
by Ashley Lyons, MPA, PA-C
Dr. Lyons is a Doctor of Medical Science in Psychiatry at Rocky Mountain University School of Health Professions in Norfolk, Virginia.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(7–9):44–47.
Abstract
Background: Patients diagnosed with major depressive disorder (MDD) who fail to respond to two or more antidepressants are often considered to have treatment-resistant depression (TRD). Many of the current options for TRD have significant side effect profiles, are expensive, and are difficult to access. There has been a revival of psychedelic research in recent years that shows promising results in the treatment of TRD.
Case Presentation: Here, the case of a 43-year-old man with TRD is presented. TRD symptoms were greatly interfering with his life. He underwent psychological testing, lab work, adequate trials of numerous medications, transcranial magnetic stimulation (TMS), and electroconvulsive therapy, all without adequate relief of his symptoms. The patient began self-administering a microdosing regimen of psilocybin and experienced significant improvement of MDD symptoms, as characterized by Hamilton Depression Rating Scale (HDRS).
Discussion: In recent years, multiple randomized, controlled trials (RCTs) have shown the benefit of psilocybin in the treatment of varying types of depression. One trial evaluated psilocybin and escitalopram as treatments for depression, and psilocybin was found to be superior.
Conclusion: This case suggests the possible benefit of psilocybin in the setting of TRD, as outlined in recent research. Additional research is needed to confirm these observations.
Keywords: Depression, psilocybin, treatment-resistant, MDD
Major depressive disorder (MDD) is one of the most pervasive mental health diagnoses world-wide. Symptoms often include depressed mood, anhedonia, appetite changes, changed sleep patterns, psychomotor agitation, fatigue/anergia, feelings of worthlessness, excessive guilt, diminished ability to focus, or recurrent thoughts of death.1 The prevalence of MDD in the adult population is remarkably significant. According to the National Institutes of Health (NIH), there were 19.4 million adults in the United States (US) who had at least one major depressive episode during 2019. This equates to 7.8 percent of all adults in the US.2
Treatment-resistant Depression
For many patients, episodes of major depression are recurrent and can be difficult to treat. This can lead to a diagnosis of treatment-resistant depression (TRD), in which a patient diagnosed with MDD fails medication treatment. There is no agreed upon definition for TRD, and as such, it can vary among practices. Because of this, it is difficult to obtain accurate prevalence rates of TRD.3,4
Patients with TRD can be challenging to treat due to the complex nature of the disorder. Treating TRD often requires a multifaceted approach, using off-label and/or augmenting agents. Patients with TRD are often willing to undergo electroconvulsive therapy (ECT) due to their multiple failed trials of medications. Often, ECT is considered the “gold-standard” in the treatment of TRD, but it has many associated challenges. There is still a notable stigma associated with ECT, and as a result, some patients are hesitant to undergo such treatments. The treatment can also be expensive and is much more invasive than traditional treatments for depression.5,6
History of Psychedelic Research
Psychedelic compounds became prominent in the field of psychiatric research in the 1940s and 1950s. Psilocybin was formally identified as the psychoactive component in hallucinogenic mushrooms in 1958 by Albert Hoffman. During the late 1960s, criticism of the research came to light due to the controversial nature of its mind-altering properties and potential for abuse. Research was gradually prohibited during this time, and ultimately, in 1970, President Nixon signed the Controlled Substances Act, which made both lysergic acid diethylamide (LSD) and psilocybin Schedule 1 drugs, and thus illegal for public consumption.7
Purpose
The aim of this case report is to evaluate the use of psilocybin in the setting of TRD. In recent years, research in psychedelic modalities has resurfaced in multiple areas of medicine, including psychiatry.
In addition to our case presentation, we performed a literature review using both PubMed and Google Scholar. The search terms “psilocybin and mental health” and “psilocybin and depression” were utilized to evaluate current available literature on the topic. Numerous studies were evaluated, and the most appropriate were selected and are referenced below.
Case Presentation
The patient was a 43-year-old White man with a past medical history of hypertension and MDD seeking evaluation and treatment of his MDD. He was diagnosed with MDD at the age of 17 years, without any precipitating factors. He had recurrent and chronic episodes of depressed mood, anhedonia, hypersomnia, anergia, irritability, and suicidal ideations. The patient denied any history of manic or hypomanic symptoms. He previously received extensive psychotherapy, where he had a full psychiatric evaluation and was not found to have any indications for bipolar disorder or any personality disorders. The patient saw multiple prior psychiatric providers and reported unsuccessful trials of fluoxetine, escitalopram, duloxetine, venlafaxine, quetiapine, and lithium. These medications were used in various combinations, both as monotherapies and polytherapies, throughout various points in his treatment. Each of these treatments was given adequate trials with multiple doses, allowing enough time to see the optimized benefits of each dosage trial. The patient was also adherent to all medications and dosed them as prescribed. He underwent multiple rounds of transcranial magnetic stimulation (TMS) as well. The patient reported that he experienced mild improvement of his irritability with use of quetiapine, but also experienced significant weight gain, which led to his discontinuation of this medication. He also experienced mild improvement of his depressed mood and anergia with use of duloxetine. Other symptoms of anhedonia, hypersomnia, and suicidal ideation persisted with moderate severity throughout all of these medication trials. TMS and all other medications trialed were considered ineffective at improving his symptoms. The patient had multiple hospitalizations due to suicidal ideation. His most recent hospitalization was one year prior to the initial visit.
The patient had no pertinent family history. The patient was 5’10” and weighed 200 pounds. He was married, heterosexual, had a high school education, and worked as a real estate agent. He had a stable socioeconomic status. He considered himself an atheist. He admitted to an unhealthy, Western-style diet. He denied any alcohol use and other illicit drug use, but he did occasionally smoke cigarettes. He drank one cup of coffee daily and had a relatively sedentary exercise life.
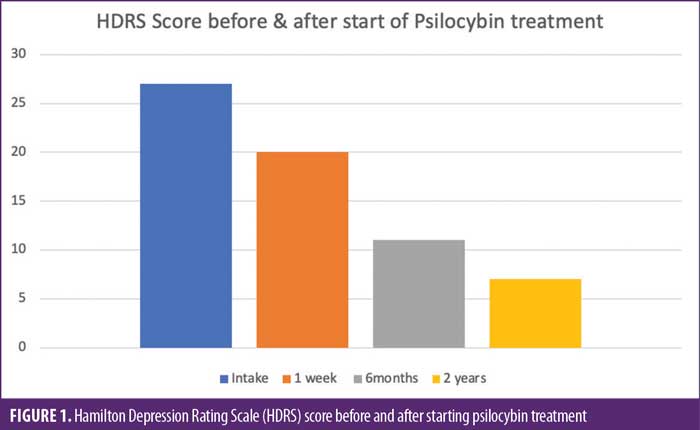
On physical exam, the patient appeared to be overweight. He had an obvious depressed mood and flat affect. There was mildly diminished insight. He was otherwise alert and oriented to time, place, and person. He was cognitively intact, with good short-term memory, long-term memory, and judgment. His gait appeared steady, and motor was grossly intact. Cranial nerves II to XII were intact. There were no obvious signs of pressured speech, response to internal stimuli, or psychomotor agitation during the time of exam. Speech was coherent, and thought process was somewhat impoverished. The patient had no known drug allergies. The patient was clinically evaluated and diagnosed with MDD based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria. The patient was administered the Hamilton Depression Rating Scale (HDRS), a 17-item, provider-administered tool to assess for depression. This scale was first administered upon initial intake, and the patient had a score of 27, which is classified as very severe depression.
After initial evaluation, the patient failed a six-month course of increasing doses of nortriptyline, which was 150mg orally every night at its highest dose, followed by a trial of lamotrigine, which maxed out at 200mg orally twice per day. Both medication trials resulted in minimal-to-no improvement of symptoms, and both were subsequently discontinued.
He underwent serum lab work to rule out any other medical etiologies, which were all within normal limits. Lastly, he underwent genetic testing for psychiatric medications, which resulted in minimal beneficial information in this patient’s case. The patient was then recommended to undergo ECT therapy. He underwent ECT treatments three times a week for four weeks, for a total of 12 ECT sessions. The patient had the most pronounced improvement after ECT, reporting moderate improvement of his depressed mood, anhedonia, anergia, and suicidal ideation. Despite these positive results, the patient felt that the treatment was expensive, invasive, and created unwanted side effects, such as headaches, confusion, memory loss surrounding sessions, and cognitive impairment. Despite being advised against the use of illicit substances, based upon his own research, the patient began to self-administer microdoses of psilocybin. This treatment was done as monotherapy, as all other psychiatric medications and treatments were discontinued.
Treatment
The patient began to grow his own hallucinogenic mushrooms, namely Psilocybe cubensis. The patient then dried out the mushrooms and ground them into a fine powder. He used the Fadiman Protocol, which is recommended for beginners to microdosing. This entails weighing out the powder, starting at 0.1g, and encapsulating it. The patient took one capsule on the morning of Day 1, followed by two days of no dosing, then took one capsule on Day 4, followed by another two days of no dosing. This cycle was repeated for eight weeks. After taking a four-week break from dosing, he restarted the eight-week cycle. With this protocol, the patient is meant to start at 0.1g, and may titrate up to 0.2g or 0.3g, if needed. The goal of microdosing is to create a sense of happiness and wellbeing, without any hallucinogenic effects. After one week, the patient maintained on the 0.2g dose, and continued this regimen for three years.
Upon taking the HDRS one week after starting self-administration of psilocybin, which entailed three total doses, the patient had a score of 20. He noted great improvement in delusions of guilt, as well as improved appetite, libido, and anxiety symptoms. The HDRS was administered six months after starting psilocybin treatment. At this point, the patient underwent two rounds of the aforementioned Fadiman Protocol and scored 11 on the HDRS. He experienced notable improvement in his anhedonia, depressed mood, and suicidal ideations. The same test was administered two years later, at which point the patient had finished eight total rounds of the protocol, and the patient scored 7 on the HDRS, which classified his depression as in remission (Figure 1). At last follow-up, the patient reported being able to function normally, and that the use of psilocybin has not affected his day-to-day life. He reported mild nausea after his first two doses but denied any other side effects.
Discussion
Psilocybin is a prodrug of psilocin 4-hydroxy-dimethyltryptamine (psilocin), a serotonin receptor agonist and classic psychedelic drug whose principal psychoactive effects are mediated by serotonin 2A (5-HT2A) receptor agonism. The mechanism of action creates a novel avenue of treatment for TRD. Research into psilocybin for the treatment of depression has been emerging in recent years, and multiple studies have shown benefits in this setting.7
A pilot study was done in 2016 by Carhart-Harris et al8 wherein patients with diagnosed TRD showed improvement of depressive symptoms one week after a two-dose treatment with psilocybin and had sustained improvement at three-month follow-up. Five of the 12 participants experienced complete remission of depression at the three-month point.8
A waitlist randomized, controlled trial (RCT) by Davis et al9 had 27 participants who were randomized to either an immediate treatment group or delayed treatment group, which started eight weeks later. Both groups underwent two different psychotherapy sessions while dosed with psilocybin. Measurements of their depressive symptoms were measured using the GRID Hamilton Rating Scale for Depression (GRID-HAMD) scale at Weeks 1 and 4 for the immediate group and at Weeks 5 and 8 for the delayed group. The findings showed statistically significant improvement of depressive symptoms based on these clinical scales. Thirteen participants were in complete remission four weeks after their respective treatments.9
The last study noted for depression was a double-blind RCT by Carhart-Harris et al10 that compared the use of psilocybin and escitalopram in the treatment of depression. The participants in this study had long standing history of moderate-to-severe MDD. There was a total of 59 patients who were randomized to either the psilocybin group or escitalopram group. Those in the psilocybin group received 25mg doses of psilocybin three weeks apart, in addition to six weeks of daily placebo. Those in the escitalopram group received two 1mg doses of psilocybin three weeks apart, in addition to six weeks of daily oral escitalopram. Outcomes were measured using the Quick Inventory of Depressive Symptomatology (16-Item) (Self-Report) (QIDS-SR-16) scale. Remission of depression, characterized by a QIDS-SR-16 score of greater than 7, was found in 57 percent of the psilocybin group, compared to 28 percent of the escitalopram group.10
Due to the nature of psilocybin being a Schedule 1 substance, it is important to evaluate the safety profile of the compound. One study by Johnson et al11 evaluated the abuse potential of psilocybin based on the eight criteria used by the Controlled Substances Act. They evaluated the scientific pharmacological effects, such as tolerance, physical dependence, toxicity, and pharmacodynamics. They also studied historical patterns of abuse of psilocybin, compared to other illicit substances. Using the Treatment Episode Datasets (TEDS), they showed that hallucinogens made up 0.1 percent of the primary substance of abuse in people aged 12 years and older from 2005 to 2015. In 2015, the percentages for opiates, cocaine, and alcohol were 33.4, 4.9, and 33.9 percent, respectively. The Drug Abuse Warning Network (DAWN) evaluated numbers of drug-related emergency department visits by comparing them between types of illicit substances. In 2011, over five million drug-related emergency department visits were recorded. Of those, over 6,000 were related to psilocybin, compared to 855,000 for opiates, 505,000 for cocaine, and 724,000 for alcohol. The literature has shown very little evidence of psilocybin-related deaths or overdoses. A study done in the United Kingdom (UK) evaluated the overdose/death potential of illicit substances, and psilocybin was rated lowest on a scale of 20 different substances.11
Limitations. The limitations of the report include very small sample size (a single patient) and a lack of formalized administration of the compound by a high-level clinician. RCTs are needed to confirm our findings.
Conclusion
In the case described here, we report a patient with severe TRD who successfully self-treated his TRD with psilocybin in microdoses. The patient had a significant reduction of depressive symptoms, as shown by his HDRS scores over time. The current published research is limited, but it shows efficacy, tolerability, and a relatively good safety profile for the compound. Because of this, psilocybin could be an alternative to ECT, which is expensive, more difficult to access, and has a harsher known side effect profile. This case study shows an individualized positive response, which, coupled with current research, indicates the need for continued exploration and further clinical trials for the use of psilocybin in the setting of TRD.
References
- National Institute of Mental Health. Major Depression. Updated Jan 2022. https://www.nimh.nih.gov/health/statistics/major-depression#:~:text=The%20prevalence%20of%20major%20depressive%20episode%20was%20highest,episode%20was%20highest%20among%20individuals%20aged%2018-25%20%2813.1%25%29. Accessed 20 Oct 2021.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(Suppl 8):17–25.
- Pandarakalam JP. Challenges of treatment-resistant depression. Psychiatr Danub. 2018;30(3):273–284.
- Goldberg JF. Electroconvulsive therapy: still the gold standard for highly treatment-resistant mood disorders. CNS Spectr. 2021;1–2. Epub ahead of print.
- Kellner CH, Greenberg RM, Murrough JW, et al. ECT in treatment-resistant depression. Am J Psychiatry. 2012;169(12):1238–1244.
- Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology. 2017;42(11):2105–2113.
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627.
- Davis AK, Barrett FS, May DG, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(5):481–489.
- Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402–1411.
- Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143–166.





