
By Katie T. B. Touma, PharmD, BCPP, BCPS, and Jonathan R. Scarff, MD
Dr. Touma is with the William Jennings Bryan Dorn VA Medical Center Community-based Outpatient Clinic in Anderson, South Carolina. Dr. Scarff is with the Department of Behavioral Health at Kenner Army Health Clinic in Fort Lee, Virginia.
Innov Clin Neurosci. 2018;15(5–6):13–16
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimer: The views expressed in this article are those of the authors and do not reflect official policy or position of the Department of Veterans Affairs or Department of the Army.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Tardive dyskinesia (TD) is a medication-induced permanent movement disorder with no United States Food and Drug Administration (FDA)-approved treatments prior to 2017. Although TD is medication-induced, patients who have responded well to antipsychotics might not be candidates for dose reduction or discontinuation due to a risk of psychiatric decompensation. Valbenazine and deutetrabenazine were recently approved by the FDA for the treatment of TD. They offer a unique mechanism of action by inhibiting vesicular monoamine transporter type 2. The objective of this review is to discuss the efficacy, tolerability, dosing, drug interactions, and precautions for valbenazine and deutetrabenazine.
Tardive dyskinesia (TD) is defined as involuntary athetoid or choreiform movements lasting at least a few weeks and generally involving the tongue, face, and/or extremities, which develops in association with a neuroleptic medication usage for at least a few months.1 TD occurs in 32.4 percent of patients who receive typical antipsychotics and in 13.1 percent of patients who receive atypical antipsychotics.2 The pathophysiology of antipsychotic-induced TD is attributed to prolonged blockade of postsynaptic dopamine receptors with resultant dopamine hypersensitivity as well as damage to gamma-aminobutyric acid and cholinergic neurons in the striatum.3 Oxidative stress and consequent damage in the striatum might also result in TD.4 Prior to 2017, the American Academy of Neurology recommended amantadine, ginkgo biloba, and clonazepam for the treatment of TD.5 Given that vesicular monoamine transporter Type 2 (VMAT-2) transports serotonin, norepinephrine, and dopamine for storage and future neurotransmission, the inhibition of VMAT-2 increases cytosolic dopamine levels and decreases synaptic dopamine release and post-synaptic receptor stimulation, thereby decreasing dyskinesia.6,7 Until recently, only one VMAT-2 inhibitor, tetrabenazine, was utilized “off-label” for the treatment of TD, but its use was limited due to plasma fluctuations, the need for frequent dosing, and adverse effects.
The United States Food and Drug Administration (FDA) has recently approved two VMAT-2 inhibitors, valbenazine (Ingrezza™; Neurocrine Biosciences, Inc., San Diego, California, USA) and deutetrabenazine (Austedo™; Teva Neuroscience, Inc., Kansas City, Missouri, USA) for the treatment of TD. In contrast to tetrabenazine, deutetrabenazine utilizes the nontoxic deuterium isotope rather than hydrogen, which is hypothesized to lead to slower metabolism and therefore fewer plasma fluctuations and adverse effects. This review addresses the efficacy, tolerability, dosing, drug interactions, and precautions for valbenazine and deutetrabenazine in patients with TD.
Efficacy
Valbenazine and deutetrabenazine were evaluated in several trials for efficacy and safety, and details are provided in Tables 1 and 2, respectively.8–15 All subjects were adults aged 18 to 85 years. Valbenazine studies designated the change from baseline to Week 6 on the Abnormal Involuntary Movement Scale (AIMS) as the primary outcome measure and the Clinical Global Impression–Tardive Dyskinesia (CGI-TD) score as the secondary outcome measure. KINECT 2 and KINECT 4 also employed the Patient Global Impression of Change (PGIC) as a secondary outcome measure, which measures the beliefs of patients about the efficacy of their treatment. KINECT 4 also relied on the Tardive Dyskinesia Impact Scale (TDIS). Studies of deutetrabenazine utilized change in AIMS score from baseline to Week 12 as the primary outcome measure, and secondary outcomes were measured by changes in CGI-TD, PGIC, and the modified Craniocervical Dystonia Questionnaire (mCDQ)-24 from baseline to Week 12. Similar to the PGIC, the mCDQ-24 is a self-report assessment; however, it measures the quality of life of patients in relation to their neurological disorder.
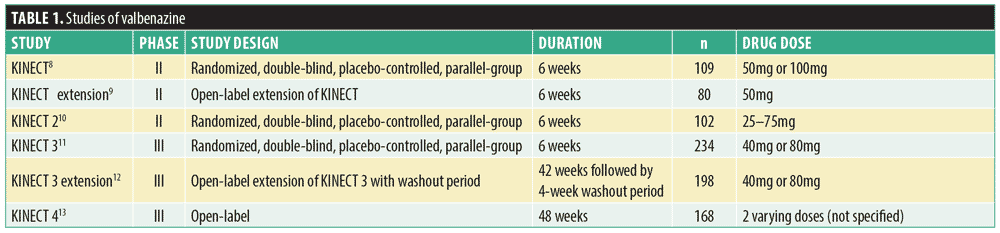

Valbenazine. In the KINECT study, primary inclusion criteria required subjects to have schizophrenia or schizoaffective disorder, have untreated neuroleptic-induced TD for at least three months, be receiving a stable dose of antipsychotic medication for a minimum of 30 days before the study’s start, or have stable psychiatric status.8 Patients with significant unstable medical conditions, substance dependence or abuse within three months (excluding nicotine and caffeine dependence), or a history of neuroleptic malignant syndrome (NMS) were excluded. Patients were also excluded if they exhibited a risk of suicidal or violent behavior or were receiving dopamine-modulating medications, such as reserpine, metoclopramide, stimulants, tetrabenazine, or other treatment for TD. There were three treatment arms: 1) placebo for six weeks; 2) valbenazine 50mg for six weeks; or 3) valbenazine 100mg daily for two weeks followed by valbenazine 50mg daily for four weeks. Although the AIMS change from baseline was -3.3 in the treatment group and -2.5 in the placebo group, the mean score change was not statistically different between groups.7 Following the completion of the KINECT study, 80 subjects entered an open-label extension study and all received valbenazine 50mg daily.9 Patients experienced a mean change of -5.8 on AIMS, and 61 percent of patients were assessed to be “much improved” or “very much improved” according to their CGI-TD score.
In the KINECT 2 study, subjects were included if they had schizophrenia, schizoaffective disorder, mood disorder with neuroleptic-induced TD, gastrointestinal disorder with metoclopramide-induced TD, moderate-to-severe dyskinesia, were psychiatrically stable (as assessed by investigator and score <50 on the Brief Psychiatric Rating Scale), and taking stable doses of concomitant medications.10 Patients were excluded if they were receiving tetrabenazine, benzodiazepines, amantadine, or anticholinergic medications. Patients received either placebo or valbenazine 25mg daily, which could be increased by 25mg every two weeks to a maximum of 75mg depending on clinician assessment of TD severity and the treatment’s efficacy and tolerability. The AIMS change from baseline between the treatment and placebo groups was statistically significant (-2.4), and 67 percent of patients were assessed as “very much improved” or “much improved” according to CGI-TD score.
In the KINECT 3 study, subjects were included if they had a diagnosis of schizophrenia, schizoaffective disorder, or mood disorder for at least three months, as well as moderate-to-severe dopamine receptor blocker-induced TD for at least three months.11 Patients were excluded if they were medically or psychiatrically unstable, had another involuntary movement disorder, a score of greater than 2 on more than two items on the Simpson-Angus scale, a history of neuroleptic malignant syndrome, or a risk of suicidal or violent behavior. Patients received either placebo, valbenazine 40mg daily, or valbenazine 80mg daily. Although reduction in TD symptoms occurred in both treatment groups, only the 80mg treatment group exhibited a statistically significant reduction (change in AIMS scores of valbenazine vs. placebo: -3.2 vs. -0.1). Although there were no statistically significant differences among groups according to CGI-TD score, subgroup analyses suggested significant improvement in those subjects with detectable plasma levels at Week 6.
Following the completion of the KINECT 3 study, 198 subjects participated in a 42-week extension study and received 40mg or 80mg daily of valbenazine.12 Both groups experienced significantly significant reductions in mean AIMS score (-3.0 in the 40mg group and -4.8 in the 80mg group). Reduction in CGI-TD and PGIC scores also demonstrated a clinically significant improvement. TD symptoms returned to baseline during a four-week discontinuation and washout period, as demonstrated by AIMS scores. The two-year safety and tolerability study (KINECT 4) was completed in March 2017, but results were unavailable at the time of this manuscript writing.13
Deutetrabenazine. Deutetrabenazine was evaluated in two trials.14,15 Participants were required to have TD for at least three months and dopamine receptor antagonist exposure for at least three months if younger than 60 years of age (vs. at least 1 month if over 60 years of age). Participants had to score greater than 6 on the AIMS. Patients were excluded if they had received any one of various dopamine-modulating medications or botulinum toxin or suffered from a neurologic condition that could interfere with the assessment of dyskinesia, had untreated or undertreated psychiatric illness, or prolonged corrected QT (QTc) interval.
In the ARM-TD study, patients in the treatment group received deutetrabenazine in divided doses for six weeks, starting at 12mg daily and titrated until efficacy was reached, adverse effects appeared, or a maximum dose of 48mg/day was achieved.14 This was followed by a six-week maintenance period and a one-week washout. Patients in the treatment group experienced significantly reduced AIMS scores compared to those in the placebo group (-3.0 vs. -1.6). However, a notable placebo effect was observed on AIMS ratings, which was attributed to the perception or expectation of improvement by patients, as well as the inconsistent manifestation of TD symptoms, which can be influenced by external factors (e.g., stress, time of day). While deutetrabenazine was perceived as effective on CGI-TD and PGIC, these changes were not statistically significant.
In the AIM-TD study, patients in the treatment group received deutetrabenazine in divided doses starting at 12mg/day and titrated to their specific group dosage over four weeks.15 This was followed by a maintenance period of eight weeks and a one-week washout period. Patients receiving 24mg/day or 36mg/day of deutetrabenazine had statistically significant reductions in AIMS scores (-3.2 in the 24mg/day group and -3.3 in the 36mg/day group vs. -1.4 in the placebo group). Similarly, 49 percent of patients in the 24mg/day group and 44 percent of patients in the 36mg/day group were “much improved” or “very much improved” according to their CGIC score. Comparable to the ARM-TD study, a significant placebo effect was observed for the AIMS score and was attributed to the same causes. There was a statistically insignificant improvement on PGIC and mCDQ-24 scores, but results could have been confounded by an underlying psychotic disorder, which might have affected their ability to accurately assess for improvement.15
Safety and Tolerability
In all KINECT studies, there were no significant changes of vital signs, cardiac rhythm, or laboratory study results, nor was there a worsening of parkinsonism, akathisia (except in KINECT 3), or dyskinesia. In the KINECT study, adverse effects were not statistically more frequent in the treatment group compared to the placebo group.8 Serious adverse effects reported in any treatment group were chest pain, bronchitis, fall, suicidal ideation, and worsening psychosis, while fewer adverse effects of fatigue, somnolence, and urinary tract infection were also noted. In the KINECT extension study, there were no treatment-related serious adverse events or suicidal ideation.9 In KINECT 2, the most common adverse effects were fatigue and headache.10 There were no emergent mood changes or worsening psychosis. In KINECT 3, the most common adverse effects were somnolence, akathisia, and dry mouth, with no difference in suicidal ideation between the valbenazine and placebo groups.11 Somnolence and suicidal ideation led to discontinuation in three patients in the KINECT 3 extension study but not because of valbenazine use; the most common adverse effects were headache and urinary tract infection.12 Although no published data could be obtained from the KINECT 4 study, data from KINECT 1, KINECT 3, and KINECT 4 were pooled to assess long-term safety and tolerability over 48 weeks.16 The most common adverse effects were somnolence, fatigue, and dry mouth. Serious adverse effects were reported in 13 percent of subjects; of those affected, six percent required dose reduction and 14 percent discontinued treatment.
The most common adverse effects in those receiving deutetrabenazine treatment were somnolence, fatigue, insomnia, headache, diarrhea, and akathisia.14,15 Rates of depressed mood, suicidal ideation, weight change, metabolic status, and electrocardiogram changes were similar to those with the placebo. Deutetrabenazine was well-tolerated, as noted by the low need for dose reductions or study withdrawals, which were similar to the placebo group.
Metabolism, Dosing, and Precautions
After ingestion, valbenazine is metabolized by hydrolysis to the active metabolite (+)-alpha-dihydrotetrabenazine (?-HTBZ) and by oxidation by cytochrome P-450 (CYP) 3A4/3A5 to form minor metabolites; ?-HTBZ is further metabolized by CYP2D6.17 Metabolites are excreted primarily in the urine. Valbenazine reaches steady-state plasma concentrations within one week. Valbenazine is available as 40mg and 80mg capsules, and the recommended starting dose is 40mg once daily for one week, which is then increased to 80mg daily.17 Dosage adjustment is not necessary for patients with mild-to-moderate renal impairment. The once-daily dose of 40mg is recommended for patients with moderate or severe liver impairment (defined as a Child-Pugh score of 7–15) or who are taking a strong CYP 3A4 inhibitor. For patients taking a medication that is a strong CYP2D6 inhibitor, the labeling recommends considering a dose reduction based on the tolerability of valbenazine. Valbenazine is not recommended in combination with strong inducers of CYP3A4 or monoamine oxidase inhibitors (MAOIs), or in patients with severe renal impairment.17
There are no contraindications for valbenazine. Although recommended doses could insignificantly prolong the QTc interval, it should still be avoided in patients with congenital long QT syndrome or with arrhythmias that might prolong the QTc interval.17 An additional warning exists for somnolence, so patients should not perform tasks requiring alertness until they know how the medication affects this. Despite limited data on the use of valbenazine in pregnancy, animal studies found an increase in the number of stillborn offspring and postnatal mortalities at doses of less than one times the maximum recommended human dose.17 There is no information about the presence of valbenazine or its metabolites in human milk or the effects on a breastfed infant or milk production. Current recommendations are to avoid breastfeeding while taking valbenazine and for five days after the final dose.17
After ingestion, deutetrabenazine is biotransformed via carbonyl reductase to ?-HTBZ and ?-HTBZ, which are metabolized primarily by CYP2D6 and then renally excreted.18 Deutetrabenazine is available in 6mg, 9mg, and 12mg tablets. The starting dose is 12mg daily in divided doses, which can be increased by 6mg weekly to a maximum dose of 48mg daily, taken whole with food. In patients who are poor CYP2D6 metabolizers or who are receiving strong CYP2D6 inhibitors, the total daily dosage should not exceed 36mg. For patients requiring deutetrabenazine doses greater than 24mg per day and who are receiving drugs known to prolong QTc interval, it is recommended that clinicians assess the QTc interval when changing doses of either deutetrabenazine or the other drugs.18 No dose adjustment is necessary for patients with renal impairment, but it is contraindicated in those with hepatic impairment.18 It is also contraindicated in patients with suicidal ideation, untreated/inadequately treated depression, or those who are receiving MAOIs, reserpine, or tetrabenazine.18 There are no adequate data for deutetrabenazine in pregnant women, although animal studies did not adversely affect fetal development. There is no information on the presence of deutetrabenazine or its metabolites in human milk or the effects on a breastfed infant or milk production.18
Deutetrabenazine carries a boxed warning for increased risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease, as well as a warning for changes in mood, cognition, chorea, rigidity, functional capacity, risk for depression, and suicidal ideation and behaviors (in patients with Huntington’s disease), neuroleptic malignant syndrome, akathisia, agitation, restlessness, parkinsonism (in patients with Huntington’s disease), and sedation/somnolence.18 Caution is recommended when treating patients with depression or prior suicide attempts or ideation, with careful monitoring for emergence or worsening of depression, suicidality, or unusual changes in behavior.18
Conclusion
Valbenazine and deutetrabenazine offer a unique mechanism of action for the treatment of TD and are the first treatments to be FDA-approved for this condition. The high level of available evidence warrants clinical consideration for patients who are not candidates for a dose reduction or change in antipsychotic therapy with moderate-to-severe TD. It is important to note that the data on quality of life are scarce and that AIMS scores do not correlate with functional or social impairment. Additional studies and data are needed regarding the effects of valbenazine and deutetrabenazine on quality of life and their long-term benefits and safety.
References
- American Psychiatric Association. Medication Induced Movement Disorders and Other Adverse Effects of Medication. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed.. Washington DC: American Psychiatric Publishing; 2013: 709–714.
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–156.
- Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry. 2005;50(9):541–547.
- Lister J, Nobrega JN, Fletcher PJ, et al. Oxidative stress and the antipsychotic-induced vacuous chewing movement model of tardive dyskinesia: evidence for antioxidant-based prevention strategies. Psychopharmacology (Berl). 2014;231(11):2237–2249.
- Bhidayasiri R, Fahn S, Weiner WJ, et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463–469.
- German CL, Baladi MG, McFadden LM, et al. Regulation of the dopamine and vesicular monoamine transporters: pharmacological targets and implications for disease. Pharmacol Rev. 2015;67(4):1005–1024.
- Citrome L. Valbenazine for tardive dyskinesia: A systematic review of the efficacy and safety profile for this newly approved novel medication-What is the number needed to treat, number needed to harm and likelihood to be helped or harmed?. Int J Clin Pract. 2017;71(7).
- ClinicalTrials.gov. NBI-98854 for the Treatment of Tardive Dyskinesia in Subjects With Schizophrenia or Schizoaffective Disorder (KINECT Study). Available at: https://clinicaltrials.gov/ct2/show/NCT01688037. Accessed December 23, 2017.
- Jimenez R, Shiwach R, Bari M, et al. Twelve-week treatment of tardive dyskinesia with NBI-98854: 826. Mov Disord. 29(Supp 1):S304–S305.
- O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681–1687.
- Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476–484.
- Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344–1350.
- ClinicalTrials.gov. Safety and Tolerability Study of NBI-98854 for the Treatment of Tardive Dyskinesia (Kinect 4). Available at: https://clinicaltrials.gov/ct2/show/NCT02405091. Accessed December 23, 2017.
- Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003–2010.
- Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595–604.
- Remington G, Factor S, Cornella C, et al. Safety and tolerability of valbenazine (NBI-98854) in subjects with tardive dyskinesia: results of long-term exposure data from three studies. Neurology. 2017;88(16 Supplement):P2.017.
- INGREZZA™ (valbenazine) [prescribing information]. San Diego, CA: Neurocrine Biosciences, Inc.; 2017.
- AUSTEDO™ (deutetrabenazine) [prescribing information]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2017.





