 by Sangsoo Lee, MD, MSc; Chang Jin Oh, MD; and Jeong Won Seong, MD
by Sangsoo Lee, MD, MSc; Chang Jin Oh, MD; and Jeong Won Seong, MD
Dr. Lee is with the Department of Psychiatry at Ahav Hospital, South Korea; Dr. Oh is with the Department of Pain Medicine and Anesthesiology at the Seoul
Samsung Union Clinic in South Korea; and Dr. Seong is with the Department of Family Medicine at the SNEPI Research Institute, Sarang Clinic in South Korea.
Innov Clin Neurosci. 2016;13(11–12):32–36.
Funding: No funding was received for the preparation of this article.
Financial disclosures: The authors have no conflicts of interest relevant to the content of
this article.
Key words: Sympathetic nerve entrapment point injection, antireflux procedure, refractory, laryngopharyngeal reflux, reflux, autonomic regulation
Abstract: Surgical treatment is not suitable for laryngopharyngeal reflux that is refractory to proton pump inhibitors. We present a case of proton pump inhibitor-refractory laryngopharyngeal reflux that was successfully treated with sympathetic nerve entrapment point injection. The patient had previously been diagnosed with laryngopharyngeal reflux and treated with proton pump inhibitors for six months without substantial improvement. After sympathetic nerve entrapment point injection treatment, her reflux symptom index improved from 15 points to 1 point, and this response was maintained for six months. Hyperexcitability of T5 and T6 sympathetic preganglionic fibers appears to be the main cause of laryngopharyngeal reflux. Sympathetic nerve entrapment point injection may represent an alternative to anti-reflux procedures.
Introduction
Laryngopharyngeal reflux (LPR) causes chronic inflammation or mucosal injury due to reflux of stomach contents into the pharynx and larynx through the esophagus.[1,2] However, some patients complain of LPR symptoms in the absence of typical symptoms of gastroesophageal reflux disease (GERD). Recently, there has been evidence that LPR is an independent disease, although the main treatment approach remains focused on the suppression of gastric acid secretion.[3,4]
Treatment with proton pump inhibitors (PPIs) for at least three months is recommended for LPR, while longer-term prescriptions are required for GERD.[4,5] Relapses often occur after discontinuation of PPI administration, and the long-term safety remains controversial.[6] Surgical interventions for LPR are not as effective as they are for GERD, and surgery is not performed in patients with LPR that is unresponsive to PPIs.[4,7–9] Because LPR is a risk factor for laryngeal cancer, effective treatment options for patients unresponsive to PPIs are needed.[2,10] This article aims to increase understanding of the pathogenesis of LPR based on the sympathetic nerve entrapment syndrome (SNES) hypothesis. Here, we introduce the use of autonomic regulation as a new therapeutic paradigm for LPR and describe a case in which a patient withPPI-refractory LPR was successfully treated with sympathetic nerve entrapment point injection (SNEPI) treatment alone.
Clinical Vignette
A 41-year-old, unmarried, nonsmoking woman presented at our hospital with persistent cough, globus pharyngeus, and throat clearing. Her globus pharyngeus and throat clearing first occurred approximately 10 years previously. Due to the impact this had on her quality of life, she sought treatment from an otolaryngology clinic two years before presenting to our clinic, and at that time was diagnosed with LPR and prescribed PPI treatment. However, after six months of treatment, she showed no substantial improvement and discontinued treatment at her own discretion.
After PPI discontinuation, her globus pharyngeus and throat clearing became more severe. Upon presentation to our clinic, she also reported severe discomfort at work due to a persistent dry cough during speaking, which had been present for approximately nine months. She did not report typical GERD symptoms, such as heartburn and regurgitation. Her body mass index was normal (22.6kg/m2). She was not taking any drugs and did not have diabetes, hypertension, or respiratory diseases, such as tuberculosis or asthma. After obtaining informed consent, SNEPI was performed 14 times for approximately four months according to the following schedule: twice weekly from Weeks 1 to 4, once weekly from Weeks 5 to 7, every other week for the next month, and monthly for the remaining period.
At the beginning of treatment, 0.5% lidocaine was injected along the tender points of the spinal cord at T3, T4, T5, and T6 by dividing 20cc of 0.5% lidocaine into 2.5cc per injection. One month later, or from the ninth treatment session, 0.5% lidocaine was injected mainly into the multifidus at the T5 and T6 spinal cord levels by dividing 10cc into 2.5cc per injection.
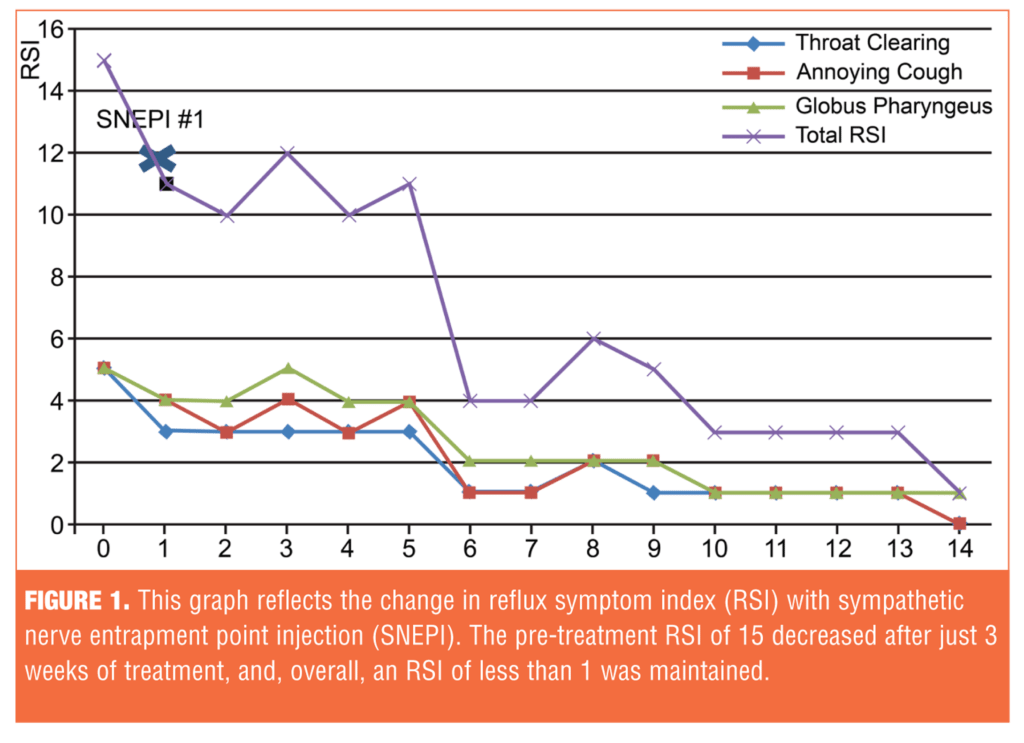
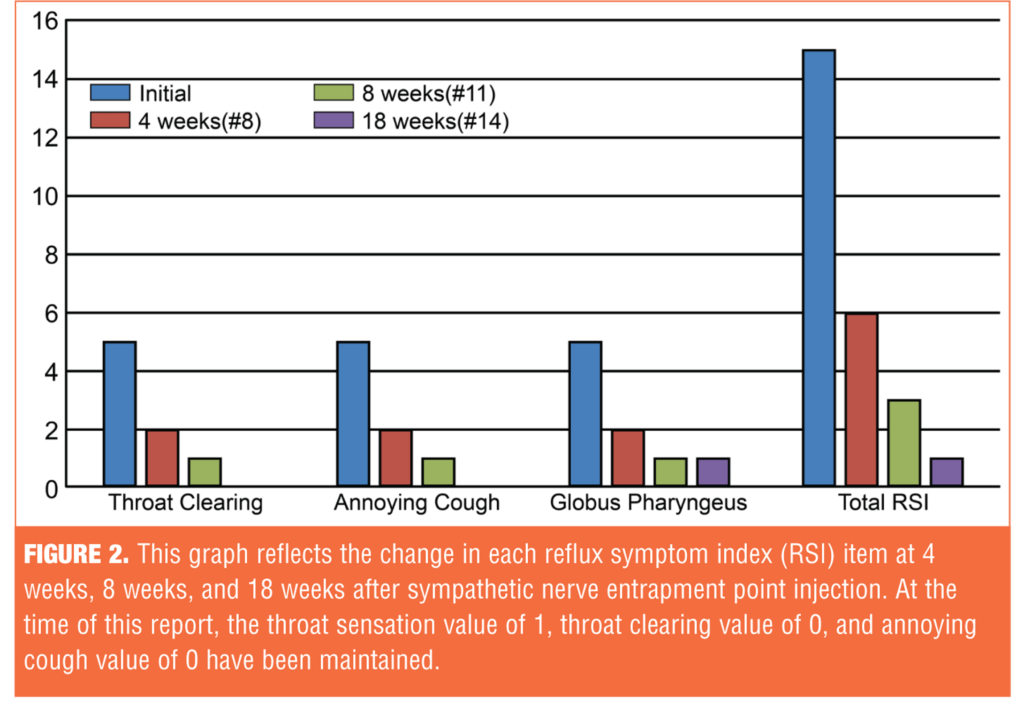
Treatment efficacy was evaluated using the Reflux Symptom Index (RSI).[11] Clinically, LPR is suspected if the RSI score exceeds 13. The initial RSI before treatment was 15; the RSI after treatment was maintained at 1 (Figure 1). Her symptoms were significantly reduced after SNEPI, and the response was maintained up to 18 weeks after the initiation of treatment (Figure 2).
Discussion
Long-term administration of PPIs for LPR treatment remains controversial, as it may lead to suppression of gastric acid secretion, resulting in impaired mineral and vitamin absorption and increased intestinal and extraintestinal infections.[6] Fundoplication, an antireflex surgery considered for patients with GERD refractory to PPI treatment, has negligible efficacy, and it is not recommended in PPI refractory patients; therefore, additional treatment approaches for PPI refractory patients with LPR are urgently needed.[8,9]
Our patient had LPR refractory to PPIs and was successfully treated with SNEPI alone.
SNEPI was designed based on the SNES hypothesis. Changes in the alignment between two adjacent vertebral bodies can lead to twisting or traction of the ramus communicans.[12] If the spinal nerve trunk in the intervertebral foramen is compressed, and consequently the vasa nervorum of sympathetic preganglionic fibers present in the spinal nerve trunk is pressed, focal ischemia can be induced. Adenosine triphosphate (ATP) production can then decrease, causing failure of the Na+/K+ ATPase pump and elevation of the extra-membrane K+ concentration. This may increase the resting membrane potential or reduce the threshold of nerve cells,[13,14] leading to membrane hyperexcitability. Hyperexcited vasomotor sympathetic preganglionic fibers can be excited by even small stimuli and can then produce abnormal excitation signals. The production of norepinephrine from the nerve terminal of sympathetic postganglionic fibers increases, which activates alpha-1 receptors present in the smooth muscles of visceral arteries. This causes the vessels to contract and may reduce blood flow to internal organs or tissues.[12]
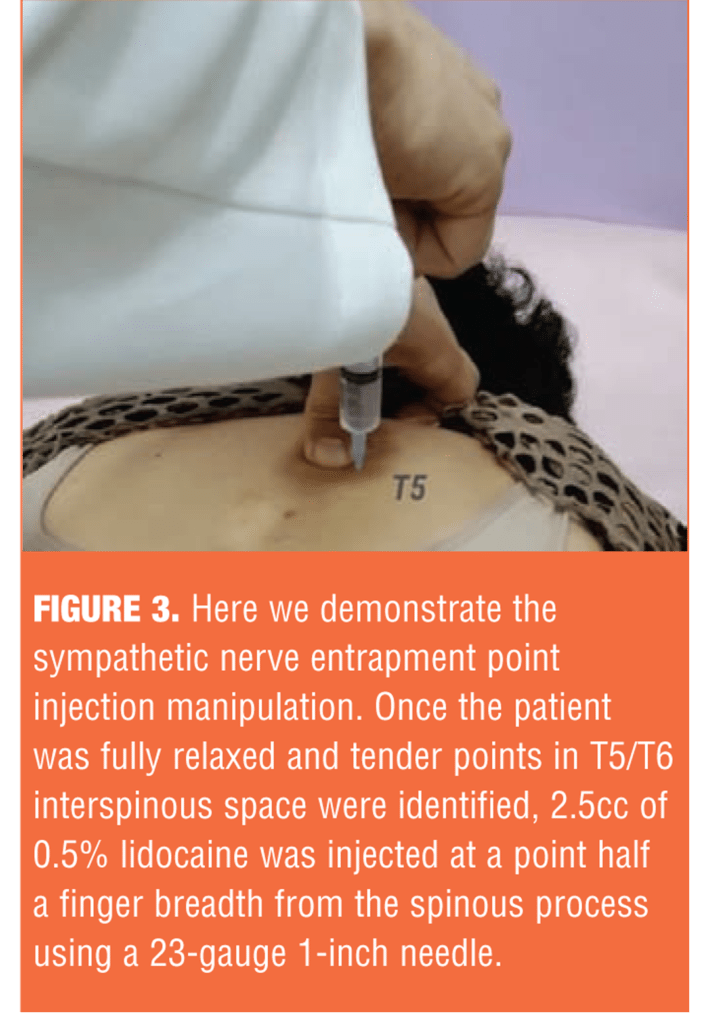
SNEPI treatment involves intramuscular injection of 0.5% lidocaine into the paraspinal deep muscles. This allows access to the treatment points that address organ dysfunction and may ultimately resolve the selective excitability of spinal nerves, including sympathetic preganglionic fibers.[12] SNEPI is an intramuscular injection administered to the multifidus, with the patient fully relaxed in a face-down position. Needle insertion sites are identified at the exact height of the spinal cord and at tender points; this can be quickly performed as an outpatient procedure (Figure 3).
The LES is composed of an asymmetric thickening of circular smooth muscles spanning 2- to 4cm, and it is controlled by parasympathetic and sympathetic nerves. Parasympathetic nerves control esophageal peristalsis through the vagus nerves.[15,16] Neuroanatomically, sympathetic preganglionic fibers carrying autonomic efferent signals from the T5 and T6 intermediolateral cell columns travel via greater thoracic splanchnic nerves that reach celiac ganglion of the nerve junctions. Postganglionic fibers receive the signals from these points to innervate the LES and vascular smooth muscles.
Sympathetic preganglionic fibers at T5 and T6 that are selectively hyperexcited in the intervertebral foramen may increase the production of norepinephrine at the nerve terminal while sending excitability signals to sympathetic postganglionic fibers. Norepinephrine then binds to alpha-1 receptors in the vascular smooth muscles of the LES, stimulating vasoconstriction. SNEPI with intramuscular injection of 0.5% lidocaine into the multifidi at T5 and T6 can reduce intervertebral pressure and relax the constriction of the vasa nervorum, which supplies the blood flow to sympathetic preganglionic fibers. This can improve the hyperexcitability of the sympathetic preganglionic fibers, which, in turn, can normalize norepinephrine levels at the nerve terminals through neurotransmission. This may increase the blood flow to the LES and eventually normalize the hyperexcitability of the parasympathetic fibers. While the normalized efferent signals of the vagus nerve in the LES may enter the autonomic nervous system in the nerve center and undergo fine adjustment through signal transmission in the parasympathetic nervous system pathological LES relaxation is assumed to be improved.[17,18]
Because the vagus nerve contains only excitatory neurons in the esophagus and LES, contraction of longitudinal esophageal muscles caused by excitatory simulation is thought to activate the inhibitory motor neurons that are responsive to stretching of the LES, and therefore lead to LES relaxation.[15]
Our case suggests that the excitatory simulation of the LES parasympathetic fibers is likely to be reduced when the hyperexcitability of sympathetic preganglionic fibers is resolved through SNEPI. There is also a possibility that the vasa nervorum, supplying blood flow to both sympathetic preganglionic fibers and parasympathetic fibers, can play a dominant role in sympathetic-parasympathetic interaction through alpha-1 adrenergic receptor-mediated norepineprine.[19] The vasa nervorum, which supplies blood to the nerves, is known to regulate blood flow through direct innervation by autonomic nervous system and is susceptible to physical presssure.[20] Our case demonstrates, in part, the possibility of autonomic regulation as a promising therapeutic paradigm, considering the close link between the autonomic nervous system.
Gastric acid that flows into the esophagus may stimulate receptors in the mucosa to induce the vagus nerve-mediated reflex, and if the pathological reflex is persistent, symptoms such as chronic cough and globus may occur.[3] It is thought that SNEPI can reduce LES relaxation as well as improve blood flow to the mucosa, subsequently contributing to the stability of the vagus reflex. In the present case, the initial SNEPI treatment points were at the T5 and T6 levels with additional SNEPI treatment points at the T3 and T4 levels, depending on the location of the tender points. With regard to the observed reduction of the cough, SNEPI treatment at the corresponding T3 and T4 levels may likely have stabilized sensory receptors in the laryngopharyngeal mucosa or increased laryngopharyngeal mucosal defense factors. A rich blood supply in the mucous epithelium is vital for the defense mechanism because acid-neutralizing bicarbonate is present in the blood, and oxygen, inflammatory cells, and phagocytic cells play a critical role in the destruction of harmful substances.[21,22] The improvement response was maintained with T5- and T6-level SNEPI treatment alone at the middle and late treatment phases; this was thought to have reduced LES relaxation in our patient.
Based on the SNES hypothesis, reflux due to pathological LES relaxation may be the main cause of LPR. The fact that this LPR patient, who had no typical GERD symptoms and showed no response to PPIs, was successfully treated with SNEPI alone highlights SNEPI as an effective alternative therapy for patients who are refractory to PPI. Because the symptoms are caused by non-acidic reflux or gas reflux events, as opposed to acidic reflux events as refluxates, treatment options are limited.[23] When the reflux pathophysiology is not clearly distinguished (whether refluxates are acid refluxes, non-acid refluxes, or gas refluxes), SNEPI may control reflux.
In our case, LPR symptoms were improved with SNEPI treatment alone, demonstrating that the pathogenesis of LPR may substantially contribute to the hyperexcitability of T5 and T6 sympathetic preganglionic fibers. It may be valuable to systemically verify the SNES hypothesis. If sympathetic preganglionic fibers are hyperexcited and the pathological hyperexcitability is consequently persistent, organ impairment controlled at the corresponding levels may be improved. Controlled studies investigating the therapeutic mechanism of SNEPI for LPR are required.
Limitation. A limitation of this study is that treatment effects were evaluated with RSI alone in the absence of a reflux finding score (RFS). The RSI is determined through a nine-question survey. Patients score their symptoms on a 6-point scale ranging between 0 to 5 points according to the degree of interference with their lives; therefore, the reliability of the patients’ subjective assessment can be a limitation. However, according to previous studies, the RFS based on laryngoscopy does not accurately reflect improvement in symptoms, and the inter-rater reliabilities of the laryngoscopic findings are low.[24] In contrast, the RSI is a subjective assessment scored by patients, and it has been shown to reflect the degree of improvement in symptoms associated with pharmacotherapy relatively well.[25]
Conclusion
Hyperexcitability of T5 and T6 sympathetic preganglionic fibers appears to be the main etiology of LPR. SNEPI can reduce LES relaxation and improve blood flow to the mucosa, thereby increasing the stability of the vagus reflex and laryngopharyngeal mucosal defense factors. To the best of our knowledge, this case represents the first report of a patient with LPR refractory to PPIs who was successfully treated with SNEPI alone. SNEPI can be considered as an alternative to anti-reflux procedures.
References
1. Vakil N, van Zanten SV, Kahrilas P, et al. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidencebased consensus. Am J Gastroenterol. 2006;101:1900–1920.
2. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78.
3. Campagnolo AM, Priston J, Thoen RH, et al. Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Int Arch Otorhinolaryngol. 2014;18:184–191.
4. Portnoy JE, Gregory ND, Cerulli CE, et al. Efficacy of super high dose proton pump inhibitor administration in refractory laryngopharyngeal reflux: a pilot study. J Voice. 2014;28:369–377.
5. Hanson DG, Jiang JJ. Diagnosis and management of chronic laryngitis associated with reflux. Am J Med. 2000;108:112S–119S.
6. Lodato F, Azzaroli F, Turco L, et al. Adverse effects of proton pump inhibitors. Best Pract Res Clin Gastroenterol. 2010;24:193–201.
7. Ford CN. Evaluation and management of laryngopharyngeal reflux. J Am Med Assoc 2005;294:1534–1540.
8. Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433–441.
9. Abou-Ismail A, Vaezi MF. Evaluation of patients with suspected laryngopharyngeal reflux: a practical approach. Curr Gastroenterol Rep. 2011;13:213218.
10. Tae K, Jin BJ, Ji YB, et al. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: a preliminary report. Clin Exp Otorhinolaryngol. 2011;4:101–104.
11. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274–277.
12. Seong JW. Principle and Insights into Pain, First Edition. Seoul South Korea: Koonja Press, 2015: 287-325.
13. Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123:2542–51.
14. Han SE, Lin CS, Boland RA, Kiernan MC. Nerve compression, membrane excitability, and symptoms of carpal tunnel syndrome. Muscle Nerve. 2011;44:402–409.
15. Jiang Y, Sandler B, Bhargava V, et al. Antireflux action of Nissen fundoplication and stretch sensitive mechanism of lower esophageal sphincter relaxation. Gastroenterology. 2011;140:442–449.
16. Shim K. Esophageal sphincter: normal and abnormal. J Neurogastroenterol Motil. 2006;12:1–7.
17. de Morree HM, Rutten GJ, Szabo BM, et al. Effects of insula resection on autonomic nervous system activity. J Neurosurg Anesthesiol. 2016;28:153–158.
18. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244.
19. Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–107.
20. Amenta F, Mione MC, Napoleone P. The autonomic innervation of the vasa nervorum. J Neural Transm. 1983;58:291–297.
21. Rourk RM, Namiot Z, Sarosiek J, et al. Impairment of salivary epidermal growth factor secretory response to esophageal mechanical and chemical stimulation in patients with reflux esophagitis. Am J Gastroenterol. 1994;89:237–244.
22. Woo J. Update of pathophysiology in GERD/LPR. J Neurogastroenterol Motil. 2010;16:83–90.
23. Kawamura O, Aslam M, Rittmann T, et al. Physical and pH properties of gastroesophagopharyngeal refluxate: a 24-hour simultaneous ambulatory impedance and pH monitoring study. Am J Gastroenterol. 2004;99:1000–1010.
24. Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope. 2002;112:1019–1024.
25. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope. 2001;111:979–981.





