
by Rocco Salvatore Calabrò, MD, PhD
Dr. Calabrò is with IRCCS Centro Neurolesi “Bonino-Pulejo” in Messina, Italy.
Funding: No funding was provided for this article.
Disclosures: The author has no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(7–9):12–16.
Abstract
Stroke is a leading cause of death and disability throughout the world. Although physical and cognitive impairments after stroke have been well studied, little is known about sexual functioning and satisfaction in these patients, despite being crucial aspects of quality of life. Post-stroke sexual dysfunctions seem to be common; in men affected by stroke, a decline in libido and poor or curtailed erection and ejaculation are frequently observed. Sexual disorders after stroke are thought to be due to multiple etiologies, including both organic (e.g., lesion localization, premorbid medical conditions, medications) and psychosocial (e.g., fear of recurrences, loss of self-esteem, role changes, anxiety and depression) etiologies. Thus, the exploration of sexual dysfunction and sexual counseling by trained professionals should be part of stroke rehabilitation.
Keywords: Sexuality, stroke, counseling, sexual coach
The most difficult aspect of having a stroke is living with the disability caused by this condition. Stroke is associated with high morbidity rates, meaning that many patients experience both physical and mental disability following the event. Stroke morbidity is the leading cause of decreased independence and lowered quality of life among adults.1,2
Sexuality is an integral and important part of quality of life, and patients affected by this neurological disability should also be investigated and treated for sexual dysfunction (SD). The impact of SD on patients with recent stroke is substantial; however, even though they suffer from sexual impairment, patients usually do not ask for counseling, and, moreover, they are not commonly investigated for this issue by physicians.3,4
Sexual Dysfunction: Types, Etiology, and Epidemiology
Sexual function relies on a complex network of peripheral and central pathways involving the participation of autonomic and somatic nerves and the integration of numerous spinal and supraspinal sites in the central nervous system (CNS), with the hypothalamic and limbic regions playing a pivotal role.5
Neurological diseases have long been recognized as causing SD through an altered processing of sexual stimuli that precludes arousal, decreases or increases desire, or curtails genital engorgement.6 This could explain why various studies have shown a significant decrease in sexual satisfaction after stroke. Indeed, all phases of the sexual response cycle can be affected by the disease, as a decline in libido, poor or curtailed erection, and ejaculation are frequently observed after a stroke.7–11 Libido is frequently impaired after stroke, and the reported prevalence of post-stroke diminished sexual desire varies from 17 to 42 percent.5 Korpelainen et al11 showed a significant decline in libido, sexual arousal, and satisfaction with sexual life in both male and female patients following stroke, although the frequency of patients who ceased having sexual intercourse was not high, with 28 percent at two months and only 14 percent at six months following the acute event. The same authors demonstrated that SD was strictly related to the presence of sensory hemisyndrome. Indeed, tactile stimulations are extremely important in sexual arousal and orgasm during foreplay and intercourse. However, a significant decline in coital frequency, sexual satisfaction, libido, sexual arousal, and orgasm has been demonstrated among stable patients with stroke with mild or no disability.13
It is noteworthy that, during the natural course of the disease, it is possible to achieve a partial or full resolution of the sexual problem, even without formal treatment. This could be due to the effect of the neuroplastic changes following the brain injury and/or positive psychological response to the functional recovery. This issue, however, deserves investigation.
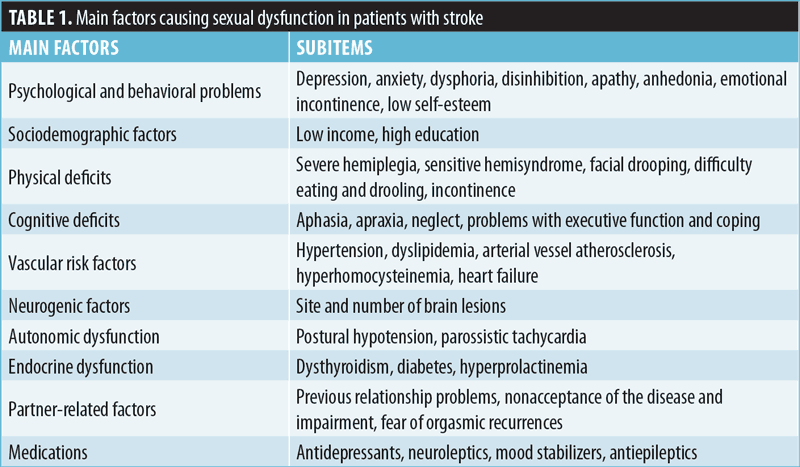
The cause of SD is often multifactorial, with a complex interplay between psychological and organic factors (Table 1). In fact, sexual problems seem to be related to various factors, such as general attitude toward sexuality, incipient depression with anxiety after stroke, or prior medical conditions, such as hypertension, diabetes mellitus, or the use of specific drugs.6
Some researchers have postulated a relationship between the location of the lesion and sexual changes.14,15 SD appears to be more frequent when the right hemisphere is involved.15 It has been reported that patients with unilateral stroke with a stroke lesion in the right cerebral hemisphere experienced a significant decrease in sexual desire and frequency of intercourse.16 Libido and ability to achieve erection might require activation of specific limbic and cortical structures, and the right hemisphere seems to be dominant for attention/activation functions and in processing emotions. Moreover, the right hemisphere dominance for male sexual activity might be related to the specific control of the hypothalamus-pituitary axis, as suggested by the observation of altered sexual behaviors in patients with right temporal lobe epilepsy.16
Nevertheless, to date, few studies have attempted to determine the correlations between the sexual function of patients with stroke and location of lesions. Erectile dysfunction (ED) has been shown to be associated with either middle cerebral artery or posterior cerebral artery infarction; a high prevalence of SD was also present in basal ganglia and brain stem infarction, further demonstrating how the brain’s control of erection is very complex and involves many areas and pathways.6 Patients with multiple brain lesions had a significant decrease of erectile function, compared to those with one lesion. In particular, a decrement of sexual desire was associated with a stroke lesion on the left basal ganglia; patients with lesions in the right cerebellum experienced significant ejaculatory disorders, and patients with lesion in the right pons were associated with a decrease in International Index of Erectile Function (IIEF)-5 scores.17,18
Physical impairment could have an important role in the etiology of long-term sexual problems. As in severe brain trauma, the effects of a devastating stroke might influence body positioning and movement and challenge the ability to embrace and stimulate the partner during sexual intercourse.19,20 Obvious drawbacks are drooling, bladder and bowel incontinence, and other potentially unattractive behaviors.21,22 Facial dropping, speech and memory problems, hemiparesis, difficulty eating, and incontinence might all contribute to feeling less attractive, with a consequent loss of desire and reduction in sexual intercourse.23 Right-middle cerebral artery strokes have the potential to produce not only hemianesthesia, but also perceptual neglect (i.e., the inability to interpret the left side of the environment), both of which might interfere with erotic sensations.
The role of previous medical conditions in the pathogenesis of post-stroke SD is still under debate. Bener et al24 have demonstrated that the most important comorbid factors for ED in stroke patients were diabetes, hypertension, and hypercholesterolemia, and important risk factors included smoking and obesity.
Mood disorders, such as depression, anxiety, and posttraumatic stress disorder (PTSD), are often observed after a stroke. Therefore, post-stroke depression commonly results in SD, and, conversely, changes in mood seem to be related to dependence to activities of daily living (ADL) and severity of neurological deficits.20,21 It is not a coincidence that people with more severe physical impairments experience emotional disorders and decreased sexual intercourse more frequently than people with mild impairments. Kimura et al25 reported that patients with SD after stroke had more frequent and severe depressive disorder or more impaired ADL, compared to patients without SD. Depression and fear of recurrent stroke are examples of psychological factors influencing sexual function and, in particular, sexual desire, but low self-esteem, partner refusal, and loss of work are other important issues to take into account.
The role of psychological factors is further confirmed by the observation that SDs are reported not only by patients, but also by their partners. The illness is often experienced as a critical life event, and the impact of stroke on the psychological health of caregivers is relevant.26 Nevertheless, little information is available about the consequences of stroke on sexual behaviors and attitudes of the spouses of patients with stroke, although they are very important in terms of stroke survivors’ wellbeing.27,28 Korpelainen et al10 revealed a significant decline in libido, coital frequency, sexual arousal, and satisfaction with SD, which was significantly associated with various psychological factors, such as general attitude toward sexuality, fear of stroke recurrence, and ability to discuss sexuality.
The sexuality of stroke survivors is commonly affected by motor, sensory, and autonomic dysfunction, but for people with aphasia, SD is often more related to their communication disorder, since adequate communication skills are essential for forming and maintaining social and sexual relationships. Aphasia also represents a formidable barrier to talking about sexuality with healthcare professionals, especially when they are mute on this issue.29
Interestingly, sexual intercourse has been described in some cases as an unusual trigger of stroke.30
Diagnostic Work-up
Diagnostic assessment of SD in male patients consists of three phases: anamnesis, physical examination, and instrumental investigation.19,30,31
Anamnesis, or medical history, is the key element of the clinical approach. It leads to the identification of risk factors (e.g., personal habits, including smoking, alcohol intake and use of psychoactive drugs; endocrine-metabolic diseases; psychological and/or social stressors) to look into either the organic or psychological pathogenesis of SD and acts as a guide for further diagnostic evaluation.
A psychological screening for depression and anxiety disorders should always be performed, using validated scales, such as the Hamilton Rating Scale for Depression and Anxiety, when determining SD to rule out potential psychological/psychiatric causes.
Medication history plays an important role in SD diagnosis, since there are many drugs commonly used in patients with stroke, such as β-blockers, diuretics, antidepressants, neuroleptics, and sedatives, which might lead to sexual side effects.19
Although SD is common in male patients with stroke, its quantification is limited by the paucity of validated, user-friendly scales. Sexual functioning may be measured using the Arizona Sexual Experience Scale (ASEX), a brief five-item scale designed to assess the core elements of sexual function (i.e., drive, arousal, penile erection/vaginal lubrication, ability to reach orgasm, and satisfaction with orgasm), or the IIEF-15, a standardized and validated 15-item self-evaluation scale that provides pre- and post-treatment clinic evaluations of erectile function, orgasmic function, sexual desire, satisfaction in sexual intercourse, and general satisfaction.19,32
General, neurological, and urogenital examination is necessary to point out medical comorbidities. ED can be the first clinical sign of an unknown and untreated cardiovascular disease, so an accurate evaluation of the heart and main arteries should be done in select individuals.
A full endocrine and metabolic workup, including serum levels of testosterone and thyroid function, might be of some help in some cases.
The instrumental investigation is built up to confirm the suspicion made by history and physical examination and, typically, used to evaluate erectile function and capacity since the other aspects of normal male sexual response are better assessed by a psychological approach and better diagnosed using Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) and International Classification of Diseases, 10th Revision (ICD-10).33,34
It is well known that some erectile episodes are present during rapid eye movement (REM) sleep. The neural mechanism of this sleep related erection (SRE) remains largely unknown, although the involvement of several structures of the brainstem, such as the spinal center of the erection or hypothalamic preoptic area, and other diencephalic structures was hypothesized.35 The screening of SRE through Nocturnal Penile Tumescence REM sleep Monitoring (NPTRM) is widely used to differentiate the psychogenic ED from the organic one.36,37 When NPTRM is inconclusive, a penile color duplex ultrasound (PCDU) should be requested to investigate abnormalities or disease of penile vessels leading to ED. This will help determine the psychogenic or physical etiology of the erectile dysunftion.33,34 While basal PCDU can show arterial or venous abnormalities of the penis, a dynamic PCDU, with intracavernous injection test, studies the hemodynamic changes that occur during pharmacological erection.35 In patients affected by neurological disease, including stroke, and experiencing ED and/or ejaculatory disorders, a diagnosis of involvement of neural and muscular structures related to sexual function might be strengthened, refined, and documented by neurophysiological testing. The dorsal penile nerve conduction gives information about the speed of sensitive nervous conduction through an orthodromic sensitive nerve conduction performed by distending the penis and applying two electrodes to the extremities. The bulbocavernosus reflex is the neurophysiological correlate of the elicited bulbocavernosus reflex during the neurological examination, often requested when the response to physical stimuli is not clear; it is performed in patients through stimulation of the dorsal penile nerve and detection of the response in the pelvic floor muscle through the aid of concentric single fiber needle electromyography (EMG). The pudendal somatosensory evoked potential (SEP) evaluates the speed of conduction to the cortex of stimuli applied at the peripheral level, with a percutaneous bipolar electrode placed on the penile shaft, giving information on whether the site of the lesion is peripheral or central.35–37
Other helpful neurophysiological tests include the pelvic floor muscle EMG, sympathetic skin response, and cardiovascular reflex tests to rule out autonomic dysfunction, which is frequent in patients with right hemispheric stroke.
Finally, penile angiography, cavernosometry and cavernosography are three diagnostic tools that are considered as third-level investigations and used to better evaluate arterial and venous pathologies. They are performed only when the PCDU exam is inconclusive to refine the diagnosis of a vascular ED, especially in young subjects who are candidates for surgical repair.5–38
Treatment of SD
ED. Significant advances in the understanding of the physiology and pathophysiology of male sexual function, as well as in the methods of its investigation and treatment, have been attained during the past decades. Since SD is common in male patients affected by neurological diseases, including stroke,19 neurologists should be aware of sexual problems and their treatment to improve patient quality of life.
Oral pharmacotherapy is currently the mainstay of treatment for ED.39,40 Although a number of oral prescription drugs might have the potential to be used to treat ED, most of these drugs act centrally, and they are not so effective in this regard and have a number of side effects.
Significant advances in the pharmacologic treatment of ED have occurred in recent years, most notably after the introduction of sildenafil, the first oral selective phosphodiesterase type 5 (PDE5) inhibitor, in 1998. Sildenafil quickly gained acceptance by the medical community and public because of its broad efficacy for different types of ED and ease of use.41 Other PDE5 inhibitors, including vardenafil, tadalafil, and the more recent avanafil, have since joined sildenafil to compete in the ED market.42 Common adverse events with all the PDE5 inhibitors include headache, flushing, nasal congestion, myalgia/back pain, and dyspepsia. However, these adverse events are generally mild, self-limited after long-term use, and not associated with treatment discontinuation. Lastly, the possible relationship between nonarteritic anterior ischemic optic neuropathy (NAION) and PDE5 inhibitor use has raised important questions; nevertheless, to date, there is no epidemiological evidence that the incidence of NAION is higher in patients receiving PDE5 inhibitors.41,42 PDE5 inhibitors have proven effective in the treatment of several neurological disorders, including spinal cord injury and multiple sclerosis (MS), and the compounds could be taken into consideration in treating stroke-induced SD, especially in young or middle-aged patients with few comorbidities. Although in patients with diabetes or hypertension, a dose of sildenafil 50mg does not appear to produce detrimental effects on cerebral blood flow, individuals with a history of stroke might be at increased risk of hemodynamic impairment after the use of the drug.43 Thus, having an accurate medical history is fundamental for patients with stroke before prescribing PDE5 inhibitors, starting with low doses.
Injectable and intraurethral agents were relegated to second-line therapy after the appearance of the effective oral PDE5 inhibitor. However, the local delivery of medication (i.e., prostaglandin E1 [PGE1] and papaverine) remains useful, as in about 25 to 30 percent of patients with ED, PDE5 inhibitors are ineffective.42 Therefore, these second-line therapies could be a valid alternative in patients with more severe stroke.
About 40 percent of patients with ED have evidence of abnormal arterial flow, only partially involving aortoiliac bifurcation, since most men with major vessel disease rarely present with impotence. Conversely, the majority of vascular patients with ED have pathological changes in the small vessels of the penis and, generally, revascularization for such small arteries is challenging. Penile prosthesis offers a valid therapeutic alternative for patients who fail vasoactive drugs and vacuum-constrictive devices and who are not candidates for vascular reconstruction procedures.30,39,40
Ejaculatory disorders. Since premature ejaculation (PE) is mostly due to a psychogenic etiology, psychosexual treatment is considered the mainstay, with high rates of success.44 Dapoxetine, a short-acting selective serotonin reuptake inhibitor (SSRI), could treat some patients,45 whereas some trials have evaluated the use of sertraline, with inconclusive results.30
Since the most common etiology of anorgasmia is the intake of psychotropic agents, regaining the orgasmic sensation might be achieved with discontinuation and/or substitution of the inciting drug. In cases of anejaculation, vibratory stimulation might be helpful, but intact dorsal penile nerves are necessary for the ejaculatory response.30
Ejaculatory pain represents a component of SD that has received little attention in the literature so far. Postorgasmic pain is associated with prostatitis, chronic pelvic pain syndrome, benign prostatic hyperplasia, ejaculatory duct obstruction, prostate radiation, and radical prostatectomy. However, it could rarely be related to CNS lesions, including stroke. In that case, the treatment options vary from antidepressants, such as amitriptyline; antiepileptics, such as pregabalin and topiramate; muscle relaxants; and even surgical procedures, such as pudendal nerve decompression, in the most severe cases.47
Nonpharmacological treatment. Besides pharmacological treatment, one of the most important, although often underestimated, success factors of SD therapy is undoubtedly the involvement of the patient’s partner in expert counseling. Psychosexual and relational counseling could be of help, especially when psychogenic factors overcome the organic ones.
Specific pelvic floor muscle training or individualized sexual rehabilitation might also be used in some cases, but data are insufficient to provide any reliable indication of benefit or risk to guide clinical practice.30
Conclusion
Sexuality is one of the most complex aspects of human life. Sexual expression is dependent on functioning anatomical and physiological systems, which are influenced by cognitive and emotional processes. To assess and treat problems in this area requires knowledge of those factors influencing both the dynamics of the relationship and the physical and psychological aspects of sexual functioning. Neurological diseases, including stroke, have long been recognized as causing SD, through complex and multifaceted mechanisms. Nevertheless, practicing neurologists and physiatrists have not traditionally paid much attention to SD in their patients, in part because therapeutic possibilities were scant. With an emerging awareness of the primary importance of quality of life as an indicator of good patient management and the advent of more effective SD treatments, ignoring this very important dimension of life is no longer acceptable.
References
- Mansfield A, Inness EL, Mcilroy WE. Stroke. Handb Clin Neurol. 2018;159:205–228.
- Darlington AS, Dippel DW, Ribbers GM, et al. Coping strategies as determinants of quality of life in stroke patients: a longitudinal study. Cerebrovasc Dis. 2007;23(5–6):401–407.
- Calabrò RS, Bramanti P. Post-stroke sexual dysfunction: an overlooked and under-addressed problem. Disabil Rehabil. 2014;36(3):263–264.
- Low MA, Power E, McGrath M. Sexuality after stroke: exploring knowledge, attitudes, comfort and behaviours of rehabilitation professionals. Ann Phys Rehabil Med. 2022;65(2):101547.
- Pistoia F, Govoni S, Boselli C. Sex after stroke: a CNS only dysfunction? Pharmacol Res. 2006; 54(1):11–18.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512–525.
- Boldrini P, Basaglia N, Calanca MC. Sexual changes in hemiparetic patients. Arch Phys Med Rehabil. 1991;72(3):202–207.
- Tamam Y, Tamam L, Akil E, et al. Post-stroke sexual functioning in first stroke patients. Eur J Neurol. 2008;15(7):660–666.
- Giaquinto S, Buzzelli S, Di Francesco L, Nolfe G. Evaluation of sexual changes after stroke. J Clin Psychiatry. 2003;64(3):302–307.
- Korpelainen JT, Nieminen P, Myllylä VV. Sexual functioning among stroke patients and their spouses. Stroke. 1999;30(4):715–719.
- Korpelainen JT, Kauhanen ML, Kemola H, et al. Sexual dysfunction in stroke patients. Acta Neurol Scand. 1998;98(6):400–405.
- Kautz DD, Van Horn ER. Sex and intimacy after stroke. Rehabil Nurs. 2017;42(6):333–340.
- Cheung RT. Sexual functioning in Chinese stroke patients with mild or no disability. Cerebrovasc Dis. 2002;14(12):122–128.
- Koehn J, Crodel C, Deutsch M, et al. ED (ED) after ischemic stroke: association between prevalence and site of lesion. Clin Auton Res. 2015;25(6):357–365.
- Winder K, Seifert F, Köhrmann M, et al. Lesion mapping of stroke-related ED. Brain. 2017;140(6):1706–1717.
- Daniele A, Azzoni A, Bizzi A, et al. Sexual behavior and hemispheric laterality of the focus in patients with temporal lobe epilepsy. Biol Psychiatry. 1997;42(7):617–624.
- Jung JH, Kam SC, Choi SM, et al. Sexual dysfunction in male stroke patients: correlation between brain lesions and sexual function. Urology. 2008;71(1):99–103.
- Jeon SW, Yoo KH, Kim TH, et al. Correlation of the ED with lesions of cerebrovascular accidents. J Sex Med. 2009;6(1):251–256.
- Calabrò RS, Gervasi G, Bramanti P. Male sexual disorders following stroke: an overview. Int J Neurosci. 2011;121(11):598–604.
- Grenier-Genest A, Gérard M, Courtois F. Stroke and sexual functioning: a literature review. NeuroRehabilitation. 2017;41(2):293–315.
- Boller F, Agrawal K, Romano A. Sexual function after strokes. Handb Clin Neurol. 2015;130:289–295.
- Rosenbaum T, Vadas D, Kalichman L. Sexual function in post-stroke patients: considerations for rehabilitation. J Sex Med. 2014;11(1):15–21.
- Kautz DD. Hope for love: practical advice for intimacy and sex after stroke. Rehabil Nurs. 2007;32:95–103(3); discussion 132.
- Bener A, Al-Hamaq AO, Kamran S, Al-Ansari A. Prevalence of ED in male stroke patients, and associated co-morbidities and risk factors. Int Urol Nephrol. 2008;40(3):701–708.
- Kimura M, Murata Y, Shimoda K, Robinson RG. Sexual dysfunction following stroke. Compr Psychiatry. 2001;42(3):217–222.
- Prior S, Reeves N, Peterson G, et al. Addressing the gaps in post-stroke sexual activity rehabilitation: patient perspectives. Healthcare (Basel). 2019;7(1):25.
- Calabrò RS. Post-stroke sexual dysfunction at a young age: time to act! Eur J Neurol. 2014;21(5):e48.
- Bugnicourt JM, Hamy O, Canaple S, et al. Impaired sexual activity in young ischaemic stroke patients: an observational study. Eur J Neurol. 2014;21(1):140–146.
- Lemieux L, Cohen-Schneider R, Holzapfel S. Aphasia and sexuality. Sex Disabil. 2001;19:253–266.
- Stratton H, Sansom J, Brown-Major A, et al. Interventions for sexual dysfunction following stroke. Cochrane Database Syst Rev. 2020;5(5):CD011189.
- Richards A, Dean R, Burgess GH, Caird H. Sexuality after stroke: an exploration of current professional approaches, barriers to providing support and future directions. Disabil Rehabil. 2016;38(15):1471–1482.
- Del Popolo G, Cito G, Gemma L, Natali A. Neurogenic sexual dysfunction treatment: a systematic review. Eur Urol Focus. 2020;6(5):868–876.
- Baumann F, Hehli D, Makaloski V, et al. ED– overview from a cardiovascular perspective. Vasa. 2017;46(5):347–353.
- Yafi FA, Jenkins L, Albersen M, et al. ED. Nat Rev Dis Primers. 2016;2:16003.
- Kandeel FR, Koussa VK, Swerdloff RS. Male sexual function and its disorders: physiology, pathophysiology, clinical investigation, and treatment. Endocr Rev. 2001;22(3):342–388.
- Lundberg PO, Ertekin C, Ghezzi A, et al. Neurosexology. Guidelines for neurologists. European Federation of Neurological Societies Task Force on Neurosexology. Eur J Neurol. 2001;8(suppl 3):2–24.
- Diemont WL, Meuleman EJ. Neurological testing in ED. J Androl. 1997;18(4):345–350.
- Meuleman EJ, Diemont WL. Investigation of ED. Diagnostic testing for vascular factors in ED. Urol Clin North Am. 1995;22(4):803–819.
- Calabrò RS, Polimeni G, Bramanti P. Current and future therapies of ED in neurological disorders. Recent Pat CNS Drug Discov. 2011;6(1):48–64.
- Calabrò RS, Polimeni G, Bramanti P. Recent advances in the treatment of neurogenic ED. Recent Pat CNS Drug Discov. 2014;9(1):41–53.
- Francis SH, Corbin JD. Sildenafil: efficacy, safety, tolerability and mechanism of action in treating ED. Expert Opin Drug Metab Toxicol. 2005;1(2):283–293.
- Feifer A, Carrier S. Pharmacotherapy for ED. Expert Opin Investig Drugs. 2008;17(5):679–690.
- Lorberboym M, Mena I, Wainstein J, et al. The effect of sildenafil citrate (Viagra) on cerebral blood flow in patients with cerebrovascular risk factors. Acta Neurol Scand. 2010;121(6):370–376.
- Calabrò RS, Polimeni G, Ciurleo R, et al. Neurogenic ejaculatory disorders: focus on current and future treatments. Recent Pat CNS Drug Discov. 2011;6(3):205–221.
- McMahon CG. Dapoxetine for premature ejaculation. Expert Opin Pharmacother. 2010;11(10):1741–1752.
- Calabrò RS, Marra A, Quattrini F, et al. Central neuropathic pain: an unusual case of painful ejaculation responding to topiramate. J Sex Med. 2012;9(12):3274–3278.


