by Sadiq Naveed, MD; Afshan Naz Amray, MD; Nusrat Jahan, MD; Fatima Bilal Moti-wala, MD; and Muhammad Hassan Majeed, MD
Dr. Naveed is with the University of Kansas Medical Center in Kansas City, Kansas. Dr. Amray is with Dow University of Health Sciences in Karachi, Pakistan. Dr. Jahan is with Rush University Medical Center in Chicago, Illinois. Dr. Motiwala is with the Texas Tech University Health Sciences Center in Lubbock, Texas. Dr. Majeed is with Johns Hopkins Hospital in Baltimore, Maryland.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Anxiety disorders are the most prevalent psychiatric disorders among youth, with prevalence rates ranging from 25 to 32 percent. These disorders are under-recognized and often undertreated in this population. Anxiety disorders in youth exhibit a chronic and persistent course of symptoms with a higher risk of comorbidities, functional impairment, and worsening of severity. The early recognition and treatment of anxiety disorders in children and adolescents are vital for better long-term outcomes. This article summarizes the evidence- based pharmacologic treatments for mixed anxiety disorders including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder in children and adolescents based on case reports, case series, open-label trials, and randomized, controlled trials.
Keywords: Anxiety, pediatric, pharmacotherapy, psychotherapeutic treatment
Innov Clin Neurosci. 2019;16(9–10):36–43
Anxiety disorders are among the most common psychiatric diagnoses in children and adolescents.1 The 12-month or lifetime prevalence of anxiety disorders in adolescents is estimated to be between 25 to 32 percent.2,3 In a National Comorbidity Survey Replication Adolescent Supplement, the prevalence of panic disorder was 35 percent, generalized anxiety disorder (GAD) was 32 percent, and separation anxiety disorder was 25 percent among people in the 13- to 17-year age group.4 Anxiety disorders in children and adolescents can have a spontaneous remission or a chronic course with rare spontaneous remission.4 In preadolescent children, the range of anxiety disorders varies widely from 2.6 to 41 percent.5,6 In a worldwide survey of mental health disorders, the prevalence of any anxiety disorder was 6.5 percent in children and adolescents.7
Children and adolescents with untreated anxiety disorders are at a higher risk of suicidal ideation, suicide attempts, academic impairment, and social dysfunction, compared to the general population.8 Moreover, youth with anxiety disorders are more likely to develop secondary anxiety disorders; depressive disorders; and nicotine, alcohol, and/or other substance dependency or use disorder.8,9 GAD in adolescents, for example, is strongly associated with panic disorders and phobias (odds ratio [OR]: 7.06; 95% confidence interval [CI]: 4.40–11.34; p<0.001) and phobias (OR: 2.40; 95% CI: 1.94–2.97; p<0.001).10 Behavioral inhibition and avoidance are general temperamental features associated with anxiety disorders in children and adolescents.11 Stressful events in life can also contribute to anxiety symptoms in children.12
The early recognition and treatment of anxiety disorders can decrease the burden of illness and improve the functional outcomes in children and adolescents.9 There are several treatment options available for pediatric anxiety disorders, including psychotherapy (e.g., cognitive-behavioral therapy [CBT], mindfulness therapy, psychodynamic therapies) and pharmacologic interventions.13 In this article, we focus on pharmacological treatments, reviewing the evidence obtained from case reports, case series, open-label trials, and randomized, controlled trials (RCTs), that supports the use of drug therapies for treatment of mixed anxiety disorders in children and adolescents, including GAD, social anxiety disorder, and separation anxiety disorder. These three disorders were specifically selected for our review because, historically, they have been studied together in clinical trials, with similar responses to different treatment options. Additionally, as a group, these three anxiety disorders have higher rates of comorbidities, compared to other childhood-onset anxiety disorders, such as obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD).14
Methods
This article reviews the evidence for use of fluoxetine, escitalopram, fluvoxamine, sertraline, guanfacine, clomipramine, atomoxetine, clonazepam, and N-acetylcysteine (NAC) in the treatment of mixed anxiety disorder, GAD, social anxiety disorder, and separation anxiety disorder among children and adolescents. In April 2018, two electronic databases (PubMed and Scopus) were systematically searched for relevant publications using the following search terms: anxiety disorder AND psychopharmacology OR medications AND children OR adolesce OR youth. The search term adolesce was used to account for adolescence and adolescent search terms. Only original research in humans were selected. Two independent investigators then performed a manual review of the selected articles’ reference sections using the same search terms, which yielded additional articles. A total of 274 articles were selected. Search results were imported to Endnote X7 (Clarivate Analytics, Philadelphia, Pennsylvania) to remove duplicates. Two independent investigators reviewed the titles and abstracts (when available) and then the full text of each article, using predetermined eligibility criteria. In cases of ambiguity, a decision to include or exclude an article was reached through consensus among the investigators or under guidance from a senior reviewer (S.N.). Our inclusion criteria included case reports, case series, open-label trials, and RCTs. Abstract-only articles, conference papers without original data, review articles, theses, posters, book chapters, editorials, letters, and commentaries were excluded. No restrictions on language, country, publication year, age, sex, or ethnicity of patients were applied.
Results
Sixteen articles met our inclusion criteria, comprising seven RCTs, four open-label trials, one double-blind cross-over study, one chart review, one case series, and one case report. Two secondary analyses of the two RCTs were also included. Table 1 provides a summary of these studies.

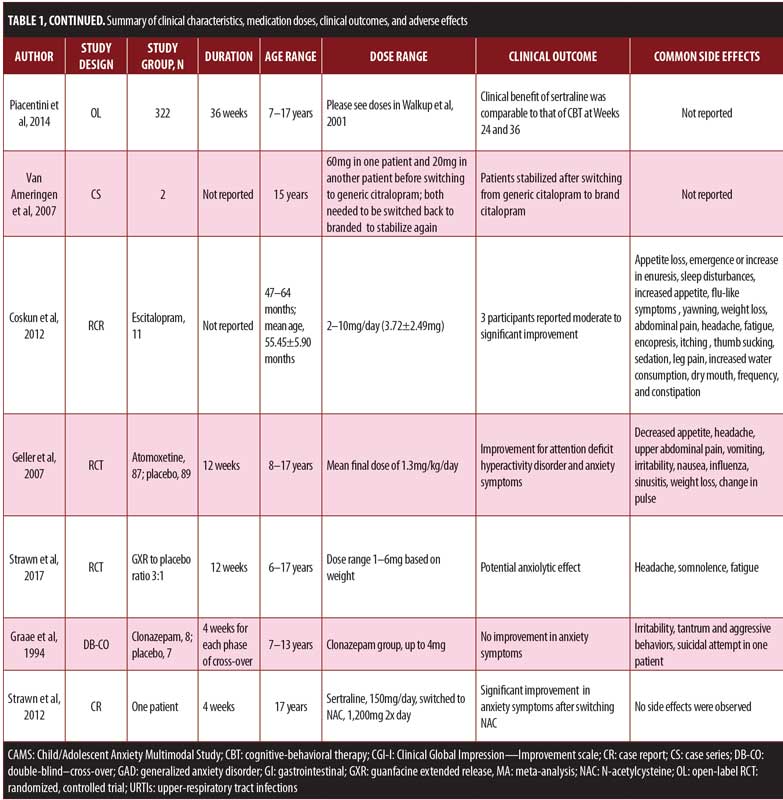
Fluoxetine. In a 12-week RCT, youths ranging in age from 7 to 17 years with a diagnosis of GAD, social anxiety disorder, or separation anxiety disorder and with significant clinical impairment were randomized to receive fluoxetine (n=37) or placebo (n=37).15 Fluoxetine was initiated at 10mg/day and titrated to 20mg/day, if it tolerated. The Clinical Global Impression-Improvement (CGI-I) scale was used to measure improvement, while the CGI-Severity (CGI-S) scale was used to measure severity; response was defined as a CGI-I score of two points or less.9 About 61 percent of the participants in the fluoxetine group showed improvement, compared to 35 percent in the placebo group. Among the participants with social phobia, 76 percent in the fluoxetine group achieved a CGI-I score of two points or less compared to 21 percent of the placebo group. For GAD, about 67 percent of the fluoxetine group achieved the target outcome versus 36 percent of the placebo group. However, there was no significant difference between fluoxetine (54%) and placebo (45%) for improvement in social anxiety disorder symptoms. Fluoxetine was better tolerated than the placebo in this study. Mild transient headaches and gastrointestinal symptoms were observed in the fluoxetine group; five children on fluoxetine discontinued the study due to significant agitation.15
In an open-label, long-term extension of an RCT, Clark et al16 assessed the efficacy of fluoxetine for treatment of childhood anxiety disorders for one year, which included youths ranging in age from 7 to 17 years with a diagnosis of GAD, social anxiety disorder, or separation anxiety disorder. Of 74 youths from the original RCT, 56 participated in the open-label extension trial. The greatest improvement was observed in the patients who were switched from placebo in the RCT to fluoxetine in the open-label study (patient number and dosages not provided). The greatest worsening in severity was observed in participants who received the placebo in the acute period followed by no medication during the follow-up period. The proportion of participants with a CGI-S score indicating ‘‘normal, not at all ill’’ was highest among the RCT-fluoxetine/follow-up-fluoxetine group, followed by the RCT-placebo/follow-up-fluoxetine group; no participants from the RCT-fluoxetine/follow-up-no medication group or RCT-placebo/follow-up-no medication group achieved this score. Separately, the proportion of participants achieving the defined CGI-I response score reflecting ‘‘very much improved’’ was highest among the RCT-fluoxetine/follow-up-fluoxetine group, followed by the RCT-placebo/follow-up-fluoxetine group, then RCT-fluoxetine/follow-up-no medication group, with the RCT-placebo/follow-up-no medication group last.16
In an open-label, 6- to 9-week trial, Fairbanks et al17 assessed the efficacy and tolerability of fluoxetine in 16 patients ranging in age from 9 to 17 years with overanxious disorder, avoidant disorder, social anxiety disorder, panic disorder with or without agoraphobia, or selective mutism. Participants with a history of exposure to psychotherapy were included. For children under 12 years of age, the initial dosage was 5mg/day, which was increased to 10mg/day at Week 2, 15mg/day at Week 3, and 20mg/day at Week 4, with a maximum dose of 40mg/day for the remaining four weeks of the study. For adolescents 12 years or older, the initial dose was 5mg/day, which was titrated to 10mg/day at Week 2 and 20mg/day at Week 3. In adolescents with no improvement by Week 5, fluoxetine was titrated to 40mg/day and then to 60mg/day at Week 6 and finally to 80mg/day for the remainder of the trial. The CGI-S scale showed clinically significant improvements in severity of anxiety after five weeks of treatment with fluoxetine. Clinical improvement (CGI-I score less than 3) was reported in 100 percent of patients with separation anxiety disorder, 80 percent of those with social anxiety disorder, 67 percent with specific phobia, 60 percent with panic disorder, and 15 percent with GAD. The most common adverse effects were drowsiness, difficulty falling asleep or staying asleep, decreased appetite, nausea, abdominal pain, and easy excitability. Other rare side effects were dizziness, heartburn, sweating, lethargy, and irritability.17
Fluoxetine versus clomipramine. In a 12-week, randomized, double-blind, placebo-controlled trial, da Costa18 compared the efficacy of clomipramine (n=9), fluoxetine (n=10), and placebo (n=11) in children/adolescents (age range: 7–17 years). These participants were diagnosed with GAD and/or separation anxiety disorder and/or social anxiety disorder. The dosages of fluoxetine ranged from 10 to 40mg/day for children and up to 60mg/day for adolescents. Clomipramine dosages varied from 25 to 150mg/day (5mg/ kg/day) for children and 225mg/day for adolescents (average clomipramine and fluoxetine doses at Week 12 were 118.8mg and 35mg, respectively). The treatment response rates (CGI-I score of 2 or 1) were 87.5 percent in the clomipramine group, 100 percent in the fluoxetine group, and 77.7 percent in the placebo group. The remission rate followed the same pattern, with 100 percent in the fluoxetine group, 75 percent in the clomipramine group, and 44.4 percent in the placebo group. Reported feelings of confusion were more prevalent in the clomipramine group compared to fluoxetine and placebo groups. Children taking fluoxetine reported side effects that included sedation, malaise, abdominal discomfort, excessive salivation, tachycardia, and excessive sweating.18
Fluvoxamine. In an eight-week double-blind RCT, Pine et al19 compared fluvoxamine (n=63) to placebo (n=65) in children/adolescents (age range: 6–17 years) with social anxiety disorder, GAD, and/or separation anxiety disorder who did not improve after three weeks of psychotherapeutic interventions. Supportive psychotherapy was continued throughout the trial. In adolescents, fluvoxamine was titrated 50mg/week to a maximum dose of 300mg/day. For children 6 to 11 years of age, the maximum dose was 250mg/day. Improvements in Pediatric Anxiety Rating Scale (PARS) scores (data not provided) were observed in the fluvoxamine-treated patients. Global improvement was reported on the CGI-I scale for a score of 3 or less. Five patients discontinued treatment in the fluvoxamine group due to adverse effects that included sedation, somatic discomfort, and hyperactivity; one patient in the placebo group discontinued due to irritability. An additional five patients in the fluvoxamine group discontinued the study due to nonadherence and/or inability to swallow medications. Side effects included abdominal discomfort, increased motor activity, headache, nausea, upper respiratory infection, vomiting, sore throat, fatigue, muscle or joint pain, and decreased appetite.19
The RCT on fluvoxamine19 was followed by an open-label trial (6 months) by Walkup et al20 that compared the efficacy of fluvoxamine, fluoxetine, and placebo for participants aged 6 to 17 years. All subjects from the RCT were invited to participate in this open-label trial. Treatment response was defined using the CGI-I scale. Participants with a CGI-I score of 3 or less were considered responders, whereas participants who exhibited scores CGI-I scores greater than 3 were considered nonresponders. In this open-label trial, responders to fluvoxamine (n=35) were continued on fluvoxamine, fluvoxamine nonresponders were switched to fluoxetine (n=14), and placebo nonresponders were switched to fluvoxamine (n=48). The CGI-I scale was chosen as the primary outcome measure of improvement, and response was defined by relative change from baseline to study endpoint.20
For fluvoxamine responders, the average dose was 131±86mg/day and 33 of 35 patients in the open-label trial continued to report improvements.20 Two fluvoxamine participants withdrew from the open-label study due to lack of efficacy. Fluvoxamine nonresponders from the original RCT were started on fluoxetine in the open-label trial at 10mg/day for one week, followed by 20mg/day for an additional five weeks. The dose of nonresponders was increased to 40mg/day, and average final dose in the open-label trial was 24±11mg/day. About 71 percent of this group met the criteria of response based on CGI-I scales scores. Placebo nonresponders from the original RCT were started on fluvoxamine, which was increased by approximately 50mg/week until Week 6 to a maximum dosage of 50mg/day in children and 300mg/day in adolescents. The final dose was 135±88mg/day in this group. About 56 percent of the participants in this group were considered responders at the end of the open-label study.20 Based on these results, fluvoxamine appears to be the safe choice for the treatment of pediatric anxiety disorders with fluoxetine as an alternative
Fluvoxamine and methylphenidate. Children and adolescents aged 6 to 17 years with attention-deficit/hyperactivity disorder (ADHD) and co-occurring anxiety disorders (separation anxiety disorder, GAD, social anxiety disorder) participated in a four-phase study by Abikoff et al.21 In Phase 1, participants (n=42) who were previously stable on stimulants underwent a washout period of five days to assess baseline symptoms of ADHD and anxiety. In Phase II, participants (n=36) were started on immediate-release methylphenidate, and the dose was optimized to maximum of 40mg/day for children weighing less than 25kg, or 50mg/day for children weighing more than 25kg. These dosages were adjusted based on clinical response and side effects. Phase III was the stabilization phase with an assessment for response to ADHD treatment and presence of ongoing impairment due to anxiety symptoms. In Phase IV, participants were randomized to an eight-week trial of continued methylphenidate + fluvoxamine or methylphenidate + placebo. Parents and teachers reported ADHD symptoms at baseline and throughout the study by using the Swanson, Nolan, and Pelham Rating Scale (SNAP-IV) and the Conners, Loney, and Milich Rating Scale. The clinician-administered PARS was used to assess for anxiety. Clinical improvement was measured using the CGI-I scale, and participants who exhibited a score of 3 or less were categorized as responders. After stabilization of ADHD symptoms, 25 participants were randomized into fluvoxamine (n=15) or placebo (n=10) groups. These participants continued to use methylphenidate and either fluvoxamine or placebo during this phase. Three participants withdrew due to side effects that included headache, nausea, vomiting, fever, sedation, and rash, where as one patient had worsening of anxiety and one was lost to follow-up. The methylphenidate-fluvoxamine group did not differ significantly from the methylphenidate-placebo group in the way of baseline PARS total and SNAP composite scores. The average daily dosages in the methylphenidate-fluvoxamine and methylphenidate-placebo groups at the end of Phase IV were 145.4mg and 202.4mg, respectively. The methylphenidate-fluvoxamine group reported side effects such as stomachache and muscle pain, whereas crying, biting nails, rash, and dry mouth were commonly reported in the methylphenidate-placebo group.21
Sertraline. The Child/Adolescent Anxiety Multimodal Study (CAMS),13,14,22 an RCT that included 488 children and adolescents, ranging in age from 7 to 17 years, with separation anxiety disorder, GAD, and/or social anxiety disorder, randomized participants into four treatment groups: 1) sertraline, 2) CBT, 3) sertraline and CBT, or 4) placebo. At Week 12 of the RCT, the mean dose of sertraline in the sertraline-only group was 146±60.8mg/day and in the combination group 133.7±59.8mg/day. Improvement (CGI-I score <2) was observed in about 80 percent of patients in the combination group, 59.7 percent in the CBT-only group, 54.9 percent in the sertraline-only group, and 23.7 percent in the placebo group. The combination treatment was superior to all of the other treatments studied, and sertraline and CBT monotherapies achieved better results than placebo (effect size: 0.86 for combination therapy, 0.45 for sertraline, and 0.31 for CBT). Side effects, including fatigue, sedation, and restlessness, were common in the sertraline group, while symptoms of suicidal and/or homicidal ideation (not reported at baseline) did not significantly differ between groups.13 Of the 488 particpants, 322 (78.2%) completed the study.22
In the CAMS six-month extension study by Piacentini et al,23 325 (78.9%) of the participants from the 12-week RCT completed the 36-week assessment. At Week 36, 82.69 percent of the participants in the combination group continued to show improvement. Responders in the combination (83%), sertraline (82%), and CBT (80%) groups continued to have a consistent positive response at Weeks 24 and 36. At Week 36, the effect size of 0.34 to 0.41 suggests small clinical benefits of combination treatment over monotherapy. The clinical advantage of sertraline was comparable to that of CBT at Weeks 24 and 36. The investigators assessed the reported adverse events in the CAMS RCT using 1) a standard questionnaire that was completed by the study coordinator and reviewed by the primary clinician and 2) the Physical Symptom Checklist, a self-report questionnaire. The PSC scores were not shared with the primary clinician. Investigators observed no difference between the sertraline and placebo groups in terms of individual or total physical and/or psychiatric adverse effects. When the researchers controlled for the number of reporting opportunities, insomnia, fatigue, and sedation occurred more frequently in patients treated with sertraline monotherapy than those who received the combination therapy. New physical symptoms like abdominal pain, difficulty breathing, numbness, and tingling of the legs or arms were observed more frequently in the placebo group. There was a decline in total PSC scores over time, without any significant differences between the treatment arms. The rates of adverse events, including suicidal and homicidal ideations, were comparable between placebo and sertraline groups. Side effects like fatigue, sedation, restlessness, irritability, and homicidal ideations were observed more frequently in the sertraline groups compared to CBT monotherapy. Other adverse effects in the sertraline group included headache, gastric distress, insomnia, cold symptoms, body aches, and sedation. Psychiatric symptoms such as disinhibition, increased motor activity, and disobedient and defiant behaviors were reported in the combination group.24
Citalopram. A case series by van Ameringen et al25 reviewed the potential exacerbation of symptoms in 20 patients between the ages of 15 and 55 years after being switched from branded to generic formulations of citalopram. There were two adolescents in this case series. One of the adolescenets, diagnosed with panic disorder and agoraphobia, was taking 60mg/day of branded citalopram before the switch and was stabilized on the same dose after two weeks. Increased irritability was reported after 2.5 weeks in this patient, necessitating the change from generic citalopram to brand citalopram. The other patient, who was diagnosed with panic disorder, agoraphobia, and social phobia, and was taking 20mg/day of branded citalopram before the switch and was stabilized on 30mg after six weeks. Increased depressive symptoms were reported after four weeks in this patient, necessitating the change from generic citalopram to branded citalopram.25
Escitalopram. The efficacy and tolerability of escitalopram in preschool children was evaluated in a retrospective chart review by Coskun et al,26 which included 11 children (8 girls, 3 boys, 55.45±5.90 months in age; range: 47–64 months) who were taking escitalopram (2–10mg/day, 3.72±2.49 mg).26 Diagnoses included the following (some children had more than one diagnosis): OCD (n=6), PTSD (n=3), separation anxiety disorder (n=2), social anxiety disorder (n=2), specific phobia (n=2), and GAD (n=1) by clinical interview. Three participants showed moderate to significant improvement in symptoms of separation anxiety disorder, GAD, social anxiety disorder, and specific phobias (e.g., lightening, animals). Of the 11 children, five exhibited significant behavioral disinhibition following initiation of escitalopram, of which three children dropped out of the study. Other reported side effects included appetite loss (n=4), emergence or increase in enuresis (n=3), sleep disturbances (n=3), increased appetite (n=2), flu-like symptoms (n=2), yawning (n=2), weight loss (n=2; 1,000–3,000 grams), abdominal pain (n=2), headache (n=2), fatigue (n=1), encopresis (n=1), itching (n=1), thumb-sucking (n=1), sedation (n=1), leg pain (n=1), increased water consumption (n=1), dry mouth (n=1), frequency (n=1), and constipation (n=1).26
Atomoxetine. In a double-blind, 12-week RCT, the efficacy of atomoxetine was assessed for GAD and social anxiety disorder with comorbid ADHD in children/adolescents ranging in age from 8 to 17 years.27 In this study, participants were randomly assigned to either atomoxetine (n=87) or placebo (n=89). About 75 percent of the recruited participants completed the 12-week-long trial. After randomization, 26 participants dropped out due to adverse effects (n=2), lack of efficacy (n=11), personal conflict (n=6), protocol violation (n=4), relocation of participant (n=1), sponsor’s decision (n=1), and lack of contact (n=1). Atomoxetine was initiated at 0.8mg/kg/day (for 3 days), titrating to 1.2mg/kg/day with a mean final dose of 1.3mg/kg/day. For patients with residual ADHD symptoms, the dose was maximized at 1.8mg/kg/day at the sixth visit with a maximum daily dose of 120mg. Atomoxetine-treated patients significantly improved in terms of anxiety and ADHD ratings. Specificly, atomoxetine was well-tolerated, and the most common side effects included decreased appetite, headache, abdominal pain, vomiting, irritability, nausea, influenza, sinusitis, weight loss, and increased heart rate.27
Guanfacine (extended release) (GXR). GXR was evaluated in a 12-week, double-blind, RCT of children/adolescents aged 6 to 17 years with GAD, separation anxiety disorder, and/or social anxiety disorder.28 Eighty-three patients were randomized 3:1 to GXR (n=62) or placebo (n=21). GXR was initiated at a dose of 1mg/day and was flexibly dosed to a maximum of 0.12mg/kg/day weekly (dose range: 1–6mg), based on clinical response, tolerability, and investigator judgment. During the dose-optimization and maintenance phases, 54.2 percent of participants in the GXR group reported improvement (CGI-I scale score of 2 or less) compared to 31.6 percent of placebo-treated participants, as reported by the clinical investigator. Inadequately powered study and unbalanced randomization were important limitations of this study. Participants in both the GXR group and placebo group exhibited exhibited similar decreases, from baseline to study endpoint, in heart rate, blood pressure, and electrocardiogram readings. Suicidal ideation occurred in four (6.5%) participants taking GXR and three (14.3%) on placebo, with no suicide attempts. The most commonly reported adverse events were tachycardia, blurred vision, fatigue, dizziness, postural dizziness, anxiety, emotional disorder, mood-related changes, and panic attack. The most frequent side effects of GXR were headache (35.5%), somnolence (27.4%), and fatigue (21.0%).28
Clonazepam. In a double-blind, crossover pilot study, the efficacy and safety of clonazepam in 15 children (7–13 years old) with an anxiety disorder for at least six months were evaluated.29 Participants were randomly assigned to clonazepam (n=8) or placebo (n=7) for four weeks. Clonazepam was started at 0.25mg/day and increased in increments of 0.25mg over three days to reach a dose of 1mg/day. After this, it was increased in increments of 0.25mg every two days to reach a maximum dose of 2mg/day, unless side effects or adherence issues occurred. After four days at the maximum dose, the medication was tapered to zero by the end of each phase. Children were closely monitored weekly for dose adjustment, clinical symptoms, and any adverse events. Two children dropped out due to increased irritability, tantrum, and aggressive behaviors (one of which attempted suicide), and one dropped out due to nonadherence. Twelve children completed the trial, with nine children demonstrating moderate to significant clinical improvement and three reporting no improvement. At the end of the study, six children reported complete remission (assessment method not provided). However, there was no significant difference in anxiety symptoms on the Diagnostic Interview Schedule for Children and the Children’s Manifest Anxiety Scale among both arms. The incidence of side effects was higher with clonazepam compared to placebo.29
N-acetylcysteine. In a case report of a 17-year-old adolescent with GAD and social anxiety disorder who failed to respond to sertraline monotherapy (150 mg/day), adjunctive N-acetylcysteine (1,200mg twice daily) resulted in significant improvement in his anxiety symptoms (a CGI-I scale score <2) and was well-tolerated.30
Discussion
Based on our review of the literature, in pediatric patients with anxiety disorders, the most frequently studied pharmacotherapies are selective serotonin reuptake inhibitors (SSRIs). Additionally, our results indicate that the effects of pharmacotherapy appear to be amplified by the addition of psychotherapy. The combination of sertraline and CBT in CAMS was associated with a large effect size of 0.86.13 While combination therapy has demonstrated better treatment prognosis, the dosages of SSRIs were less in combination therapy than when used as a monotherapy, without any psychosocial interventions.13 The results of CAMS suggest CBT plays a favorable role in the treatment of anxiety disorders in children and adolescents. SSRIs and selective serotonin-norepinephrine reuptake inhibitors (SNRIs) appear to be efficacious in this population as well.
Strawn et al31 found that antidepressant-related treatment response is usually seen within the first two weeks of treatment in pediatric patients with anxiety, and it is significantly different by six weeks of treatment compared to placebo. High doses of SSRIs appear more likely to achieve a rapid treatment response than lower doses.
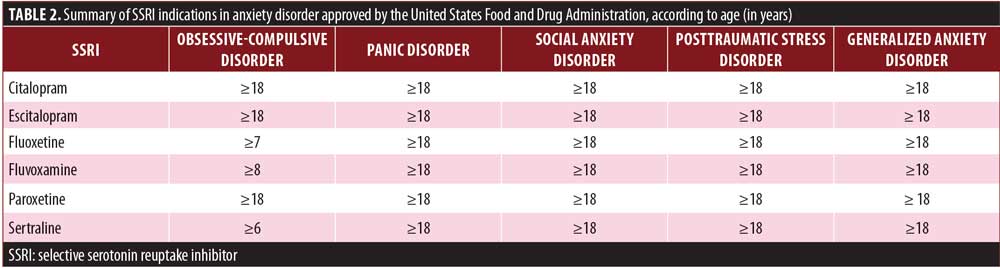
The clinical efficacy of SSRIs has been established in long-term studies (9-month study by Birmaher et al15 and 1-year extension study by Clark et al.16 Several SSRIs have been approved by the United States Food and Drug Administration (FDA) for the treatment of anxiety disorders in children/adolescents, and include the medications citralopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline (Table 2). Moreover, a more rapid and greater response has been observed using SSRIs in the pediatric population, compared to SNRIs.31
For pediatric anxiety disorders, the recommended duration of pharmacologic treatment is 6 to 9 months.32 However, some clinicians continue pharmacologic treatment for 12 months based on adult literature. Older age, female sex, minority status, low socioeconomic status, comorbid internalized disorders, social anxiety disorder, the severity of baseline anxiety symptoms, and adverse life events adversely impact the level of response and long-term remission rate of anxiety disorders in children and adolescents. Functional recovery is an indicator of clinical remission and is an important factor to consider when deciding the duration of pharmacological therapy in this patient population.32
Use of SSRIs or SNRIs for treating anxiety disorders in pediatric patients requires monitoring for transient and mild side effects including headache, gastric distress, insomnia, fatigue, and sedation.13 Suicidal ideations are less frequent but are a serious side effect of SSRIs and SNRIs.33 Certain anxiolytics in children/adolescents can cause behavioral activations (e.g., irritability, excitability, agitation, aggression), which may require or cause discontinuation of these medications.34 Reported side effect risks are greater in SNRIs and clomipramine than in SSRIs, of particular note is treatment-emergent suicidality, which has been reported with venlafaxine.18,31 Electrocardiogram and blood-level monitoring is sometimes required when using tricyclic antidepressants (TCAs).35 There is a higher incidence of sudden death with TCAs as well.36
Conclusion
Anxiety disorders can develop early in childhood and can run a protracted course into adulthood, increasing the risk of secondary anxiety and mood disorders. Early identification and management of anxiety disorders in children/adolescents is important. Psychotherapy, particularly CBT, is usually the first line of treatment for several anxiety disorders in children/adolescents. SSRIs and SNRIs have consistently demonstrated a moderate positive effect size in the treatment of anxiety disorders in child and adolescent populations. A combination of psychotherapy and pharmacologic agents usually results in better treatment outcomes, compared to monotherapy, in this patient population.
References
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631–648.
- Avenevoli S, Avenevoli S, Costello EJ, et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):372. doi:10.1001/archgenpsychiatry.2011.160.
- Merikangas KR, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. Adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989.
- Kessler RC, Avenevoli S, Costello J, et al. Severity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):
381–389. - Paulus FW, Backes A, Sander CS, Weber M, von Gontard A. Anxiety disorders and behavioral inhibition in preschool children: a population-based study. Child Psychiatry Hum Dev. 2015;46(1):150–157.
- Cartwright-Hatton S, McNicol K, Doubleday E. Anxiety in a neglected population: prevalence of anxiety disorders in pre-adolescent children. Clin Psychol Rev. 2006;26(7):817–833.
- Polanczyk G V., Salum GA, Sugaya LS, et al. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–365.
- Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence.
J Am Acad Child Adolesc Psychiatry. 2001;40(9):
1086–1093. - Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578.
- Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47.
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, et al. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Harv Rev Psychiatry. 1(1):2–16.
- Majeed MH, Khokhar MA, Abid M, et al. Frequency and correlates of symptoms of anxiety and depression among young caregivers of cancer patients: a pilot study. BMC Res Notes. 2018;11(1):631.
- Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):
2753–2766. - Compton SN, Walkup JT, Albano AM, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health. 2010;4(1):1.
- Birmaher B, Axelson DA, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423.
- Clark DB, Birmaher B, Axelson D, et al. Fluoxetine for the treatment of childhood anxiety disorders: open-label, long-term extension to a controlled trial.
J Am Acad Child Adolesc Psychiatry. 2005;44(12):1263–1270. - Fairbanks JM, Pine DS, Tancer NK, et al. Open fluoxetine treatment of mixed anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 1997;7(1):17–29.
- da Costa CZG, de Morais RMCB, Zanetta DMT, et al. Comparison among clomipramine, fluoxetine, and placebo for the treatment of anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23(10):687–692.
- Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med. 2001;344(17):1279–1285.
- Walkup J, Labellarte M, Riddle MA, et al. Treatment of pediatric anxiety disorders: an open-label extension of the Research Unit on Pediatric Psychopharmacology Anxiety Study. J Child Adolesc Psychopharmacol. 2002;12(3):175–188.
- Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):
418–427. - Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365.
- Piacentini J, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297–310.
- Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: Evaluating Safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190.
- Van Ameringen M, Mancini C, Patterson B, Bennett M. Symptom relapse following switch from Celexa to generic citalopram: an anxiety disorders case series. J Psychopharmacol. 2007;21(5):472–476.
- Cokun M, Öztürk M, Zorolu S. Escitalopram treatment in preschool children with anxiety disorders: a case series. Klin Psikofarmakol Bülteni-Bulletin Clin Psychopharmacol. 2012;22(3):262–267.
- Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1119–1127.
- Strawn JR, Compton SN, Robertson B, et al. Extended release guanfacine in pediatric anxiety disorders: a pilot, randomized, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2017;27(1):
29–37. - Graae F, Milner J, Rizzotto L, Klein RG. Clonazepam in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1994;33(3):372–376.
- Strawn JR, Saldaña SN. Treatment with adjunctive N-acetylcysteine in an adolescent with selective serotonin reuptake inhibitor-resistant anxiety. J Child Adolesc Psychopharmacol. 2012;22(6):472–473.
- Strawn JR, Mills JA, Sauley BA, Welge JA. The impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2018;57(4):235–244.e2.
- Hathaway EE, Walkup JT, Strawn JR. Antidepressant treatment duration in pediatric depressive and anxiety disorders: how long is long enough? Curr Probl Pediatr Adolesc Health Care. 2018;48(2):31–39.
- Zisook S, Trivedi MH, Warden D, et al. Clinical correlates of the worsening or emergence of suicidal ideation during SSRI treatment of depression: An examination of citalopram in the STAR*D study. J Affect Disord. 2009;117(1-2):63–73.
- Ferguson JM. SSRI Antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22–27.
- Preskorn SH, Jerkovich GS. Central nervous system toxicity of tricyclic antidepressants: phenomenology, course, risk factors, and role of therapeutic drug monitoring. J Clin Psychopharmacol. 1990;10(2):
88–95. - Biederman J. Sudden death in children treated with a tricyclic antidepressant. J Am Acad Child Adolesc Psychiatry. 1991;30(3):495–498.





