 by Eileen Trigoboff, RN, DNS; Jeffery Grace, MD; Herman Szymanski, MD; Jaspinder Bhullar, MBBS; Claudia Lee, MD; and Thomas Watson, RN, MSN
by Eileen Trigoboff, RN, DNS; Jeffery Grace, MD; Herman Szymanski, MD; Jaspinder Bhullar, MBBS; Claudia Lee, MD; and Thomas Watson, RN, MSN
Drs. Trigoboff, Grace, and Lee are from the Buffalo Psychiatric Center in Buffalo, New York, and the State University of New York at Buffalo; Mr. Watson is from the Buffalo Psychiatric Center in Buffalo, New York; and Dr. Szymanski is from the State University of New York at Buffalo.
Innov Clin Neurosci. 2013;10(5–6):20–27
Funding: There was no funding for the interviews, development, and writing of this article.
Financial disclosures: The authors do not have conflicts of interest relevant to the content of this article.
Key words: Aspiration pneumonia, clozapine, dosing, hypersalivation, sialorrhea
Abstract: This case study compares two different clinical outcomes for a patient with a long-standing psychotic disorder prescribed clozapine on two occasions. During the first trial, clozapine was used at a higher dose for this patient (350–450mg/day) and included clinically significant sialorrhea, pneumonia, and pneumonia-like illnesses requiring immediate medical intervention including hospitalization. There were also patient complaints of fatigue, cough, choking, and constipation leading to poor adherence. Clozapine was discontinued when the patient withdrew his consent due to side effects, despite his awareness of its benefits, including reduction of command hallucinations and irritability. The second clozapine trial was associated with lower daily doses and therapeutic serum blood levels. The patient was actively participating in and adhering to the medication plan. A very narrow window of clozapine dose was exceeded for two days and the patient complained of hypersalivation, cough, and lethargy. He was subsequently hospitalized for a two week period to treat aspiration pneumonia. This hospitalization helped establish the ideal daily dose of clozapine for this patient and also brought the relationship between aspiration pneumonia and clozapine to the attention of the psychiatrist and medical specialist. Once the appropriate dosage for this patient was established, his psychotic and affective symptoms were controlled, he was not hampered by adverse side effects, and he started to actively participate in social and recreational activities and plans that culminated in discharge from a state psychiatric facility to a supportive community residence. It is our hope that the lessons we have learned from our shared experience with this patient will be of benefit to other clinicians and patients.
Introduction
This case study compares two different clinical outcomes for a patient with a long-standing psychotic disorder prescribed clozapine on two occasions, from 2005 to 2009 and again from 2010 to 2012. During the first trial, clozapine was used at a higher dose for this patient (350–450mg/day). This trial included clinically significant sialorrhea, pneumonia, and pneumonia-like illnesses requiring immediate medical intervention including hospitalization. There were also patient complaints of fatigue, cough, choking, and constipation leading to poor adherence. Clozapine was discontinued when the patient withdrew his consent, due to side effects, despite his awareness of its benefits, which included a reduction of command hallucinations and irritability.
The second clozapine trial was associated with lower daily doses and therapeutic serum blood levels. The patient was actively participating in and adhering to the medication plan. Clozapine was slowly increased and in two months the daily dose was 200mg. When the daily dose was increased to 225 mg for two days and 250mg for an additional two days the patient complained of hypersalivation, cough, and lethargy. He was subsequently hospitalized for a two-week period to treat aspiration pneumonia. This hospitalization helped establish the ideal daily dose of clozapine for this patient and also brought the relationship between aspiration pneumonia and clozapine to the attention of the psychiatrist and medical specialist. Clozapine was then augmented with quetiapine resulting in the significant clinical improvement of psychotic symptoms (relative to baseline). Lithium was also added to the medication regimen to control affective disorder symptoms. With his psychotic and affective symptoms controlled he started to actively participate in social and recreational activities and plans that culminated in discharge from a state psychiatric facility to a supportive community residence. It is our hope that the lessons we have learned from our shared experience with this patient will be of benefit to other clinicians and patients.
The Patient
This 56-year-old, single, never-married Native-American man had a long history of a psychotic disorder complicated by substance abuse, borderline intellectual functioning, antisocial and borderline personality disorders, and criminal behaviors. He had multiple admissions to civil and forensic psychiatric facilities since 1967 for the evaluation and treatment of depression, suicidal thoughts and/or self-mutilation, self-isolation, and impulsivity. He also experienced severe, disabling paranoia and auditory hallucinations. In the past he made deep cuts (requiring stitches) to his wrists and arms in response to command hallucinations, as well as several suicide attempts including at least one overdose with over-the-counter medications.
Just prior to this patient’s transfer to our state psychiatric center, he had served 15 years in prison for rape and assault with a weapon (knife). He was due to be paroled, but he admitted to correctional officers that he felt more comfortable in prison and that if he were released he had anxiety and trepidation that he would rape again and/or kill someone. He was admitted to a forensic psychiatric facility to evaluate his statements and then transferred to our state psychiatric facility, on parole, for continued evaluation, treatment and discharge planning.
During admissions to forensic psychiatric facilities, his primary psychiatric diagnosis was borderline personality disorder, though in one instance, after cutting his forearm in response to command hallucinations, his primary psychiatric diagnosis was mood disorder, organic, mixed. Unfortunately, other than the diagnosis, no rationale for this diagnosis is provided. Upon admission to our psychiatric center he was diagnosed with major depression recurrent with psychotic features; polysubstance dependence by history; antisocial personality disorder; and borderline personality disorder. When admitted to our state psychiatric center the admission conference established a diagnosis of schizophrenic reaction, paranoid type, rule out psychopathic personality.
The patient had been offered multiple trials of antipsychotic medication, including perphenazine, thiothixene, risperidone, ziprasidone, and quetiapine. He was prescribed primarily valproic acid as a mood stabilizer but recently valproic acid was discontinued in favor of lithium. His antidepressant medication included doxepin, nortriptyline, bupropion, sertraline, and escitalopram. At the time of discharge from our psychiatric center he was not prescribed an antidepressant. Reportedly he experienced serious side effects with chlorpromazine and thioridazine in the past, and he is considered “allergic” to these medications, although his medical records do not list these medications as having been prescribed.
The patient had the following health issues: positive purified protein derivative (PPD) test (a skin test used to diagnose tuberculosis) since 1991 (treated with isoniazid [INH]), edentulous with no hard fruits or vegetables (annual choking/aspiration screens performed over the past seven years show that the edentulous patient increased the risk for choking and/or aspiration), status post-surgery (S/P) for cataracts in 2004, and S/P prostatectomy in 2008. A computed tomography (CT) scan in 2010 indicated “normal pressure hydrocephalus (NPH) versus mild obstructive hydrocephalus.” Consultation with neurosurgery did not recommend surgery. The absence of ataxia and dementia ruled out the diagnosis of NPH. He had a 20-year history of smoking one pack of cigarettes per day until our facility became smoke-free in January of 2007. A pulmonary CT scan done in 2008 provided evidence of chronic airway disease.
Treatment Course
First clozapine trial, 2005 to 2009. In 2005, this patient was admitted to our state psychiatric center, given medical clearance, and, with the patient’s consent, started on clozapine. Clozapine was titrated over the next two months to 450mg daily. The most immediate side effect, and most notable, was sialorrhea. The patient also reported significant lethargy and constipation. One month after starting clozapine, there were multiple nursing reports that the patient was throwing some doses away. Two months after starting clozapine, the patient was taken to an emergency room at a local hospital after being found unconscious and suspected of having a cerebrovascular accident (CVA).
Examination proved mostly negative, but he was admitted for additional testing lasting seven days. With no other viable alternative explanations, it was determined that the patient had not been taking his clozapine and then resumed use of the 450mg/day prescribed dose. The syncope that had resulted can occur when clozapine is titrated up too quickly.[1] Clozapine was discontinued by the attending physician at the local hospital and the patient was returned to the state psychiatric center.
Shortly after the patient’s return, clozapine was reordered and titrated to 300mg daily. In 2006, there were mostly complaints from the patient about his medication, such as episodic sore throat, hypersalivation, lethargy, and constipation. For the most part, he was able to manage these side effects with throat lozenges, additional rest, and laxatives. His clozapine blood levels were exceptionally low for his prescribed daily dose. Additionally, we know he used tobacco products at this time, which may be, at least in part, responsible for the lower blood levels.[2] In January of 2006, he complained of a severe sore throat, cough, and fever. Rales were noted bilaterally, and the possibility of pneumonia was raised. Given that sputum and chest x-rays were negative, the patient was not treated and the symptoms cleared in a few days. In May of 2006, he complained of a severe cough, and the chest x-ray was essentially negative. Again, his cough cleared in a few days. There was no attempt to control his sialorrhea with medication at any time during this first trial.
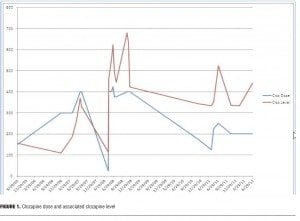
In 2007, additional references to hypersalivation, cough, lethargy, and constipation were noted in his record on daily doses of 350 to 400mg. The facility became a tobacco-free zone during 2007, although most patients were able to obtain tobacco for the remainder of 2007. Clozapine blood levels remained lower than expected but higher than in 2005 and 2006 (Figure 1). In May, the patient was admitted for two days to a local hospital with chest pain. Scattered rales were noted bilaterally. Hypersalivation, cough, and lethargy were patient complaints. Pneumonia was considered but x-rays were negative. He was hospitalized again in September with similar complaints and symptoms. In addition to sialorrhea, cough, and lethargy, he had a fever. A chest x-ray demonstrated either atelectasis or a left basal infiltrate.
In 2008, daily doses of 350 to 400mg were prescribed for this patient. He continued to have sialorrhea, cough, and lethargy. There were also two hospitalizations for pneumonia in 2008. The hospital smoking ban was in full effect by this time, and his serum blood levels continued to move up slowly (Figure 1). In January of 2008, the patient was transferred to a local hospital complaining of shortness of breath, cough, fever, and malaise. The CT scan showed “infiltrate within the right lower lobe.” He was diagnosed with right lower-lobe pneumonia. He was treated and returned to the state psychiatric center. In September of 2008, the patient was again transferred to a local hospital for nine days to treat right lower-lobe pneumonia. He had a productive cough, fever, and chills. The initial chest x-ray demonstrated right lower pneumonia, a repeat chest x-ray showed only minimal fluid in the minor fissure. Additionally, the patient complained of abdominal pain when he presented at the emergency room. A CT scan suggested a significant fecal impaction. The patient often complained of constipation secondary to clozapine.
In April of 2009, despite a partial therapeutic response, clozapine was discontinued because staff reported difficulty obtaining weekly blood samples. He was started on a low dose of haloperidol, which was gradually increased in an effort to control his psychotic symptoms. Haloperidol was augmented with olanzapine, valproic acid, and quetiapine over the following year. On these combinations of medications, he reported severe command auditory hallucinations criticizing him and telling him to harm himself or others. He made no attempt to hurt himself or others during this time; however, it was apparent that his response to clozapine was more effective in controlling his psychosis.
Literature review. Kane and Correll[3] demonstrate two central points: that an effective approach considers current symptoms, past therapeutic response, adverse effects, patient choice and expectations, and that clozapine is the only evidence-based treatment for refractory patients. Farka and Azorin[4] add that clozapine is the treatment of choice for patients with schizophrenia who are refractory to treatment, display violent behaviors, or at high risk for suicide.
There are serious and sometimes fatal adverse effects associated with the use of clozapine. Agranulocytosis, seizure activity, myocarditis, cardiomyopathy, metabolic syndrome, diabetes mellitus, fever, and neuroleptic malignant syndrome (with clozapine alone or when used in combination with lithium or other central nervous system [CNS] drugs) are the more well-known and severe adverse effects. Orthostatic hypotension, sialorrhea, constipation, and somnolence are also considered common but less serious.[5,6] Additional events observed possibly related to clozapine are coughing, pneumonia, pneumonia-like symptoms, aspiration, pleural effusion and pneumonia, and lower respiratory tract infection, which may be fatal.[6]
The adverse effects of sialorrhea, aspiration pneumonia, and pneumonia-like illnesses are central to our discussion. Pathophysiology and management of clozapine-induced sialorrhea are reviewed by Praharaj, Arora, and Gandotra and Hussaini.[7,8] Sheriff et al[9] note that there is still room for additional research on clozapine-induced hypersalivation. Aspiration pneumonia and pneumonia-like illnesses and their association to clozapine are increasingly being addressed in the literature.[10–13]
Hypersalivation is the most prominent side effect associated with the use of clozapine. Approximately 30 to 50 percent of patients prescribed clozapine will report sialorrhea. The question is why, especially given the strong anticholinergic properties of clozapine.[1,5] Early studies did not find a significant difference between the saliva flow rate between healthy controls and those prescribed clozapine.[14,15]
More recently, the evidence seems to suggest that clozapine does in fact increase the saliva flow rate; however, there was no correlation between salivary flow rate and mean clozapine dose.16 Ekström, Godoy and Riva’s animal study found the overall actions of clozapine suggested salivation increased during sleep and at rest, but decreased during meals.[17] Clozapine also caused secretion by direct action on muscarinic (M1) receptors of acinar cells, a response independent of central nervous mechanisms, presynaptic intraglandular events, and circulating catecholamines. It appeared likely that the net effect is an increase in saliva production with clozapine, which may increase the risk for aspiration pneumonia, especially for those who also experience swallowing difficulties, a side effect also often associated with clozapine.
When Rabinowitz et al[15] found that clozapine did not increase saliva flow rate, they hypothesized that drooling, choking, and aspiration may result from interference disrupting signals to the brain that the oral cavity needs to be cleared in order to swallow. Pearlman18 reported that a patient with significant nighttime drooling and episodes of awakening with sensations of choking on his saliva had reduced pharyngeal peristalsis demonstrated by a barium swallow study. However, he noted that instruction in swallowing two or three times without inhaling alleviated the choking sensation without affecting the sialorrhea, leading him to suggest that the risk of choking would be better handled by patient education regarding swallowing than by treating sialorrhea.
Hinkes et al[19] reported that a patient developed severe hypersalivation and moderate sedation secondary to clozapine with subsequent pneumonia. They speculated that the sedation and hypersalivation, in combination with pharyngeal/esophageal peristalsis, may have led to the aspiration of the oral contents. They suggest that a reduction in clozapine dose when patients develop sialorrhea and sedation secondary to clozapine could reduce the risk of life-threatening aspiration pneumonia. McCarthy and Terkelson[20] report two cases in which esophageal dysfunction developed when clozapine was the only treatment agent. One case was complicated by hypersalivation; one was not. Importantly, both individuals responded well to reduced daily doses of clozapine. Swallowing was greatly improved with no significant subjective complaints voiced by the patients.
Kuo’s group[10] looked at the association between second-generation antipsychotics and the risk of pneumonia requiring hospitalization in non-geriatric patients with schizophrenia. Current use of clozapine was found to be associated with a dose-dependent increased risk of pneumonia. Quetiapine, olanzapine, and risperidone were also found to be associated with increased risk of pneumonia with no clear dose-dependent relationship. There was also an increased risk of pneumonia if clozapine was used in combination with quetiapine, olanzapine, or risperidone. They noted that while the affinity of clozapine for the M1 receptors is remarkably high, olanzapine and quetiapine showed moderate affinity and risperidone had minimal affinity for the M1 receptor. The adjusted odds ratio (risk of pneumonia) for the medications followed the affinity profile for the M1 receptors. They also note that clozapine, olanzapine, and quetiapine had high affinity for the histaminergic-1 (H1) receptor and risk ratios for pneumonia, while risperidone had a low affinity for the H1 receptor and a low risk ratio for pneumonia. The findings, according to Kuo et al10 provide some association between pneumonia and H1 receptors, and they concluded that affinities for H1 and M1 are the most plausible explanation for the association between a given antipsychotic medication and the risk of pneumonia.
Kuo’s perspective significantly adds to the understanding of the association of atypical antipsychotic medication and the risk of pneumonia.10 If the Ekström, Godoy, and Riva[17] animal model is applicable to human physiology and clozapine actually causes secretion by the direct action on M1 receptors of the acinar cells, instead of dry mouth we would have increased salivation. We are not alone in concluding that sialorrhea combined with esophageal hypomotility and sedation could, in certain individuals, lead to aspiration pneumonia.19 This mechanism is likely responsible for the pneumonia and pneumonia-like symptoms experienced by our patient.
Second clozapine trial 2010 to 2012. Due to clear therapeutic effects of clozapine for this patient, especially reduction of auditory hallucinations and absence of lethal command hallucinations, in October of 2010, the patient was approached with the idea of a low-dose clozapine trial. The patient voiced his concerns about the side effects he had experienced with clozapine and the difficulty in obtaining blood samples from him but acknowledged the positive effects of the medication. He agreed to a trial of clozapine at doses around 200mg/day.
As the clozapine was initiated and titrated he was observed closely. Adherence to taking clozapine was excellent. One month after restarting clozapine at a daily dose of 200mg, the patient was not complaining of lethargy, sialorrhea, or constipation. The dose was titrated to 225mg for two days and then to 250mg for two days. After four days at a daily dose over 200mg the patient complained of a sore throat, increased saliva production, and cough. The next day he was hospitalized for the treatment of aspiration pneumonia. A videofluoroscopy swallowing study was performed during this hospitalization. It demonstrated residual matter in the patient’s oropharynx, prolonged pooling of the bolus, and a delay in the swallowing reflex. Recommendations to prevent repeated aspiration pneumonia for this patient included a percutaneous endoscopic gastrostomy (PEG) tube (no oral feedings) or use of a pureed diet. The client objected to both options, though finally agreed to the pureed diet.
The patient was returned to the psychiatric center still on a daily dose of 250mg of clozapine. Immediately the dose was lowered to 75mg daily and gradually increased to 200mg daily. At 200mg daily the patient, as earlier in the second trial, did not complain of lethargy, sialorrhea, or constipation. However, a year later at a time when he was experiencing increased auditory hallucinations and irritability, the dose was increased once again to 250mg for two days. By the second day, the patient reported cough and lethargy. His temperature was 101.1° F. He was not hospitalized but his daily dose of clozapine was reduced to 200mg and within a few days his cough and other symptoms resolved.
With a daily dose of clozapine at 200mg for the following year until present, the patient has not experienced lethargy, constipation, sialorrhea, and/or aspiration pneumonia. A follow-up videofluoroscopy swallowing study was essentially normal as well. Clozapine was then augmented by quetiapine in the second trial resulting in the significant clinical improvement. Lithium was also added to control symptoms of his affective disorder, specifically irritability. With his psychotic and affective symptoms controlled, he started to actively participate in social and recreational activities as an inpatient. He was discharged to a supportive community residence on clozapine 200mg/day, quetiapine 100mg/day, and lithium 600mg HS. He had been on this combination for at least the past year.
Discussion and Conclusion
Use of a lower clozapine dose. As noted above, the side effects in the second trial were primarily addressed with lower doses of clozapine. Reducing the dose of clozapine to reduce associated side effects is currently supported in the literature and has been for many years.[1,5,19,21,22]
While there was significant improvement in the client’s symptoms of psychosis, he still reported occasional hallucinations. Therefore, with the patient’s consent, the dose was increased over four days to 250mg PO daily. Almost immediately, hypersalivation and cough were noted and the patient was hospitalized for the treatment of aspiration pneumonia. Hinkes[19] reported a case of aspiration pneumonia in a patient taking 250mg of clozapine daily as well. Substantial symptom reduction in this case occurred with the lower dose of clozapine and without the untoward effect of aspiration.
Therapeutic drug monitoring of clozapine serum levels. Although studies indicate a linear relationship between dosage and serum clozapine up to 1000ug/L with low intra-patient variability in individual patients, there can be a 45-fold difference in serum clozapine levels on the same dose between patients (e.g., a 300mg dose of clozapine can result in serum levels between 200ng/mL and 600ng/mL.[23–25] Previous studies mostly agree with Nielsen et al[21] that in case of partial response or non-response, the use of therapeutic drug monitoring of clozapine is recommended. A minimal plasma level above the therapeutic threshold of 350 to 420ng/mL is necessary to determine nonresponse to clozapine.[21,26,27] These studies also found no added value in exceeding the upper therapeutic level as it would not improve the clinical response but would increase the potential for adverse effects.
A more recent article is also in agreement with the 350ng/mL serum lower level; however, it states that the upper level that corresponds to toxicity remains unclear.[28] They suggest that levels between 350 to 1000ng/mL achieved through slow titration are likely to be effective and less likely to cause toxicity. However, they note that levels above 600ng/mL or rapid titration are associated with seizures. They recommend ongoing monitoring when a patient is prescribed higher doses of clozapine (i.e., >600mg daily), there has been a change in the patient’s concomitant medication or cigarette use, and when there is suboptimal response.
During the first three years (2005–2007) the patient’s serum levels were low considering the prescribed dose. The patient’s partial adherence and use of cigarettes are probable explanations. In 2008, the clozapine levels are elevated, though still in the therapeutic range, at doses similar to what was prescribed in 2007. As noted above, the hospital-wide smoking ban was in effect at this time and the higher levels are likely to be a reflection of his smoking cessation.[2,29,30] It was in 2008 that he developed serious respiratory issues including pneumonia and aspiration pneumonia requiring hospitalization. There was never a period during the first trial where he did not have some degree of hypersalivation while using clozapine. It is likely that the upper level that is tolerable before serious side effects for this individual is well below the quasi-established upper limit of 1000ng/mL.
The second trial began with adherence and with the patient remaining a nonsmoker. Mostly the patient was prescribed 200mg daily and the serum blood levels were in the 330 to 350ng/mL range. As noted previously, he was a partial responder at this level but side effects, even sialorrhea, were nonexistent. Two attempts to increase his clozapine to 250mg daily did not significantly improve his clinical presentation, but did cause develop serious side effects including one instance of aspiration pneumonia requiring hospitalization. Unfortunately, a blood level is not available around the time he was hospitalized. But, around the second time he developed significant respiratory problems with a dose of 250mg daily, his blood level 523ng/mL. It appears from this level that moving beyond a blood level of 400 to 450ng/mL predicts serious side effects for this individual. Maintaining serum levels that are in the lower acceptable limits, even though only partially therapeutic, limits the side effect burden for him.
Augmentation with another antipsychotic medication. Goff and Dixon31 state when patients fail to respond to an adequate dose of an antipsychotic, clozapine is the only option with established efficiency. The problem is that there are a substantial number of patients, estimated to be around 30 to 50 percent, refractory to antipsychotics who will also be refractory or only partial responders to clozapine.[21,32,33] In this case, how does one proceed? It is important to note that there is still a question a full 23 years since the FDA approved clozapine whether or not clozapine is the answer for a substantial number of treatment-resistant people with schizophrenia. Goff and Dixon[31] suggest that in these situations clinical judgment combined with patient preference must take over when treatment algorithms fall short.
Clinical experience holds that when clozapine is ineffective or not an option for a patient, the art of psychopharmacology comes into play. Given that clozapine is typically used in treatment-resistant schizophrenia, combinations of medications that include clozapine are often tried if clozapine monotherapy is unsuccessful. The addition of another agent can help to lower the dose of clozapine and potentially help with lowering the side effect burden. Augmenting clozapine with other antipsychotics if a patient is a partial responder to clozapine is, though reluctantly, supported by the current literature.[21,22,34–36]
After discussing the rationale for augmentation with our patient, he agreed to a low dose of quetiapine in the second clozapine trial. There is some support for the use of quetiapine in combination with clozapine for treatment-resistant patients.[35] The choice of quetiapine, however, is strictly the prerogative of the prescriber. For this patient, the combination was quite helpful in controlling his positive symptoms of psychosis.
Lithium was also added to the medication plan primarily to address the affective component of our patient’s psychiatric illness and not necessarily to augment the clozapine. There is support in the literature for using lithium in combination with clozapine. Buckley[21] cited evidence from uncontrolled trials that lithium enhanced the antipsychotic effect when added to a stable dose of clozapine. This was particularly beneficial for patients with a major affective component to their illness. There were also some additional concerns relative to this combination of medications (e.g., seizures and neurotoxicity even with lithium levels <0.5mEq/L). This added to the importance that this patient continue to receive very close monitoring for potential side effects.
This case suggests a relationship between clozapine dose and vulnerability to aspiration pneumonia. Individual responses to medications have an impact on clinical efficacy and the presence and severity of associated side effects. Further research is needed to clarify this association. Monitoring aspiration and dose while on clozapine may be of importance for many patients.
As a result of our clinical experience with this patient we have ascertained that using tightly controlled pharmacological monitoring and prescribing a finely tuned titration of clozapine dose can result in controlling sialorrhea, and that an effort to rechallenge a patient on clozapine is feasible even when severe sialorrhea and complications arising from it have occurred previously.
References
1. Lieberman J, Safferman A. Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. 1992;63:1:51–70.
2. Meyer J. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21:6:569–574.
3. Kane J, Correll C. Past and present progress in pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71,9:1115–1124.
4. Fakra E, Azorin J. Clozapine for the treatment of schizophrenia. Expert Opin Pharmacother. 2012;13:1925–1935.
5. Young C, Bowers M, Mazure C. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24,3:381–390.
6. Novartis Pharmaceuticals Corporation. East Hanover, New Jersey 07936, © Novartis. Clozaril Package Insert. T2011-123, October 2011.
7. Praharaj S, Arora M, Gandotra S. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology. 2006;185:265–273.
8. Hussaini T. Clozapine-induced sialorrhea. For Your Inpharmation: Pharmacy Newsletter. Riverview Hospital. 2003;23,4:1–3.
9. Sheriff RJS, Au K, Cahill C, et al. Pharmacological interventions for clozapine-induced hypersalivation. Schizophr Bull. 2008;34,4:611–612.
10. Kuo C, Yang S, Liao Y, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2012. http://schizophreniabulletin.oxfordjournals.org doi:10.1093/schbul/sbr202.
11. Nielsen J, Foldager L, Meyer J. Increased use of antibiotics in patients treated with clozapine. Eur Neuropsychopharmacol. 2009;19,7:483–486.
12. Liang C, Hsieh T. Myoclonus as a indicator of infection in patients with schizophrenia treated with clozapine. J Psychiatry Neurosci. 2011;36(1):E1.
13. Taylor D, Douglas-Hill P, Olofinjana B, et al. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry. 2009;194:165–167.
14. Ben-Areyh H, Jungeman T, Szargel R, et al. Salivary flow-rate and composition in schizophrenic patients on clozapine: subjective reports and laboratory data. Biol Psychiatry. 1996;39(11):946–949.
15. Rabinowitz T, Frankenburg F, Centorrino F, Kando J. The effect of clozapine on saliva flow rate: a pilot study. Biol Psychiatry. 1996;40(11):1132–1134.
16. Pearlman C. Clozapine, nocturnal sialorrhea, and choking. [Letter] J Clin Psychopharmacology. 1994;14(4):283.
17. Praharqaj S, Jana A, Goswami K, et al. Salivary flow rate in patients with schizophrenia on clozapine. Clin Neuropharmacology. 2010;33(4):176–178.
18. Ekström J, Godoy T, Riva A. Clozapine: Agonistic and antagonistic salivary secretory actions. J Dental Res. 2010;89(3):276–280.
19. Hinkes R, Quesada T, Currier M, Gonzalez-Blanco M. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. [Letter]. J Clin Psychopharmacology. 1996;16(6): 462–463.
20. McCarthy R, Terkelsen K. Esophageal dysfunction in two patients after clozapine treatment. J Clin Psychopharmacology. 1994;14(4):281–283.
21. Buckley P, Miller A, Olsen J, et al. When symptoms persist: clozapine augmentation strategies. Schizophr Bull. 2001;27(4):615–628.
22. Nielsen J, Damkier P, Lublin H, Taylor D. Optimizing clozapine treatement. Acta Psychiatr Scand. 2011;123(6):411–422.
23. Thompson D. Clozapine serum levels: a review of the literature, part I. For Your Inpharmation: Pharmacy Newsletter. Riverview Hospital. 2003;23(5):1–3.
24. Thompson D. Clozapine serum levels: a review of the literature, part II. For Your Inpharmation: Pharmacy Newsletter. Riverview Hospital. 2003;23(6):1–7.
25. Potkin S, Bera R, Gulasekaram B, et al. Plasma clozapine concentrations predict clinical response in treatment resistant schizophrenia. J Clin Psychiatry. 1994;55(Suppl B):133–136.
26. Perry P, Miller D, Arndt S, Cadoret R. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Amer J Psychatry. 1991;148(2):231–235.
27. Vanderswaag C, McGee M, McEvoy J, et al. Response of patients with treatment-refractory schizophrenia to clozapine within three serum levels ranges. Amer J Psychiatry. 1996;153(12):1579–1584.
28. Stark A, Scott J. A review of the use of clozapine levels to guide treatment and determine cause of death. Austral NZ J Psychiatry. 2012;46(9):816–825.
29. Ashir M, Petterson L. Smoking bans and clozapine levels. Adv Psychiatr Treat. 2008. doi: 10.1192/apt.14.5.398b.
30. de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491–493.
31. Goff D, Dixon L. Antipsychotic polypharmacy: Are two even better than one? Am J Psychiatry. 2011;168(7):667–669.
32. Ziegenbein M, Sieberer M, Kuenzel H, Kropp S. Augmentation of clozapine with amisulpride in patients with treatment-resistant schizophrenia: an open clinical study. German J Psychiatry. 2006. Downloaded from http://www.gjpsy.uni-goettingen.de/gjp-article-ziegenbein.pdf
33. McIlwain M, Harrison J, Wheeler A, Russell B. Pharmacotherapy for treatment-resistant schizophrenia. Neuropsychiatr Dis Treat. 2011;7:135–149.
34. Taylor D, Smith L, Gee S, Nielsen J. Augmentation of clozapine with a second antipsychotic: a meta-analysis. Acta Psychiatr Scand. 2012;125:15–24.
35. Genc Y, Taner E, Candansayer S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1–13.
36. Kerwin R, Bolonna A. Management of clozapine-resistant schizophrenia. Adv Psychiatric Treatment. 2005;11:1001–1006.