 by Alicia Barnes, DO, MPH; Maggie M. Wang, BS; Jordan Feltes, BS; Je Ko, MD, PhD; and Miguel A. Guzman, MD
by Alicia Barnes, DO, MPH; Maggie M. Wang, BS; Jordan Feltes, BS; Je Ko, MD, PhD; and Miguel A. Guzman, MD
Drs. Barnes and Ko are with the Department of Psychiatry, Saint Louis University School of Medicine in St. Louis, Missouri. Ms. Wang and Feltes are with the Saint Louis University School of Medicine in St. Louis, Missouri. Dr. Guzman is with the Department of Pathology, Saint Louis University School of Medicine in St. Louis, Missouri.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Recent studies have shown that psychiatric symptoms might be the only primary manifestation of central nervous system malignancy. As a result, clinicians might overlook a brain tumor diagnosis due to overlying psychiatric symptoms. We herein describe an adolescent female patient without tuberous sclerosis clinical features whose primary presentation for a subependymal giant-cell astrocytoma was restrictive eating disorder, urinary incontinence, and depression. The behavioral symptoms improved significantly following tumor resection. This case illustrates that compression of the frontal lobes and hypothalamus can manifest as primary psychiatric symptoms of subacutely manifested anhedonia, appetite loss, low energy, flat affect, and depressive mood. An absence of tuberous sclerosis clinical features should not preclude a diagnosis of subependymal giant-cell astrocytoma. Clinicians should maintain an index of suspicion for a brain tumor in patients with sudden-onset restrictive eating disorder and depression.
Keywords: Feeding and eating disorders, brain neoplasms, astrocytoma, depression, adolescent
Innov Clin Neurosci 2020;16(10–13)
Although rare, brain tumors have been known to present initially with only psychiatric symptoms. Frontal temporal-lobe tumors have typically been associated with abulia, personality change, and depression.1,2 Anorexia is often associated with lesions in the hypothalamus.2 The prefrontal cortex, especially the right hemisphere, is also implicated in the cognitive control of food intake.2 Trauma to the right anterior cerebral hemisphere has been correlated with the “gourmand” syndrome of preoccupation with food and fine dining.3 From 1970 to 2017, there were approximately 13 cases of eating disorders associated with cerebral hemisphere lesions reported.1,2 In all but one of these cases, patients had frontotemporal epilepsy in addition to psychiatric symptoms. Only three cases have reported third-ventricle tumors with anorexia.2,4–6 Subependymal giant-cell astrocytoma (SEGA) typically presents in patients with tuberous sclerosis complex (TSC) as hydrocephalus, increased intracranial pressure, and seizures. Few case reports to date have documented SEGA in the absence of TSC clinical signs or symptoms.7–10 Previous case reports have even documented SEGA with negative genetic testing for TSC, though there is speculation this phenomenon might be due to genetic mosaicism.7 This case report is the first to describe SEGA and lateral ventriculomegaly with an initial presentation of a restrictive eating disorder and depression.
Case Presentation
Our patient was a 17-year-old female individual who initially experienced appetite loss, limiting food intake to “a handful of grapes” per day. The emergency department (ED) history revealed her symptoms began three months prior to presentation to the hospital. Her guardian reported that, despite best efforts to entice her to eat, she would eat only “a handful of food” daily. After a break-up with her boyfriend two-and-a-half months prior to presentation to the ED, her symptoms included psychomotor retardation, anhedonia, hypersomnolence (up to 20 hours daily), and depressed mood without suicidal ideation. According to the patient’s caregiver, shortly after her break-up with her boyfriend, she also started having sudden near-syncopal episodes of blackening of the visual fields, followed by generalized weakness, with no loss of consciousness. Each episode lasted approximately 5 to 10 minutes. One month prior to presentation to the ED, she began to experience nausea, intermittent headaches, and nocturnal enuresis, which warranted a visit to her primary care provider. With no identifiable organic cause then, her symptoms met criteria A and B for major depressive disorder (MDD) and criteria A, B, and C for restrictive eating disorder according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5).11
She was diagnosed with MDD and an eating disorder by her primary care provider (PCP), who prescribed imipramine, ondansetron, and oxybutynin for her constellation of symptoms, including nausea, nocturnal enuresis, and depression. The family was unsure if the patient took any of the prescribed medications. She revisited her PCP due to unremitting symptoms and was prescribed paroxetine for depression days before her visit to the ED.
At this point, her near-syncopal episodes increased, which concerned her family, who ultimately decided to bring her to the ED. At the emergency room, vital signs were within normal limits. Her body mass index was 19.6kg/m2. She was admitted and placed on an eating disorder protocol. When interviewed by psychiatry, the patient was oriented to person and place as well as the year and season but not to the month. She conversed cooperatively with providers but maintained a flat affect throughout the interview. Her responses were often limited to two to three words. There was no pertinent past psychiatric history, and her past medical history was unremarkable. The only previous medication aside from the recent prescriptions were oral contraceptives. Her family history was only concerning for multiple sclerosis in the mother, diagnosed at the age of 30 years.
We noticed dilated but equally briskly reactive 7mm pupils. Our concern for her dilated pupils led to us conducting a thorough neurologic examination, revealing hyper-reflexia in the lower limbs bilaterally, upgoing Babinski reflex bilaterally, intention tremor, incoordination (finger–nose–finger and heel–shin tests), and a positive Romberg sign. Fundoscopy showed Grade 3 to 4 papilledema.
Initial laboratory investigation included complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, and erythrocyte sedimentation rate, which were all normal. Urinalysis showed 3+ blood with trace ketones.
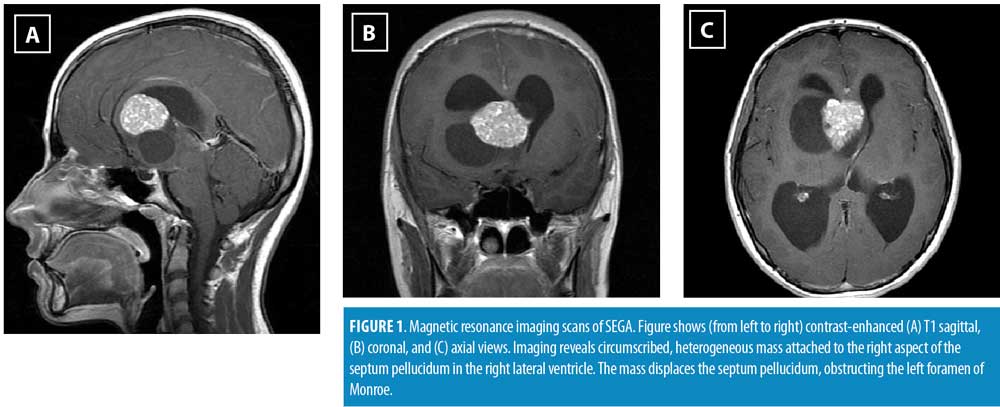
A magnetic resonance imaging brain and spinal cord scan with/without contrast was ordered, which revealed a circumscribed midline tumor with solid (3.0 × 3.7 × 2.6cm) and cystic (4.1 × 4.5 × 3.9cm) components within the frontal horn of the right lateral ventricle arising from the right aspect of the septum pellucidum, causing obstructive hydrocephalus (Figure 1). The differential diagnosis at the time included astrocytoma, ependymoma, and atypical meningioma. Neurosurgery placed bilateral external ventricular drains and later resected the tumor via an interhemispheric approach. The tumor was histologically confirmed to be a SEGA (Figure 2). Histopathology showed large tumor cells with abundant fine granular, eosinophilic cytoplasm, arranged in fascicles, sheets, and nets, with positive staining for S100 and neuron-specific enolase. The tumor cells additionally showed patchy positive staining for glial fibrillary acidic protein (GFAP) and for synaptophysin. Necrosis, vascular (endothelial proliferation), or significant mitotic activity were not seen.
The patient’s appetite returned within a day of tumor resection. She finished her hospital food tray as well as a bag of goldfish crackers and asked her family to bring in more food. Her guardian said, “I have my little girl back” because she was smiling, laughing, and answering questions in full sentences. She was animated and excited about plans for the rest of summer. Depressive symptoms quickly improved within the following days, including energy, concentration, interest in activities, and mood.
After hospital discharge, the patient followed up with neurosurgery, genetics, and the tuberous sclerosis clinic. Genetic testing was not pursued due to family cost prohibitions.
Discussion
Brain tumors are a major cause of cancer-related deaths in adolescents.12 SEGA is a World Health Organization Grade I glioma originating in the lateral ventricles that occurs typically in cases of tuberous sclerosis. We herein describe the first case of a SEGA presenting as a restrictive eating disorder and depression in an adolescent patient.
Tuberous sclerosis is an inherited autosomal dominant neurocutaneous disorder that presents with features such as hamartomas, brain lesions, and dermatologic manifestations. SEGA is considered a major feature of TSC, according to the 2012 International TSC Consensus Conference.13 Although SEGA is pathognomonic for TSC, many cases of SEGA have been reported in the absence of TSC clinical features or confirmatory genetic testing.7–10 One case report showed SEGA presenting with negative TSC genetic testing.7 Kwiatkowska et al14 hypothesized that somatic mosaicism, which occurs in 2 to 10 percent of de novo TSC cases, is responsible for exclusive neurological manifestations of SEGA. No studies have examined the long-term prognosis for patients presenting with SEGA in the absence of clinical features of TSC. Even genetic testing in these cases can be inaccurate because the absence of common clinical features might be attributable to somatic mosaicism. At our institute, genetic testing is pursued with further discussion between the clinician and patient. Our case re-illustrates that clinicians should include SEGA in the differential diagnosis for lateral ventricle tumors even in the absence of any TSC clinical features or confirmatory TSC genetic testing.
The annual incidence of primary intracranial tumor in children age 0 to 19 years is estimated at 5.6 per 100,000 people.15 SEGA represents only 1 to 2 percent of pediatric brain neoplasms.16 In comparison, the prevalence of eating disorders in adolescents is estimated at 3.8 percent.17 MDD is thought to affect eight percent of adolescents in the United States annually.18 It is not surprising that, given the patient’s initial presentation, she was treated for MDD and restrictive eating disorder.
Behavioral symptoms such as anhedonia, hypersomnolence, appetite loss, and flat affect might overlap with multiple primary psychiatric or medical disorders. Nevertheless, psychiatric symptoms might be the only initial presentation for a brain tumor, as was seen in this case. Hence, the DSM-5 places a critical criterion for many primary psychiatric disorders; criterion C for MDD and criterion D for avoidant/restrictive food intake disorder require that “the episode/eating disturbance is not attributable to the physiologic effects of a substance or to another/concurrent medical condition.”11 We believe that the initial diagnosis of MDD and eating disorder was erroneous. By the strictest standard for diagnostic assessment, a patient has not met DSM-5 criteria until their neurologic symptoms have been explored and ruled out. Interestingly, the patient’s persistent urinary incontinence was termed as “enuresis” (which also requires a criterion to rule out physiological effects of substances or another medical condition) and then attributed to an isolated psychological regression. The patient had no other personality changes, psychological reward, or relief from anxiety or responsibility to indicate she was undergoing a relapse into infantilism. Patients should receive further work-up for new neurologic symptoms or etiologies even following diagnosis of psychiatric illness.
This case builds upon previous work that correlates eating disorders and depression to local damage to the frontal cortex and ventricular system.6,19 In the past, Trummer et al19 reported three cases of intracerebral frontal-lobe damage associated with weight loss, compulsive behavior, and anxiety. Lin et al6 reported the only case of central tumor resulting in anorexia nervosa. Only three cases of central lesions in the ventricles have been associated with depression.20–22 In our case, we observed the simultaneous early presentation of restrictive eating disorder and depression. Clinical manifestations of SEGA include increased intracranial pressure, hydrocephalus, and seizures. This is the first time SEGA has presented with primary psychiatric symptoms. It is likely that the psychiatric symptoms were an early manifestation of right frontal-lobe or hypothalamus compression.
The early diagnosis and treatment of brain tumors are quintessential to recovery, yet understanding of the relationship between psychiatric symptoms and brain tumors is underappreciated. The early detection of SEGA in patients with known TSC has been shown to decrease long-term neurologic sequelae.23 With this case, according to the neurosurgery team, each fainting episode was likely the manifestation of increased intracranial pressure that could have resulted in brain herniation and catastrophic outcomes. Earlier detection could have reduced psychiatric symptoms and the risk of catastrophic outcomes. A previous study by Keschner et al24 reported psychiatric symptoms as the primary manifestation of brain tumor in 18 percent of 530 cases. Gupta and Kumar25 found that 21 percent of patients with meningioma had only psychiatric symptoms during initial presentation. Based on these studies, clinicians should maintain an index of suspicion for brain tumor in new-onset psychiatric symptoms, especially if followed by neurological symptoms, rather than attributing new symptoms to regression. Neuroimaging should be considered in patients who present with atypical psychiatric symptoms so that delays in the diagnosis and treatment of any serious neurological disorder, such as a brain tumor, might be avoided.
Conclusion
Clinical presentations of brain tumors can be subtle. Physicians might misinterpret symptoms of malignancy or another serious neurological disorder as being due to a psychiatric condition (e.g., MDD, eating disorder) and, thus, might not monitor symptom progression with the necessary vigilance. We present a clinical vignette wherein the diagnosis of SEGA was delayed by an initial diagnosis of a psychiatric illness and lack of neurologic work-up. New-onset eating disorder and depression, especially with a set of progressive neurological symptoms, can indicate the presence of frontal hemisphere and hypothalamus compression by a brain tumor. This case also demonstrates again that SEGA can present without TSC-specific clinical features. The absence of tuberous sclerosis complex clinical features or confirmatory genetic testing should not prevent the diagnosis of SEGA.
References
- Uher R, Treasure J. Brain lesions and eating disorders. J Neurol Neurosurg Psychiatry. 2005;76(6):852–857.
- Madhusoodanan S, Ting MB, Farah T, Ugur U. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273–285.
- Regard M, Landis T. “Gourmand syndrome”: eating passion associated with right anterior lesions. Neurology. 1997;48(5):1185–1190.
- Berek K, Aichner F, Schmutzhard E, et al. Intracranial germ cell tumor mimicking anorexia nervosa. Klin Wochenschr. 1991;69(10):440–442.
- Vad Winkler L, Andersen M, Hørder K, et al. Slow‐growing craniopharyngioma masquarading as early‐onset eating disorder: two cases. Int J Eat Disord. 2009;42(5):475–478.
- Lin L, Liao SC, Lee YJ, et al. Brain tumor presenting as anorexia nervosa in a 19-year-old man. J Formos Med Assoc. 2003;102(10):737–740.
- Beaumont TL, Godzik J, Dahiya S, Smyth MD. Subependymal giant cell astrocytoma in the absence of tuberous sclerosis complex: case report. J Neurosurg Pediatr. 2015;16(2):134–137.
- Kim JY, Jung TY, Lee KH, Kim SK. Subependymal giant cell astrocytoma presenting with tumoral bleeding: a case report. Brain Tumor Res Treat. 2017;5(1):37–41.
- Azam M, Rath S, Khurana R, et al. Rare case of subependymal giant cell astrocytoma without clinical features of tuberous sclerosis: case report and literature review. Precis Radiat Oncol. 2017;1(3):108–112.
- Elousrouti LT, Lamchahab M, Bougtoub N, et al. Subependymal giant cell astrocytoma (SEGA): a case report and review of the literature. J Med Case Rep. 2016;10:35.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington DC: American Psychiatric Association Publishing; 2013.
- Curtin SC, Miniño AM, Anderson RN. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. NCHS Data Brief. 2016;(257):1–8.
- Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–254.
- Kwiatkowska J, Wigowska-Sowinska J, Napierala D, et al. Mosaicism in tuberous sclerosis as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340(9):703–707.
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17 Suppl 4:iv1–iv62.
- Roth J, Roach ES, Bartels U, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr Neurol. 2013;49(6):439–444.
- Flament MF, Buchholz A, Henderson K, et al. Comparative distribution and validity of DSM-IV and DSM-5 diagnoses of eating disorders in adolescents from the community. Eur Eat Disord Rev. 2015;23(2):100–110.
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105.
- Trummer M, Eustacchio S, Unger F, et al. Right hemispheric frontal lesions as a cause for anorexia nervosa report of three cases. Acta Neurochir (Wien). 2002;144(8):797–801.
- Kohler CG, Burock M. ECT for psychotic depression associated with a brain tumor. Am J Psychiatry. 2001;158(12):2089–2089.
- Upadhyaya AK, Sud PD. Psychiatric presentation of third ventricular colloid cyst: a case report. Br J Psychiatry. 1988;152(4):567–569.
- Burkle JFM, Lipowski ZJ. Colloid cyst of the third ventricle presenting as psychiatric disorder. Am J Psychiatry. 1978;135(3):373–374.
- Torres OA, Roach ES, Delgado MR, et al. Early diagnosis of subependymal giant cell astrocytoma in patients with tuberous sclerosis. J Child Neurol. 1998;13(4):173–177.
- Keschner M, Bender MB, Strauss I. Mental symptoms associated with brain tumor: a study of 530 verified cases. JAMA. 1938;110(10):714–718.
- Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316–319.





