 by Farid Ramzi Talih, MD
by Farid Ramzi Talih, MD
Dr. Talih is Assistant Professor of Psychiatry at the Department of Psychiatry of the American University of Beirut Medical Center, Beirut, Lebanon.
Innov Clin Neurosci. 2013;10(5–6):28–31
Funding: There was no funding for the interviews, development, and writing of this article.
Financial disclosures: The authors do not have conflicts of interest relevant to the content of this article.
Key words: Visual hallucinations, Lhermitte’s syndrome, peduncular hallucinosis, atypical antipsychotics, psychosis
Abstract: Peduncular hallucinosis is a rare phenomenon that has been reported in case reports and case series. The exact pathophysiology is unclear, but is postulated to involve disruption and dysregulation of the visual neuronal pathways in the brain. A case of complex visual hallucinations in an elderly man with no previous history of psychosis is discussed. Magnetic resonance imaging of the patient’s brain revealed a discrete peduncular lesion that was the likely cause of the hallucinations. The lesion was not present on a previous brain magnetic resonance imaging that predated the onset of the hallucinations. The patient was started on low-dose risperidone, which resulted in the complete resolution of the visual hallucinations. It is important to consider discrete rostral brainstem lesions as a potential cause of new onset visual hallucinations in patients being evaluated for visual hallucinations.
Introduction
Visual hallucinations (VHs) are a complex neuropsychiatric phenomenon with varied etiologies. Peduncular hallucinosis (PH) is a relatively rare cause of complex visual hallucinations.1 Lhermitte was the first to describe this syndrome almost a century ago.[2] PH secondary to ischemic lesions in the cerebral peduncles (CP) is considered rare.[1] I present a report of a patient with PH secondary to an ischemic lesion in the left CP that was not present on previous brain imaging.
Case Report
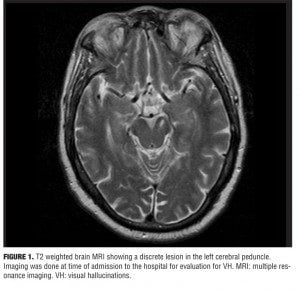
A 70-year-old man was admitted to our psychiatric unit for a four-month history of hallucinations. Additionally, insomnia with sleep fragmentation and daytime hypersomnia were reported. His history included dyslipidemia, hypertension, and coronary artery disease. There was no history of psychiatric disorders or substance abuse. No recent head trauma, neurological deficits, visual impairment or febrile illnesses were reported. The patient lived alone with minimal psychosocial support. The hallucinations reported were purely visual. VHs were stereotyped, complex, and occurred daily, usually in the evening while the patient was awake. VHs were described as human figures, of average size, dressed in what appeared to be “space suits.” They would climb through the window and silently walk around the room, and at times seemed to congregate around the patient. The figures did not talk or interact with him. No auditory, tactile, gustatory, or olfactory hallucinations were reported. In approximately 30 to 40 minutes the VHs would subside. The patient retained insight, but at times wondered if the VHs could be “aliens.” He delayed reporting the VHs due to fear of being labeled mentally ill, but eventually the distress and anxiety from the VHs overcame his fear of stigmatization. Upon admission, medications included a statin, aspirin, and a calcium channel blocker. Evaluation included an electrocardiogram, chest x-ray, complete blood count, complete metabolic panel, urine analysis and toxicology, thyroid function, vitamin B-12 levels, thiamine, and screening for syphilis (rapid plasma reagin). The results of the above work up were negative. The physical exam was unremarkable with no focal neurological deficits. Mental status examination and psychiatric evaluation were normal with the exception of the VHs. There was no clinical evidence of delirium and no evidence of confusion, delusions, or memory impairment. The patient scored 28 on the mini-mental state examination.[3] Magnetic resonance imaging (MRI) of the brain was ordered and showed a sub-acute ischemic lesion in the left cerebral peduncle area (Figure 1). The patient was started on low-dose risperidone at 0.25mg orally twice daily. The patient reported a short-lived VH that evening in his hospital room and did not have any further VHs throughout the remaining seven-day hospital stay. No adverse medication effects were noted. He was discharged in a stable condition to an assisted living facility on the same dosage of risperidone.
Discussion
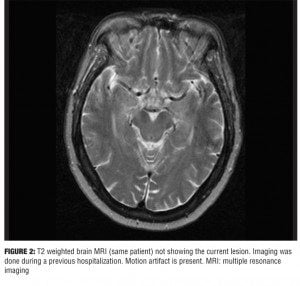
This patient’s presentation is likely due to PH. Supporting evidence is from a previous MRI (Figure 2) at a time when the patient did not have any VHs. The previous MRI does not show the current lesion. The MRI was done to evaluate for symptoms suggestive of transient ischemic attacks and predates the current MRI by four years. The abrupt onset of symptoms in this patient supports a vascular etiology. Symptoms usually start within days of the ischemic infarct.[4] The occlusion most likely involved the perforating branches of the rostral part of the posterior circulation.[1] The hallmarks of PH are vivid and stereotyped VHs. The syndrome also includes fragmented sleep and hypersomnia during the day. Additionally, oculomotor abnormalities are associated with this syndrome.[1,5] PH is attributed to lesions in the rostral brainstem—mainly the pons, cerebral peduncle, midbrain area, and occasionally the thalami. The causes of VHs, in general, include, but are not limited to, drug use (licit and illicit), intracranial pathology (tumors), psychiatric disorders (psychotic disorders), neurological disorders (epilepsy, narcolepsy, dementia, migraines), ophthalmological disorders (Charles-Bonnet syndrome), and hypnagogic/hypnopompic hallucinations.[4,6] The pathophysiology of sleep disturbances and VH in PH is thought to be related to two mechanisms.[5] The first being disruption of transmission of the ascending reticular arousal system (ARAS) to the cortex causing arousal abnormalities. The second mechanism is the loss of brainstem input to the regions of the thalamus involved in suppression of the visual cortices (occipital and parts of the temporal lobe) resulting in a release phenomenon.[1,4] VHs are related to a dysregulation of the inhibitory control of the ponto-geniculate-occipital system leading to visual experiences theoretically similar to those of rapid eye movement (REM) sleep.[1,4] Additionally, it is hypothesized that loss of serotonergic inhibition from the raphe nuclei to the visual pathways may lead to unopposed excitatory cholinergic activity to the visual cortices.[5]
There is no specific treatment for PH. Cases have been reported to respond to various classes of psychotropics, such as atypical antipsychotics, selective serotonin reuptake inhibitors, and anti-convulsants.[5–10] It is likely that the atypical antipsychotics, which modulate serotonergic activity in addition to dopamine antagonism, may have a beneficial role in such patients.[5,10]
It is crucial to keep in mind that PH is essentially a diagnosis by exclusion, since it cannot be definitely confirmed, and a thorough evaluation is required to rule out the multitude of conditions that can present with similar symptomatology. Limitations and exclusions in this report include the following: 1) There was no electroencephalogram performed to exclude seizures as a cause of VH (there was no clinical suspicion of a seizure disorder); 2) Delirium rating scales were not used in this case (delirium was unlikely in this patient, since the general medical evaluation was normal and the patient did not exhibit any fluctuations in his level of consciousness throughout his hospital stay); 3) Dementia can be a cause of VH, though in our case this was unlikely since the MMSE was normal; 4) Our patient did not undergo a neuro-ophthalmological evaluation. The presence of neuro-ophthalmological findings would be additional evidence for a brainstem lesion that is causing PH. Due to the lack of ophthalmological testing, although the patient did not report visual impairment, the possibility of Charles-Bonnet syndrome causing the VH cannot be completely excluded; 5) Narcolepsy was ruled out clinically, since cataplexy and sleep paralysis were not present in this patient, and the onset of hypersomnia was relatively recent. Although our case did not present with the typical neurological deficits (other than sleep-wake disturbances) associated with PH, a case without associated neurological deficits and similar MRI findings has been reported in the literature.[11] It can be argued that the strongest evidence suggesting the diagnosis in this patient is the overall similarity to other reported cases of PH in the literature. Long-term follow up of this patient was not possible due to relocation after discharge from the hospital. In conclusion, it is important for clinicians to consider PH due to discreet brainstem lesions among the varied and sometimes rare diagnoses (Table 1) of VH.[12]
References
1. Benke T. Peduncular hallucinosis: a syndrome of impaired reality monitoring. J Neurol. 2006;253(12):1561–1571.
2. Lhermitte J. Syndrome de la callote du pedoncule cerebral. Le troubles psycho-sensoriels dans les lesions du mesocephale. Rev Neurol. 1922;2:1359–1365
3. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98
4. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819–1840.
5. Spiegel D, Barber J, Somova M. A potential case of peduncular hallucinosis treated successfully with olanzapine. Clin Schizophr Relat Psychoses. 2011;5(1):50–53.
6. Roser F, Ritz R, Koerbel A, Loewenheim H, Tatagiba MS. Peduncular hallucinosis: insights from a neurosurgical point of view. Neurosurgery. 2005;57(5):E1068.
7. Maiuri F, Iaconetta G, Sardo L, Buonamassa S. Peduncular hallucinations associated with large posterior fossa meningiomas. Clin Neurol Neurosurg. 2002;104(1):41–43.
8. O’Neill SB, Pentland B, Sellar R. Peduncular hallucinations following subarachnoid haemorrhage. Br J Neurosurg. 2005;19(4):359–360.
9. Vertrungo R, Vella A, Mascalchi M, et al. Peduncular hallucinosis: a polysomnographic and spect study of a patient and efficacy of serotonergic therapy. Sleep Med. 2009;10(10):1158–1160.
10. Spiegel DR, Lybeck B, Angeles V. A possible case of peduncular hallucinosis in a patient with Parkinson’s disease successfully treated with quetiapine. J Neuropsychiatry Clin Neurosci. 2009;21(2):225–226.
11. Howlett DC, Downie AC, Banerjee AK, et al. MRI of an unusual case of peduncular hallucinosis (Lhermitte’s syndrome). Neuro-radiology. 1994:36:121–122.
12. Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry. 2009;11(1):26–32.