deficits associated with brain injury and disease since the early 1980s.23 A large body of scientific literature on the clinical utility of these biomarkers demonstrates that ERPs offer significant diagnostic utility to investigate sensory deficits, cognitive impairment, and motor dysfunction associated with concussion.24–27 These diagnostic domains are referenced in several of the recommended testing modalities from the ACOEM guidelines, including attention, executive function, and reaction time.14 Additional clinically relevant brain-based biomarkers, such as speed of brain processing28–30 and network activation asynchronies associated with white matter damage,31 can also be measured using ERPs.
ERPs are especially important in the detection of subtle deficits in information processing in patients who present with otherwise normal clinical findings.24–26, 32 While most of the scientific literature reports on the diagnostic utility of ERPs for concussion, there is strong evidence to support postinjury prognosis as well.26
Of particular importance for the assessment of concussion is the active auditory oddball ERP paradigm.26 In this paradigm, an infrequent (target) tone is played occasionally during a stimulus sequence of frequent (standard) stimuli. A third unexpected (distractor) tone can also be present. The test subject is instructed to respond by pressing a button on the handset as fast as they can when the infrequent target tone is heard, and reaction time is quantified.
The auditory oddball paradigm generates ERP waveforms with peaks, including the N200 and P300 (P3a and P3b) that reflect aspects of information processing involved in stimulus discrimination, evaluation, and categorization.22 The morphology of these peaks is sensitive to cognitive deficits associated with concussion. The reaction times of the button-press responses also provide sensitive measures of cognitive and motor networks within the brain and are sensitive to concussion-related injury to the cortex.33 Additional details on the “cognitive” peaks from the active auditory oddball ERP paradigm and their involvement in concussion are discussed in Box 1.34–56
As has been described previously, standard ERP testing evaluates attention, executive function, and reaction time, all of which are testing domains that are recommended in the ACOEM guidelines.14 The guidelines assert that cognitive ERP testing “has evidence of diagnostic efficacy and is recommended for diagnosis of cognitive impacts of TBI.”
Concerning attention, the ACOEM guidelines state:
Recent studies have shown that various aspects of attention are affected following TBI, especially after severe TBI. These deficits include the ability to attend to and encode information, information processing speed, maintain focus, shift attention, attention span, supervisory attentional control, focused/selective attention, and sustain attention. [Tests of attention m]ay be used to target specific cognitive rehabilitation strategies. [They m]ay help to determine the end of healing and extent of residual deficits, if any.14
Concerning tests of executive function, the AECOM guidelines state:
[Executive function testing i]s not invasive, has no adverse events, is low cost, has some evidence of diagnostic efficacy, and is thus recommended for evaluation of TBI patients. [It c]an identify and measure executive function difficulties, potentially allowing better tailoring of therapy(ies) to address any deficits.14
Concerning reaction time, the AECOM guidelines suggest a supportive role for diagnosis of TBI, stating that reaction time testing “[i]s low cost, has evidence of diagnostic efficacy, and is recommended for diagnosis of TBI.”14
Timing and frequency of neurophysiological evaluations. ACOEM guidelines recommend ERP testing for baseline evaluation of TBI, and to possibly “evaluate progress and/or residual cognitive deficits.”14 Indications for discontinuations include “[s]ufficient recovery, plateau, [and] end of healing.”14 A neurophysiological assessment should be performed soon after a concussion to promptly detect and quantify abnormalities in brain function.
After an initial baseline evaluation, the frequency of follow-up testing is ultimately the decision of the treating clinician based on the patient’s clinical course. While some patients might not require further studies, follow-up assessment may be appropriate at 90 or 180 days, or even 1 to 3 years after the initial study, based on clinical progression and clinician’s judgment. Ultimately, each case should be evaluated independently, with follow-up testing conducted only if it is expected to provide valuable clinical and/or management information.
A major factor limiting patient access to effective concussion diagnostics and therapy is the scarcity of practical, ancillary, or supportive concussion diagnostics that can be used by private-practice physicians. This requires technologies that are effective, affordable, and can be operated by nonspecialist technicians in a standard office environment.
Over the past few years, a number of companies have introduced practical, United States Food and Drug Administration (FDA)–cleared products that can detect neurophysiological deficits in patients with brain injury. One clinically validated product is the COGNISION® System (from Neuronetrix Solutions, LLC dba Cognision).57,58 This turnkey system includes all necessary hardware and software to order, perform, analyze, and report on a battery of neurodiagnostic procedures that can help physicians affirmatively detect abnormal brain activity caused by a concussion. With this system, a physician can perform audiometry, EEG, and ERP testing during a single one-hour session. Furthermore, these tests can be performed in a standard office environment with minimal patient discomfort, making these procedures practical to perform by private-practice physicians or their clinical technicians.
This battery of tests provides objective physiologic measures of various brain functions, including auditory processing, attention, working memory, executive function, and reaction time. These brain processes are sensitive measures of abnormal brain function that can provide objective evidence of injury.
At the end of the testing session, a patient report is produced that graphically displays an audiogram, EEG frequency spectrum, and ERP waveforms, along with a table of the important measures numerically quantified from each test. At this point, the physician can add their clinical interpretations of the reported biomarkers along with overall clinical findings that will be included in the patient report.59 Since the measures are objectively quantified, they can be useful in tracking a patient’s recovery over time.26
Conclusion
Many patients suffering from concussion receive insufficient and ineffective diagnostic workups. This is primarily due to the difficulty of directly evaluating complex brain processes, especially when the injury mostly involves cellular and synaptic structures within the brain.
The use of office-based neurophysiological assessments can assist in a more objective diagnosis of cognitive dysfunction in patients with concussion. This, in turn, can potentially lead to earlier treatment, thus reducing or resolving the cognitive dysfunction that occurs with acute concussion and significantly reducing the likelihood of PPCS.
References
- Centers for Disease Control and Prevention. U.S. Department of Health & Human Services. 2019 [cited 2023 Sep 6]. p. 1–3 TBI: Get the Facts | Concussion | Traumatic Brain Injury | CDC Injury Center. Available from: https://www.cdc.gov/traumatic-brain-injury/about/index.html
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention; 2003.
- Silverberg ND, Iverson GL; ACRM Brain Injury Special Interest Group Mild TBI Task Force members. The American Congress of Rehabilitation Medicine diagnostic criteria for mild traumatic brain injury. Arch Phys Med Rehabil. 2023;104(8):1343–1355.
- Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802.
- Gallagher CN, Hutchinson PJ, Pickard JD. Neuroimaging in trauma. Curr Opin Neurol. 2007;20(4):403–409.
- Hurley RA, McGowan JC, Arfanakis K, Taber KH. Traumatic axonal injury: novel insights into evolution and identification. J Neuropsychiatry Clin Neurosci. 2004;16(1):1–7.
- McAllister TW, Sparling MB, Flashman LA, Saykin AJ. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol. 2001;23(6):775–791.
- Neil J, Miller J, Mukherjee P, Hüppi PS. Diffusion tensor imaging of normal and injured developing human brain – a technical review. NMR Biomed. 2002;15(7–8):543–552.
- Stillman A, Alexander M, Mannix R, et al. Concussion: Evaluation and management. Cleve Clin J Med. 2017;84(8):623–630.
- Gosselin N, Bottari C, Chen JK, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg Focus. 2012;33(6):E7:1–7.
- Rauchman SH, Pinkhasov A, Gulkarov S, et al. Maximizing the clinical value of blood-based biomarkers for mild traumatic brain injury. Diagnostics (Basel). 2023;13(21):3330.
- University of Pittsburgh Medical Center. Concussion causes, symptoms, and treatment. Reviewed 12 Mar 2025. Accessed 25 Jun 2025. https://www.upmc.com/services/sports-medicine/services/concussion/about/facts-statistics
- Kureshi S, Mendizabal M, Francis J, Djalilian HR. Conservative management of acute sports-related concussions: a narrative review. Healthcare (Basel). 2024;12(3):289.
- American College of Occupational and Environmental Medicine. Traumatic Brain Injury. Editor-in-Chief: Hegmann KT; Evidence-based Practice Panel Chair: Parks P. Elk Grove Village, IL: Reed Group, Ltd; 2017. Available at: https://new.mdguidelines.com/Resources/ACOEM-Practice-Guidelines/Disorders/Traumatic-Brain-Injury.
- Bansal S, Preetam C, Patnaik A, Sahu RN. Assessment of hearing loss in minor head injury: a prospective study. Asian J Neurosurg. 2022;17(4):595–599.
- Alpsoy MY, Sönmez S, Orhan Z, et al. Evaluation of patients with post-traumatic hearing loss: a retrospective review of 506 cases. J Int Adv Otol. 2021;17(3):239–44.
- Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66.
- Kerasidis H, Simmons J. Quantitative EEG analysis in clinical practice: concussion injury. Clin EEG Neurosci. 2021;52(2):114–118.
- Thornton KE. The role of the quantitative EEG in the diagnosis and rehabilitation of the traumatic brain injured patients. In: Slobounov SM, Sebastianelli WJ (eds). Concussions in Athletics: From Brain to Behavior. Springer; 2014:345–361.
- Slobounov S, Sebastianelli W, Hallett M. Residual brain dysfunction observed one year post-mild traumatic brain injury: combined EEG and balance study. Clin Neurophysiol. 2012;123(9):1755–1761.
- Luck SJ. An Introduction to the Event-Related Potential Technique, second edition. The MIT Press; 2014.
- Key APF, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Dev Neuropsychol. 2005;27(2):183–215.
- Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal cortex lesions on event-related potentials during auditory selective attention. Electroencephalogr Clin Neurophysiol. 1981;52(6):571–582.
- Broglio SP, Moore RD, Hillman CH. A history of sport-related concussion on event-related brain potential correlates of cognition. Int J Psychophysiol. 2011;82(1):16–23.
- Dockree PM, Robertson IH. Electrophysiological markers of cognitive deficits in traumatic brain injury: a review. Int J Psychophysiol. 2011;82(1):53–60.
- Duncan CC, Summers AC, Perla EJ, et al. Evaluation of traumatic brain injury: brain potentials in diagnosis, function, and prognosis. Int J Psychophysiol. 2011;82(1):24–40.
- Rapp P, Keyser DO, Albano A, et al. Traumatic brain injury detection using electrophysiological methods. Front Hum Neurosci. 2015;9:11.
- Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on mild traumatic brain injury. J Rehabil Med. 2004;36(Suppl 43):61–75.
- Washnik NJ, Anjum J, Lundgren K, Phillips S. A review of the role of auditory evoked potentials in mild traumatic brain injury assessment. Trends Hear. 2019;23:233121651984009.
- Brush CJ, Ehmann PJ, Olson RL, et al. Do sport-related concussions result in long-term cognitive impairment? A review of event-related potential research. Int J Psychophysiol. 2018;132(Pt A):124–134.
- Maruta J, Mallott JM, Sulioti G, et al. Concussion disrupts normal brain white matter microstructural symmetry. Front Neurol. 2020;11:548220.
- Levy-Reis I. Use of ERP markers in patients with whiplash and concussion with cognitive complains. J Neurol Sci. 2017;381:757.
- Eckner JT, Kutcher JS, Broglio SP, Richardson JK. Effect of sport-related concussion on clinically measured simple reaction time. Br J Sports Med. 2014;48(2):112–118.
- Patel SH, Azzam PN. Characterization of N200 and P300: selected studies of the event-related potential. Int J Med Sci. 2005;2(4):147–54.
- McGeown WJ, Cecchi M, Fadem K. Neuropsychological and neuroanatomical correlates of event-related potentials in patients with Alzheimer’s disease. Presented at Alzheimer’s Association International Conference; 17 Jul 2017; London, United Kingdom.
- Bennys K, Portet F, Touchon J. Diagnostic value of event-related evoked potentials N200 and P300 subcomponents in early diagnosis of Alzheimer’s disease and mild cognitive impairment. J Clin Neurophysiol. 2007;24(5):405–12.
- Duncan CC, Kosmidis MH, Mirsky AF. Event-related potential assessment of information processing after closed head injury. Psychophysiology. 2003;40(1):45–59.
- Duncan CC, Kosmidis MH, Mirsky AF. Closed head injury-related information processing deficits: an event-related potential analysis. Int J Psychophysiol. 2005;58(2–3):133–157.
- Sarno S, Erasmus LP, Frey M, Lippert G, Lipp B. Electrophysiological correlates of active and passive attentional states after severe traumatic brain injury. Funct Neurol. 2006;21(1):21–29.
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148.
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11(03):357.
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biol Psychol. 1982;14(1):1–52.
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197(4305):792–795.
- Baillargeon A, Lassonde M, Leclerc S, Ellemberg D. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj. 2012;26(3):211–220.
- Doi R, Morita K, Shigemori M, et al. Characteristics of cognitive function in patients after traumatic brain injury assessed by visual and auditory event-related potentials. Am J Phys Med Rehabil [Internet]. 2007;86(8):641–649.
- Dupuis F, Johnston KM, Lavoie M, et al. Concussions in athletes produce brain dysfunction as revealed by event-related potentials. Clin Neurosci Neuropathol. 2000;11(18):4087–4092.
- Pratap-Chand R, Sinniah M, Salem FA. Cognitive evoked potential (P300): a metric for cerebral concussion. Acta Neurol Scand. 1988;78(3):185–189.
- Gosselin N, Thériault M, Leclerc S, et al. Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery. 2006;58(6):1151–1161.
- Segalowitz SJ, Bernstein DM, Lawson S. P300 event-related potential decrements in well-functioning university students with mild head injury. Brain Cogn. 2001;45(3):342–356.
- Clayton G, Davis N, Holliday A, et al. In-clinic event related potentials after sports concussion: a 4-year study. J Pediatr Rehabil Med. 2020;13(1):81–92.
- Li H, Li N, Xing Y, et al. P300 as a potential indicator in the evaluation of neurocognitive disorders after traumatic brain injury. Front Neurol. 2021;12:690792.
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. 2001;14(1):25–40.
- Vecchio F, Määttä S. The use of auditory event-related potentials in Alzheimer’s disease diagnosis. Int J Alzheimers Dis. 2011;2011:653173.
- Thériault M, De Beaumont L, Gosselin N, et al. Electrophysiological abnormalities in well functioning multiple concussed athletes. Brain Inj. 2009;23(11):899–906.
- Moore RD, Lepine J, Ellemberg D. The independent influence of concussive and sub-concussive impacts on soccer players’ neurophysiological and neuropsychological function. Int J Psychophysiol. 2017;112:22–30.
- Hoover S, Zottoli TM, Grose-Fifer J. ERP correlates of malingered executive dysfunction. Int J Psychophysiol. 2014;91(2):139–146.
- Cecchi M, Moore DK, Sadowsky CH, et al. A clinical trial to validate event-related potential markers of Alzheimer’s disease in outpatient settings. Alzheimers Dement (Amst). 2015;1(4):387–394.
- Cecchi M, Adachi M, Basile A, et al. Validation of a suite of ERP and QEEG biomarkers in a pre-competitive, industry-led study in subjects with schizophrenia and healthy volunteers. Schizophr Res. 2023;254:178–189.
- COGNISION Patient Report – Concussion.pdf. 2023
