 by Jonathan R. Scarff, MD
by Jonathan R. Scarff, MD
Dr. Scarff is a staff psychiatrist at the Veterans Affairs Outpatient Clinic in Spartanburg, South Carolina, USA
Innov Clin Neurosci. 2016;13(9–10):49–52
Funding: The author has received no financial support for the writing of this manuscript.
Financial Disclosures: The authors has no financial dislosures relevant to the content of this article.
Disclaimer: The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Key words: Schizophrenia, mania, mixed episode, bipolar, cariprazine, antipsychotic
Abstract
Schizophrenia and bipolar disorder are associated with significant morbidity and mortality. Although atypical antipsychotics reduce positive and negative symptoms of schizophrenia as well as manic or mixed episodes of bipolar disorder, they are associated with varying degrees of metabolic adverse effects. This necessitates continued development of efficacious yet metabolically favorable treatments. Cariprazine was recently approved to treat adult patients with schizophrenia and manic or mixed episodes. It was well-tolerated and adverse reactions included akathisia, extrapyramidal symptoms, nausea, or constipation. Cariprazine is taken once daily without regard to food. The dose should be adjusted in patients who receive CYP450 inhibitors, and it should not be given to patients with severe hepatic or renal disease. This article reviews mechanisms of action, efficacy, tolerability (including adverse effects), dosing, and contraindications of cariprazine.
Introduction
Schizophrenia is characterized by at least two of the following symptoms: delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, or negative symptoms.[1] Bipolar disorder consists of manic, hypomanic, or mixed episodes alternating with depressive episodes.[2] Because second-generation or atypical antipsychotics reduce both positive and negative symptoms of schizophrenia as well as manic and mixed episodes of bipolar disorder, they are recommended treatments for both conditions.[3–5] However, they are associated with adverse effects such as weight gain, dyslipidemia, and hyperglycemia, which may worsen comorbid cardiovascular conditions.[6,7] This necessitates continued development of effective, tolerable, and metabolically favorable treatments. In September 2015, cariprazine was approved to treat schizophrenia and manic or mixed episodes in adults. This article reviews mechanisms of action, efficacy, tolerability (including adverse effects), dosing, and contraindications of cariprazine.
Mechanisms of Action
Cariprazine is a partial agonist at D2 and D3 receptors, with significantly greater affinity for D3 receptors.[8] It exerts partial agonism at 5-HT1A receptors, antagonism at 5-HT2A and 5-HT2B receptors, and minimal antagonism at 5-HT2C, 5-HT7, and H1 receptors.[8] In contrast to full antagonism of dopamine receptors, partial agonism suggests lower risk of akathisia, tardive dyskinesia, extrapyramidal symptoms, and abnormal prolactin levels. Modulation of the D3 receptor exerts a procognitive effect and may reduce negative symptoms.[9,10] Lack of significant histamine receptor antagonism decreases risk for sedation and weight gain.
Efficacy
Schizophrenia. Cariprazine was evaluated for efficacy in three trials, which are detailed in Table 1. The primary endpoint was total change in the Positive and Negative Syndrome Scale (PANSS), and the main secondary endpoint was change in the Clinical Global Impressions – Severity scale (CGI-S).[11,12] All cariprazine doses demonstrated efficacy as evidenced by a reduction in PANSS total, PANSS subscales, and CGI scale.[13–15] Efficacy was noted starting at Week 1 for groups receiving higher doses and Weeks 2 or 3 for the lowest doses. Two studies used risperidone or aripiprazole as active controls to ensure assay sensitivity and did not compare their efficacy to cariprazine.[13,14]
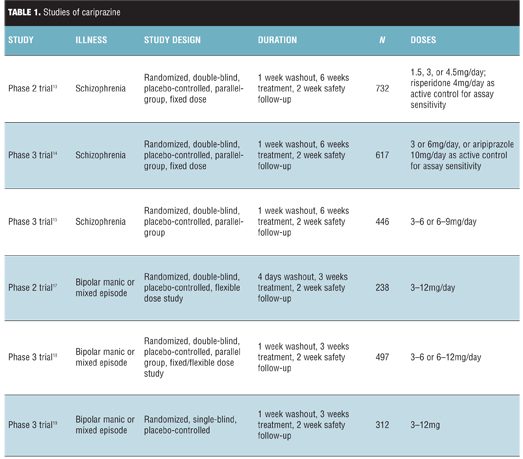
Bipolar disorder. Cariprazine was evaluated in three studies of adults with manic or mixed episodes, which are detailed in Table 1. Efficacy was assessed using the Young Mania Rating Scale and the CGI-S.[12,16] In the first study, cariprazine demonstrated efficacy within one week, and improvement on the CGI-S scale was noted as early as Day 2 of treatment; nearly twice as many patients achieved response and remission of symptoms with cariprazine.[17] In a second study, low and high doses of cariprazine reduced symptoms compared to placebo.[18] In a third study, cariprazine demonstrated significant reduction in symptoms, starting by Day 4 of treatment.[19] It was also associated with significant rates of response and remission.
Tolerability
Cariprazine was generally well-tolerated. The most common adverse effects included akathisia, extrapyramidal symptoms, nausea, or constipation. Insomnia; headache; insignificant increases in alanine aminotransferase (ALT), heart rate, or glucose; and decreased prolactin were noted infrequently. There were no differences between treatment and placebo groups on other metabolic parameters or electrocardiogram measures. However, all studies noted the short duration of treatment as a limitation, acknowledging that longer trials are needed to characterize the metabolic profile of cariprazine.
Dosing
Cariprazine is available in 1.5mg, 3mg, 4.5mg, and 6mg tablets given once daily without regard to food.20 The recommended daily dose is 1.5- to 6mg for patients with schizophrenia and 3- to 6mg for patients with manic or mixed episodes. Dosing starts with 1mg for one day, then titration in 1.5- or 3mg increments as indicated. Patients taking cariprazine and starting a CYP3A4 inhibitor should decrease the dose of cariprazine by half (decrease to 1.5mg or 3mg daily if receiving 4.5mg, and take every other day if taking 1.5mg).[20] In patients receiving a stable dose of a CYP3A4 inhibitor, cariprazine is started at 1.5mg on Days 1 and 3, with a maximum dose of 3mg. Although no dosage adjustment is required in patients with mild or moderate hepatic or renal impairment, patients should not take cariprazine with a CYP3A4 inducer or if they have severe hepatic or renal disease.[20]
Following oral administration, cariprazine reaches peak concentration within 3 to 6 hours and steady-state within 1 to 2 weeks, with a half-life of 2 to 4 days. It has a high volume of distribution and is highly protein-bound in plasma.[20] It is metabolized into two active metabolites by CYP3A4 and to lesser extent by CYP2D6 isozymes. An inactive metabolite is excreted in urine.[20]
Contraindications
Cariprazine is contraindicated in patients with known hypersensitivity to cariprazine. As with other atypical antipsychotics, cariprazine carries a “black box” warning for increased mortality and cerebrovascular events in elderly patients with dementia-related psychosis. It also includes warnings for neuroleptic malignant syndrome, tardive dyskinesia, metabolic abnormalities, low white blood cell count, orthostatic hypotension, seizures, cognitive and motor impairment, changes in body temperature, and dysphagia.[20] Prescribers are advised to monitor for delayed adverse reactions due to the long half-life of cariprazine.[20] Cariprazine is not yet assigned a pregnancy category, but neonates exposed to cariprazine in the third trimester are at risk for extrapyramidal and/or withdrawal symptoms.[20] While no data are available regarding safety in breastfeeding mothers, the manufacturer recommends that the benefits of breastfeeding should be considered along with the mother’s need for cariprazine and potential adverse effects on the infant from the medication or the underlying mental illness.[20]
Summary
Schizophrenia and bipolar disorder are debilitating conditions with significant morbidity and mortality. Many atypical antipsychotics improve functioning, but they carry risks for adverse effects. Cariprazine reduced symptoms of acute schizophrenia and manic or mixed episodes with minimal adverse effects. It is an additional option for clinicians who treat patients with schizophrenia and bipolar disorder. Further trials are warranted to evaluate its long-term efficacy and metabolic profile.
References
1. American Psychiatric Association. Schizophrenia spectrum and other psychotic disorders. In: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association;2013:87–122.
2. American Psychiatric Association. Bipolar and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association;2013:123–154.
3. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56.
4. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44.
5. National Institute for Health and Clinical Excellence. Bipolar disorder: assessment and management. September 2014. http://www.nice.org.uk/guidance/cg185/chapter/1-recommendations#managing-mania-or-hypomania-in-adults-in-secondary-care-2. Accessed November 22, 2015.
6. Hennekens CH. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. J Clin Psychiatry. 2007;68(Suppl 4):4–7.
7. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–179.
8. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328–340.
9. Zimnisky R, Chang G, Gyertyán I, et al. Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl). 2013;226(1):91–100.
10. Papp M, Gruca P, Laso?-Tyburkiewicz M, et al. Attenuation of anhedonia by cariprazine in the chronic mild stress model of depression. Behav Pharmacol. 2014;25(5-6):567-574.
11. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Rating Criteria. North Tonawanda, NY: Multi-Health Systems; 1999.
12. Guy W. ECDEU Assessment Manual for Psychopharmacology (revised). US Department of Health, Education, and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 193–198.
13. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450–457.
14. Cutler AJ, Migliore R, Mokliatchouk O, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo and active controlled trial. Presented at the 166th Annual Meeting of the American Psychiatric Association, May 18–22, 2013. San Francisco, California.
15. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, Phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–373.
16. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435.
17. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63–75.
18. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284–292.
19. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, Phase III trial. J Affect Disord. 2015;174:296–302.
20. Actavis. Vraylar (cariprazine) package insert. Actavis Pharma, Inc. Parsippany, New Jersey. September 2015.




