by John H. Greist, MD; James C. Mundt, PhD; Chad J. Gwaltney, PhD; James W. Jefferson, MD; and Kelly Posner, PhD
by John H. Greist, MD; James C. Mundt, PhD; Chad J. Gwaltney, PhD; James W. Jefferson, MD; and Kelly Posner, PhD
Dr. Greist is Clinical Adjunct Professor of Psychiatry at University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; Dr. Mundt is with the Center for Telepsychology, Madison, Wisconsin; Dr. Gwaltney is with ERT in Philadelphia, Pennsylvania; Dr. Jefferson is Clinical Adjunct Professor of Psychiatry, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; and Dr. Posner is with Columbia University, College of Physicians and Surgeons and New York State Psychiatric Institute, New York, New York.
Innov Clin Neurosci. 2014;11(9–10):23–31
Funding: ERT, a clinical services provider to the biopharmaceutical industry, supported the meta-analysis of the reported data and preparation of the manuscript for publication submission.
Financial Disclosures: Drs. Greist, Mundt, and Jefferson are shareholders of Healthcare Technology Systems, which receives royalty payments for development and use of the electronic Columbia–Suicide Severity Rating Scale (eC-SSRS) from ERT. Dr. Greist also received payment from ERT for manuscript preparation. Dr. Gwaltney is an employee of ERT. Dr. Jefferson is a co-developer and coauthor of the eC-SSRS and a principal at Healthcare Technology Systems, Inc. which receives royalties from eC-SSRS use. Dr. Posner receives royalties for development and use of the eC-SSRS from ERT through the Research Foundation for Mental Hygiene.
Key words: eC-SSRS; electronic patient-reported outcomes; suicide risk assessment, prospective risk
Abstract: Objective: Examine the ability of baseline electronic Columbia–Suicide Severity Rating Scale lifetime suicidal ideation and behavior categories to predict prospective reports of suicidal behavior in psychiatric and non-psychiatric research participants. Design: Meta-analysis of 74,406 eC-SSRS assessments completed between September 2009 and December 2012. Setting: Thirty-three clinical research studies that used the electronic Columbia–Suicide Severity Rating Scale to assess suicidal ideation and behavior at baseline and prospectively during follow-up visits. Participants: Records from 6,760 patients with psychiatric disorders (opioid dependence, generalized anxiety, major depressive, and posttraumatic stress disorders) and 2,077 nonpsychiatric disorder patients (chronic obstructive pulmonary disease, epilepsy, fibromyalgia, human immunodeficiency virus, insomnia, multiple sclerosis, osteoarthritis, pain/back pain, Parkinson’s disease, restless leg syndrome) were analyzed. Measurements: Electronic Columbia–Suicide Severity Rating Scale assessment of lifetime suicidal ideation (5 severity levels) and suicidal behavior (4 types) at baseline and prospectively reported suicidal behavior during study participation. Results: Increasingly more severe lifetime suicidal ideation at baseline was associated with a progressively greater likelihood of prospectively reported suicidal behavior during study participation. Intent to act on suicidal ideation was most predictive of reports of suicidal behavior. Reports of lifetime suicidal behaviors at baseline also predicted subsequent suicidal behavior, and multiple lifetime behaviors monotonically increased prospective risk of suicidal behavior. Baseline suicidal ideation and behavior predicted future suicidal behavior in both psychiatric and non-psychiatric trials. Conclusions: Lifetime reports of suicidal ideation and/or behavior at baseline significantly increased risk of prospectively reporting suicidal behavior during research trial participation in both psychiatric and nonpsychiatric patients. Lifetime prevalence of suicidal ideation and behavior is higher among psychiatric patients, but also presents a safety concern among nonpsychiatric patients when reported.
Introduction
Suicide is the major preventable mortality of psychiatric disorders.[1] Many who commit suicide have seen primary care or mental health specialists shortly before their deaths.[2] Recognition of risk is a prerequisite for medical intervention to prevent suicide attempts and manage subsequent risk.
Evaluation of risk conventionally rests with clinicians caring for patients. In suicide risk assessment, as with any human endeavor, variability in assessment ability is inevitable. Differences in aptitude, training, skill, experience, time pressure, fatigue, illness, beliefs, and biases are among factors affecting reliability and accuracy of clinician assessment.
In a study of “sources of unreliability in depression ratings” with the Hamilton Depression Rating Scale (HDRS), 92 percent of variance was attributable to raters and eight percent to respondents.[3] Another study provided and evaluated HDRS training for 31 raters from 15 United States clinical trial sites.[4] After training and three rating trials, seven percent of raters could not qualify. After rating in trials for one year, 42 percent of qualified raters were no longer qualified.
The common misconception that asking about suicide might evoke it can lead to critical assessment omissions.[5] Worry about managing identified suicide risk may also inhibit thorough interviewing.[6] Sensitivity of patients and professionals regarding suicide stigma may dampen inquiry and response. Candor with sensitive subjects is often compromised. Catholic confessionals have a screen between priest and penitent while analysts sit at the head of the couch. Both arrangements reduce eye contact to facilitate difficult disclosures. As Isaac Marks observed more broadly, “Fear of two staring eyes is widespread throughout the animal kingdom.”[7]
Long ago it was recognized that indirect assessment of suicide risk factors had greater sensitivity than direct assessment,[8] an observation confirmed repeatedly.[9–12] Despite these observations, there is no doubt that the clinician’s role in suicide risk assessment and management is essential and pivotal. Only clinicians can implement the range of treatments and caring that reduce suicides.
The best suicide risk assessment comes from combining systematic indirect interviewing of demonstrated prospective value with astute follow-up by clinicians. The electronic Columbia-Suicide Severity Rating Scale (eC-SSRS) is a fully-structured, computer-administered, clinical interview designed to systematically query patients regarding past and current suicidal ideation and behavior (SIB) in complete adherence to the clinical conventions of the Columbia-Suicide Severity Rating Scale (C-SSRS).[13] The psychometric characteristics of the patient-reported eC-SSRS have been assessed in multiple contexts. It has demonstrated convergent validity with the clinician-based C-SSRS when both were administered.[14] The eC-SSRS has also been shown to improve patient candor regarding SIB in epilepsy patients.[8]
From clinical and validation perspectives, it is also critically important to demonstrate that ideation and behavior ratings at one point predict ideation and behavior at a future time. However, this is difficult to accomplish with data from a single trial where base rates of ideation and behavior are low. We conducted a meta-analysis of over 35,000 eC-SSRS assessments accumulated from clinical trials conducted between 2009 and 2011. Summary indices of lifetime ideation and behavior predicted suicidal behaviors in short-term follow-up and in an additive fashion. Having both lifetime history of ideation and behavior more strongly predicted future behavior than either alone.[14]
The previous studies provide compelling initial evidence supporting the psychometric characteristics of the eC-SSRS. However, open questions remain regarding the performance of the eC-SSRS. For example, although commonly used in psychiatric populations, it is unclear if the instrument predicts future behavior in nonpsychiatric populations. Additionally, although summary indices of ideation and behavior predict subsequent behavior, it is not clear that different severity levels of ideation and behavior have predictive value. We would expect more severe ideation and greater numbers of behaviors to predict subsequent behavior more robustly.
The present study expands on the prior findings by 1) examining generalizability of the prior findings to studies involving non-psychiatric patients and 2) drilling deeper into the specific ideation and behavior items assessed by the eC-SSRS to evaluate their independent predictive relationships with prospective risk of reporting suicidal behavior during study participation.
Methods
The initial anonymized, pooled dataset for this meta-analysis included 74,884 eC-SSRS assessments initiated since September 2009. Incomplete eC-SSRS assessments, records created during system testing, and records from four studies with 10 or fewer completed assessments were excluded from further consideration, yielding a dataset of 74,406 records (99.4% of all extracted records).
A previous publication that examined the ability of summary eC-SSRS scores to predict future ideation and behavior included 35,224 (47%) of these records. They are also included here, as the goals of this study are different from the initial study: the focus in this paper is on the prediction of future ideation and behavior by individual eC-SSRS items, rather than the summary scores, and both psychiatric and nonpsychiatric samples are evaluated.20
Of the remaining 74,406 eC-SSRS assessments, 5,299 were baseline assessments not associated with subsequent prospective monitoring of SIB and 14,651 records were from prospective monitoring of SIB not associated with a prior baseline assessment of lifetime SIB. These records were also excluded from further analyses since they provide no information regarding predictive associations between baseline assessment of lifetime SIB and subsequent risk of prospectively reporting suicidal behavior during research participation.
The remaining 54,456 eC-SSRS assessment records (41% of these records were included in the 2013 publication) were retained for analysis using SPSS (V 20; IBM Corporation, Armonk, New York). Each record linked to a unique patient ID, study ID, treatment indication, assessment type (baseline of lifetime SIB or prospective assessment since last contact), date, and patient responses to the eC-SSRS queries. No patient demographic, treatment blind, or personally identifying information was available in the anonymized, pooled dataset. The final dataset included 8,837 unique patients.
Studies were classified as representing psychiatric or nonpsychiatric patients based on the treatment indication of the study. Treatment indications of opioid dependence, generalized anxiety disorder, major depressive disorder, and posttraumatic stress disorder were categorized as psychiatric studies; nonpsychiatric treatment indications included chronic obstructive pulmonary disease, epilepsy, fibromyalgia, human immunodeficiency virus, insomnia, multiple sclerosis, osteoarthritis, pain/back pain, Parkinson’s disease, and restless leg syndrome.
Baseline eC-SSRS assessments were used to characterize each patient with respect to the greatest lifetime suicidal ideation severity and prior experiences of suicidal behaviors (e.g., actual, interrupted, and/or aborted suicide attempts or behavior preparatory to an attempt). Each patient was characterized as lifetime SIB status of 1) no lifetime suicidal ideation with intent to act and no prior suicidal behavior (i.e., “None”); 2) prior suicidal ideation with intent to act, but no suicidal behavior (i.e., “Ideation only”); 3) no prior suicidal ideation with intent to act, but reported prior suicidal behavior (i.e., “Behavior only”); or 4) both suicidal ideation with intent to act and previous suicidal behavior (i.e., “Both”).
Prospective eC-SSRS reports of SIB during study participation were also used to classify each patient’s suicidal ideation and behavior as “Negative” or “Positive.” These assessments were completed at each study visit, and raters asked patients about ideation and behavior since the last assessment. Therefore, the assessed time frame does not overlap with the lifetime assessment. Patients who prospectively reported active ideation with a method and intent to act (with or without a specific plan) at any visit were categorized as “Positive” for suicidal ideation. Patients reporting any suicidal behavior during study participation, at any visit, were classified as “Positive” for suicidal behavior.
Relative risk of prospectively reporting suicidal ideation and behavior during study participation among patients reporting lifetime SIB of “Ideation only,” “Behavior only,” or “Both” was computed using Mantel-Haenszel methods; the lifetime SIB group of “None” served as the reference risk group. An analysis predicting future behavior was also conducted by Mundt et al. in 2013[20] using a subset (47%) of the data included in the current analysis. Analyses using only data that were not included in the earlier paper[20] replicated results from analyses of the full dataset and are not presented here. More detailed analyses of prospective risk associated with each level of baseline suicidal ideation severity (e.g., “Passive ideation,” “Active ideation without method,” “Active ideation with method but no intent,” “Active ideation with method and intent but no plan,” “Active ideation with method, intent, and plan”) as well as risks associated with baseline reports of individual and multiple lifetime behaviors were also evaluated.
Results
Of the 54,456 eC-SSRS assessments analyzed, 8,837 were baseline and 45,619 were prospective follow-up assessments. Of the 8,837 baseline assessments of lifetime SIB, 6,760 were from patients participating in psychiatric studies and 2,077 were from patients participating in nonpsychiatric studies. On average, patients participating in psychiatric studies had more prospective follow-up visits (mean [M]=5.63; standard deviation [SD]=2.85) than patients in nonpsychiatric studies (M=3.63; SD=2.34; t(8,835)=29.18, p<0.001); however, the follow-up period from baseline to final follow-up assessment tended to be shorter for psychiatric studies (M=72.33; SD=67.03 days) than for nonpsychiatric studies (M=97.81; SD=89.07 days; t(8,835)= -13.95, p<0.001).
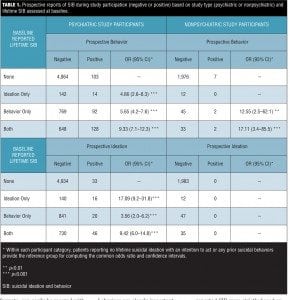
Using the baseline eC-SSRS assessments, 73.5 percent of the psychiatric patients were classified as never experiencing suicidal ideation with intent to act or any prior suicidal behaviors, 2.3 percent were classified as experiencing ideation with intent to act but no prior behaviors, 12.7 percent reported prior suicidal behavior without ideation, and 11.5 percent reported both. As expected, these percentages of most severe lifetime ideation were significantly different than the corresponding classifications of nonpsychiatric patients, which were 95.5 percent, 0.6 percent, 2.3 percent, and 1.7 percent, respectively (chi-squared(3)=578.0, p<0.001). The numbers of patients classified as negative or positive with respect to SIB during study participation, based on prospective eC-SSRS follow-up assessments and broken out by study type, are shown in Table 1. None of the nonpsychiatric study participants reported severe ideation with an intent to act during the prospective monitoring period, but 11 reported some type of suicidal behavior.
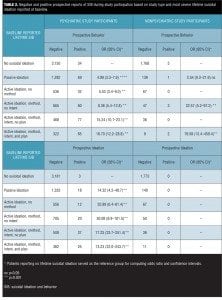
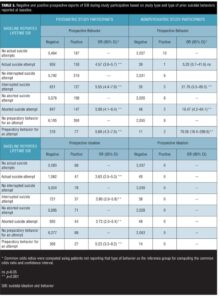
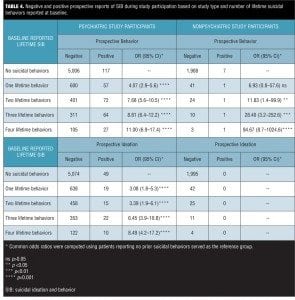
To further evaluate the extent to which lifetime SIB reported at baseline predicts prospectively reported SIB, the comparative risk associated with each level of ideational severity was examined and is presented in Table 2. Similarly, the comparative risk associated with baseline reports for each type of suicidal behavior assessed (e.g., actual suicide attempts, interrupted suicide attempts, aborted suicide attempts, preparatory behavior for making an attempt), as well as that associated with multiple lifetime behaviors are shown in Tables 3 and 4.
Of the 8,837 unique subjects, 8,059 (96.1%) did NOT report nonsuicidal self-injurious behavior (NSSIB) at baseline and 328 (3.9%) did. Of the 8,059 who did not, 294 (3.6%) went on to prospectively report a suicidal behavior compared to 7,765 (96.5%) who did not. Of the 328 subjects who did report NSSIB at baseline, 24 (7.3%) subsequently reported prospective suicidal behavior, whereas 304 (92.7%) did not. The common odds ratio for prospectively reporting suicidal behavior during trial participation for those reporting NSSIB at baseline compared to those that did not was 2.085 (95% confidence interval [CI] 1.354–3.210), p=0.001.
Of the 8,059 who did not report NSSIB as baseline, 100 (1.2%) went on to prospectively report suicidal ideation with intent to act compared to 7,959 (98.8%) who did not. Of the 328 subjects who did report NSSIB at baseline, 1 (0.3%) subsequently reported prospective suicidal ideation with intent, whereas 327 (99.7%) did not. The common odds ratio for prospectively reporting suicidal ideation with intent to act during trial participation for those reporting NSSIB at baseline compared to those that did not was 0.243 (95% CI 0.034–1.750), p=0.16.
Discussion
Concern regarding suicide risk associated with medication prompted the United States Food and Drug Administration to draft guidance recommending prospective assessment of SIB.[15–17] The primary aims of the guidance were to facilitate timely recognition and management of patients experiencing suicidal ideation and behavior and to provide risk data associated with certain medications to guide clinical expectations after the medications are marketed. The eC-SSRS addresses these aims and provides clinical investigators meaningful predictive information regarding the potential for prospective reporting of SIB during study participation.
Increased risk of emergent suicidal behavior associated with the medical history of psychiatric patients has been recognized for many years[18,19] and has been shown to be an important factor in prior analysis of eC-SSRS data.[20] The current study extends these findings to nonpsychiatric treatment indications and study participants. The prevalence of SIB in psychiatric patient populations is much greater than nonpsychiatric patients, but these analyses demonstrate that life-threatening suicidal thoughts and behaviors do occur in nonpsychiatric study participants and should be prospectively monitored to increase patient safety. Although no prospective severe suicidal ideation with intention to act was reported in the nonpsychiatric participant group, reports of lifetime ideation were present at baseline, and some of these patients reported suicidal behavior in the prospective assessments. Importantly, these analyses also show that lifetime SIB, when present in nonpsychiatric patients, does forecast risk of emergent suicidal behavior during trial participation. There is also an increase in prospective suicidal behaviors (but not ideation with intent to act) in individuals with lifetime NSSIB.
The finer-grained analyses of varying severities of lifetime suicidal ideations and the specific types and number of lifetime suicidal behaviors found that each level of ideational severity and each type of suicidal behavior contributes to the likelihood of suicidal behavior being reported during subsequent trial participation. It is particularly interesting that the risk of emergent suicidal behavior—and, to a lesser degree, ideation—during trial participation rises monotonically as a function of increasing severity of lifetime suicidal ideation as well as the number of different lifetime behaviors reported. A notable feature of computer-automated clinical interviews like the eC-SSRS, aside from improved self-disclosure, is that they ensure every question is asked in a consistent manner, answered by the patient, and documented for review, referral, and analysis. The resulting assessments are available to site staff in near real-time to facilitate further evaluation of patient safety, if needed, and are easily transferred to electronic databases. Such data, stored in consistent formats, can easily be merged with data from other studies and used to monitor the risks and/or benefits of different medications regarding SIB.
Several limitations of these analyses should be noted. First, these data reflect lifetime assessment of SIB at baseline and do not address how recent or temporally distant the reported thoughts and events were from the prospectively reported behaviors. Nonetheless, these lifetime ideations and behaviors are clearly important factors influencing the risk of prospective suicidal behaviors. If anything, assessment of more recent ideation and behavior should increase the predictive value of the scale, due to its closer proximity to the follow-up assessments. A recent revision of the eC-SSRS now assesses the recency of SIB at baseline in addition to obtaining the lifetime data analyzed in this paper. Second, the analyses of prospectively reported SIB were strictly based on self-report to the eC-SSRS and were not necessarily confirmed by subsequent clinician follow-up. Ideally, validation would be accomplished through the comparison of the eC-SSRS to other measures.[13] However, the eC-SSRS measures completed at baseline and during the subsequent visits examined different periods of time and, therefore, provide supporting evidence for the lifetime risk assessment. In addition, the analyses conducted simply evaluated the presence or absence of reported SIB at any time during study participation report, and did not evaluate the extent to which SIB may have escalated or diminished from one follow-up visit to the next. Finally, the data extracted for these analyses cannot address potential differences between specific medications or treatment conditions, since no treatment blind information is stored in the anonymized dataset.
Accurate assessment of SIB is essential in clinical trials and clinical practice. The eC-SSRS improves upon previous SIB assessment methods by directly capturing patient reports of thoughts and behaviors through a computer interview. This approach avoids error and bias that may be present when clinicians interview patients. Additionally, the electronic nature of the assessment allows for efficient, standardized collection and processing of information. Any assessment developed to measure SIB must demonstrate adequate psychometric characteristics. The current study adds to a growing evidence base supporting the reliability and validity of the eC-SSRS across patient populations and clinical contexts. These results reinforce prior findings that lifetime SIB risk predicts future suicidal behavior, and that the eC-SSRS is a valuable tool for enhancing patient safety.
References
1. Singhal A, Ross J, Seinog O, et al. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194–204.
2. Luoma JB, Martin CE, Pearson H. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159(6):909–916.
3. Kobak KA, Brown B, Sharp I, et al. Sources of unreliability in depression ratings. J Clin Psychopharmacol. 2009;29(1):82–85.
4. Kobak KA, Lipsitz J, Williams JB, et al. Are the effects of rater training sustainable: results from a multicenter clinical trial. IJ Coin Psychopharmacol. 2007;27:534–535.
5. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of your suicide screening programs: a randomized controlled trial. JAMA. 2005;293(13):1635–1643.
6. Chappell P, Mahableshwarkar AR, Alphs, L, et al. ISCTM Suicidal Ideation and Behavior Assessment Workgroup. Prospective assessment of suicidal ideation and behavior: an internet survey of pharmaceutical sponsor practices. Innov Clin Neurosci.
2014;11(9–10):14–22.
7. Marks I. Fears, Phobias, and Rituals: Panic, Anxiety, and their Disorders. New York: Oxford University Press; 1987:135.
8. Greist JH, Gustafson DH, Stauss FF, et al. A computer interview for suicide-risk prediction. Am J Psychiatry. 1973;130:1327–1332.
9. Hesdorffer DC, French JA, Posner K, et al. Suicidal ideation and behavior screening in intractable focal epilepsy eligible for drug trials. Epilepsia. 2013;54(5):879–887.
10. Levine S, Ancil RJ, Roberst AP. Assessment of suicide risk by computer delivered self-rating questionnaire: preliminary findings. Acta Psychiatr Scand. 1989;80(3):216–220.
11. Trivedi MH, Wisniewski SR, Morris DW, et al. Concise Health Risk Tracking scale: a brief self-report and clinician rating of suicidal risk. J Clin Psychiatry. 2011;72(6):757–64.
12. Vitiello B, Silva SG, Rohde P, et al. Suicidal events in the Treatment for Adolescents With Depression Study (TADS). J Clin Psychiatry. 2009;70(5):741–7.
13. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277.
14. Mundt JC, Greist JH, Gelenberg AJ, et al. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. J Psychiatr Res. 2010;44(16):1224–1228.
15. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. September 2010. https://www.federalregister.gov/articles/2010/09/09/2010-22404/draft-guidance-for-industry-on-suicidality-prospective-assessment-of-occurrence-in-clinical-trials. Accessed October 1, 2014.
16. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. August 2012. Revision 1. http://www.fda.gov/downloads/Drugs/Guidances/UCM225130.pdf. Accessed October 1, 2014.
17. Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009; 339:b2880. doi: 10.1136/bmj.b2880.
18. Beck AT, Brown GK, Steer RA, et al. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life Threat Behav. 1999;29(1):1–9.
19. Brown GK, Beck AT, Steer RA, et al.Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371–377.
20. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887–893.