
by Laura Villarreal-Martinez, MD; Laura Elia MartÍnez-Garza, MD; Iram Pablo Rodriguez-Sanchez, MD; Neri Alvarez-Villalobos, MD; Fernando Guzman-Gallardo, MD; Sulia Pope-Salazar, MD; Cynthia Salinas-Silva, MD; Maria Guadalupe Cepeda-Cepeda, MD; Alejandra Garza-Bedolla, MD; Irving Armando Dominguez-Varela, MD; Daniel ZacarIas Villarreal-Martinez, MD; Jose Humberto Treviño-Villarreal, MD; and David Gomez-Almaguer, MD
Drs. L Villarreal-Martinez, Alvarez-Villalobos, Guzman-Gallardo, Pope-Salazar, Salinas-Silva, Cepeda-Cepeda, Garza-Bedolla, Dominguez-Varela, DZ Villarreal-Martinez, Treviño-Villarreal, and Gomez-Almaguer are with Hematology Service, Hospital Universitario “Dr. José Eleuterio González” in Monterrey, Mexico. Drs. Martínez-Garza and Rodriguez-Sanchez are with the Genetics Department, Hospital Universitario “Dr. José Eleuterio González” in Monterrey, Mexico.
Funding: This study was funded by the hematology service of Hospital Universitario “Dr. José Eleuterio González.”
Disclosures: All authors are employees of Hospital Universitario “Dr. José Eleuterio González.”
Innov Clin Neurosci. 2022;19(4–6):78–86.
Abstract
Autism spectrum disorders (ASDs) are a group of neurodevelopmental pathologies characterized by social and communication deficits, for which treatments are limited. Cell therapies, including intrathecal (IT) administration of bone marrow (BM) mononuclear cells (BM-MNC), improves symptoms in patients with ASD. Twenty-four patients diagnosed with ASD, according to the Diagnostic and Statistical Manual of Mental Disorders Text Revision Fourth Edition (DSM-IV-TR) criteria, were autologously treated with IT BM-MNC, and the clinical effect was evaluated using the Childhood Autism Rating Scale (CARS) on Days 30 (n=24) and 180 (n=14) post-treatment. IT BM-MNC improved clinical outcomes by Day 30 (p=0.0039), and those benefits remained and were further accentuated by Day 180 post-treatment (n=14; p=<0.0001). Clinical benefit at Days 30 (p=0.001; r= –0.51) and 180 (p=0.01; r= –0.60) posttreatment positively correlated with the enrichment of a putative BM stem cell population expressing the cluster of differentiation 133+ (CD133+) surface marker.
Keywords: Autism, stem cells, Asperger’s syndrome, Rett’s disorder, childhood disintegrate disorder
Autism spectrum disorders (ASD) are a group of neurodevelopment pathologies that encompass disorders such as autism, Asperger’s syndrome, Rett’s disorder, and childhood disintegrate disorder. According to the Diagnostic and Statistical Manual of Mental Disorders Text Revision Fourth Edition (DSM-IV-TR), the disorders classified under ASD are characterized by a series of social/behavioral abnormalities, which includes reciprocal dysfunction in social interaction and communication, as well as the presence of repetitive and stereotypical behaviors.1,2 The current epidemiological evidence highlights an increase in the incidence of ASD, with some estimates suggesting it affects one in every 166 individuals, or close to one percent of children in some countries, including the United States (US).3–5
The etiology of ASD is unknown. However, genetic, epigenetic, immunological, neurological, vascular, and environmental risk factors can drive disorder progression. For instance, genomic and transcriptomic analyses have revealed mutations and changes in gene expression patterns for cytoarchitectural proteins, such as SH3 and multiple ankyrin repeat domains 3 (SHANK3), FMRP translational regulator 1 (FMR1), or methyl-CpG binding protein 2 (MECP2). These alterations can alter the structure of neurons and synaptic terminals and explain some characteristic defects of synaptic function, glial activation, and cytoarchitectural organization in the brains of patients with ASD.6–11 Also, defects in immunity, characterized by neuroinflammation, T cell deregulation, and excessive cytokine production, are involved in the severity of ASD. Notably, current treatments for ASD are directed at treating the social/behavioral dysfunction that derives from these pathways’ dysregulation, rather than modifying the underlying molecular basis per se.12–14
Stem cell therapies are used to treat neurological disorders sharing molecular dysfunctions, similar to those observed in patients with ASD. For instance, several stem cells produce neurotrophic factors that promote neuronal regeneration or secrete immunoregulatory cytokines to dampen inflammation and provide important pathologic processes, such as stroke, brain injuries, and multiple sclerosis (MS), among others.15–17 With this in mind, several clinical trials have been conducted to investigate the potential benefit of cell therapy to improve clinical symptomatology in patients with ASD. Most studies evaluating cell therapies in patients with ASD use the Childhood Autism Rating Scale (CARS) to determine a given intervention’s clinical effect. CARS scores 14 domains assessing behaviors associated with ASD.18,19 The different areas of evaluation include relationships with people; imitation; emotional response; use of the body; use of objects; adaptation to change; visual response; auditory response; use and response of taste, smell, and touch; fear or nervousness; verbal communication; nonverbal communication; and level of activity. By assessing changes in score before and throughout treatment, it is possible to establish various therapies’ clinical effects. With this approach, it has been documented with a certain level of confidence and reproducibility that several cell therapies, including various cell types and sources, provide clinical improvement to patients with ASD.15,17,20–22
Bone marrow (BM) is the source for cells used in most trials with patients with autism.20–23 Other sources include umbilical cord isolates and adipose tissue.22 These tissues are preferred because they are enriched in stem cells thought to mediate stem cell therapies’ benefits.23 For instance, BM contains hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC), distinguishable based on the pattern of expression of surface markers. HSC are characterized by a cluster of differentiation 45– (CD45–), CD19–, CD3–, CD56–, CD34+, CD133+, CD164+, CD117+ phenotype, while MSC bear a CD105+, CD90+ CD73+, CD34–, CD45–, CD14–, CD16–, human leukocyte antigen-DR isotype (HLA-DR) pattern of expression. Nevertheless, current cell therapy studies in patients with autism do not account or enrich for any cell subset to treat patients. They utilize unpurified BM mononuclear cell (BM-MNC) fractions, containing a variable number of stem cells that fluctuate between and within patients. The lack of consensus on any specific type of stem cell can account for variability in clinical practice and highlights the importance of standardizing protocols based on specific cell subsets of importance to mediate these therapies’ benefits.
Here, we sought to identify stem cells implicated in controlling the benefits of a cell therapy consisting of intrathecal (IT) administration of BM-MNC in patients with ASD. We characterized the BM-MNC fraction from patients with ASD for specific stem cell subsets and correlated their frequency with the benefits conferred upon the treatment with such autologous BM-cell preparation.
Materials and Methods
Study design and patient evaluation. We conducted a prospective, longitudinal, open-label clinical trial designed to evaluate the effect of BM-MNC subsets, administered intrathecally, on the clinical performance of children with ASD. Twenty-four children diagnosed with ASD were recruited at the hematology service of our hospital. We excluded patients with a history of previous cell-based therapies for autism, patients with neurodegenerative diseases, and patients with evidence of an active autoimmune and/or inflammatory/infectious disorder. This clinical trial was evaluated and approved by the ethics committee of our institution. All procedures, risks, and potential benefits were carefully explained to the parents and/or guardians of those children who qualified for the study. This trial was registered in clinialtrials.gov.
We recruited patients with a confirmed diagnosis of ASD established according to the criteria listed in the DSM-IV-TR. After providing consent/assent, the patients underwent a complete evaluation that included a clinical history, physical examination, complete blood count (CBC), blood chemistry, and a basic transplant donor evaluation. Patients also received a complete psychiatric evaluation using CARS to assess disorder severity. CARS was chosen because it is the most reliable and commonly used clinical scale to evaluate the severity of autism in most clinical studies.
A total of 24 patients with ASD were recruited and evaluated prospectively with CARS to assess clinical changes resulting from IT BM-MNC therapy. All 24 subjects were evaluated with CARS at baseline (Day 0) and 30 days after the IT BM-MNC administration. Fourteen children from this cohort were also followed continuously for 180 days to assess the long-term effects of BM-MNC administration. Although patients in the 180-day cohort were also included with the patients evaluated 30 days postinjection, no data was analyzed and/or presented twice in any datapoint, graph, or statistical analysis.
In-vivo BM stimulation and harvest. Granulocyte colony-stimulating factor (G-CSF) 10mg/kg per day was administered subcutaneously for three consecutive days before the intervention to stimulate BM cell proliferation/regeneration and increase the total number of stem cells to be harvested for the marrow aspirate.23,24 The increase in peripheral blood cells following stimulation with G-CSG was confirmed using a CBC analyzer. On the day of the procedure, patients were premedicated with hydrocortisone (10mg/kg for up to 100mg intravenously) and ondansetron (0.2mg/kg up to 8mg intravenously). Anesthesia was induced with a combination of midazolam (0.25g/kg) and sevoflurane (average alveolar concentration of 2.05%). With the patient in a prone position, we performed local anesthesia with 1% xylocaine and punctured both iliac crests with a Jamshidi needle (CareFusion Corporation; Chicago, IL, US). The BM aspirate volume was set to 8mL/kg/body weight for up to 150mL of aspirate. The harvested BM aspirate was placed into sterile 50mL corning tubes (Corning Inc.; Corning, NY, US), supplemented with an anticoagulant mix of heparin and citrate dextrose (1:100 dilutions of 1000U) and stored on ice until further processing.
BM aspirate processing. The BM aspirate was filtered through a 70µm cell strainer (Corning, Sigma-Aldrich; St. Louis, MO, US) to remove large debris and concentrate to obtain the total nucleated cell fraction. Then, the buffy coat was divided into 5mL aliquots, placed into separate tubes. We added 40mL of a sterile 1X solution of an NH4Cl buffer to lyse the red blood cells to each tube. After two sequential centrifugation steps with 50mL of sterile saline at 300×g for 10 minutes at 24 degrees Celsius, we resuspended the total BM-MNC fraction in sterile saline solution to a final volume of 10mL. This suspension was used for IT BM-MNC administration, although two aliquots of 500µL each were taken to determine viability, for microscopical examination, for bacteriological culture, and to perform flow cytometry analysis.
BM cell characterization. After counting the total cell concentration, BM cells were adjusted to 1×106 cells/100 µL and resuspended in sterile-filtered flow cytometry staining (FACS) buffer (1% bovine serum albumin [BSA], 0.5% fetal bovine serum, 5mM ethylenediaminetetraacetic acid [EDTA], 1% normal human serum, all in Dulbecco’s phosphate-buffered saline [DPBS]). Cells were incubated for 15 minutes with an Fc blocking solution (Miltenyi Biotec; Waltham, MA, US), and without washing, we added the following antibodies, at the specified concentration: fluorescein isothiocyanate (FITC) mouse anti-human CD45 (hCD45) (cloneHI30; 0.2µg/µL), allophycocyanin (APC) mouse anti-hCD34 (clone 581; 0.2µg/µL), and Brilliant Violet 421 (BV421) mouse anti-CD133 (clone 293C3; 0.3 µg/µL) (all antibodies were from BD Biosciences; San Jose, CA, US). After three extensive washes with DPBS plus 0.5% BSA, cells were resuspended in 500µL of FACS buffer, to which 10µL of a 7-amino-actinomycin D (7-AAD) viability dye solution (BD Biosciences; San Jose, CA, US) was added.
Flow cytometry analysis was performed using a BD FACS Canto instrument (BD Biosciences; San Jose, CA, US), equipped with a 405, 488, and 655nm laser system. Compensation was performed automatically with calibration beads (BD Biosciences; San Jose, CA, US), and fluorescence minus one control were used to set up the experiment’s gates on each patient determination. Analyses were performed in DIVA software (BD Bioscience; San Jose, CA, US), first by setting up the Forward Scatter Height (FSC-H) and Forward Scatter Width (FSC-W) Gate 1 to remove doubles. Then, the FSC-H and Side Scatter Pulse Height (SSC-H) Gate 2 was established to select for mononuclear cells. Finally, the FSC-H/7AAD Gate 3 was set to select the viable population. Cells were divided into CD45+ or CD45– by setting a subsequent FSC-H/CD45 Gate 4. Analysis of the CD45 population was performed from this gate by selection the CD45+ population. The CD45– population was selected as Gate 5, from which we performed the analysis of CD34 and CD133 cells. The results from our study are expressed as absolute values and correspond to cells per microliter (cells/μL) of BM.
Stem cell administration. The IT technique was performed with patients sedated as described above. A single lumbar puncture to the subarachnoid spaced at L3-L4, or L4-L5 was performed using a 25-gauge Quincke spinal needle (Spinocan, B. Braun; Melsungen, Germany). Five mL of cerebrospinal fluid (CSF) was extracted and used for cytochemical analyses and bacterial cultures. Then, a total of 10mL of BM-MNC in suspension was slowly infused at an approximate rate of 2mL/min, for a total of five minutes of infusion. Patients were kept under surveillance for two hours after the procedure to monitor adverse effects from anesthesia and/or the IT cell injection. The discharge was performed when patients were fully awake and tolerating oral fluids. Caretakers were instructed on precise indications for pain management at the puncture site, fever, headache, vomiting, and nausea. Additionally, they were instructed to motivate the patients to lay horizontally for 24 hours. The complete procedure was carried out in a single day.
Statistical analysis. Analysis for normality was performed in all our analyses using the following tests: D’Agostino-Pearson omnibus, Anderson-Darling, and Shapiro-Wilk, with the Kolmogorov-Smirnov test used to evaluate the distance from the mean of the data distribution. The alpha value was established at 0.05, and p-values above 0.05 were considered for normality. Panel 1A did not have a normal distribution and was analyzed using a two-tailed Wilcoxon matched-pairs signed-rank test for nonparametric data distribution. For all other CARS analyses, we used one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons posttest. Single grouped data, such as that in panel 1B, was analyzed using a one sample t-test and Wilcoxon signed rank test. The sum of ranked value (W) was used to determine the direction of the change, and the data were classified in different groups depending on whether they fell above or below the calculated median of the data. Pearson’s analyses were performed to identify the correlation between cells and CARS clinical scores. For these analyses, we first normalized CARS values to their baseline scores and used a one sample t-test with Wilcoxon signed rank analysis to determine significance. The mean of the distribution was calculated, and the data were separated into CARS improvement or no change, depending on whether they fell below or above this mean value, respectively. For all data, a p-value less than 0.05 was considered significant. All our analyses were done using GraphPad PRISM version 8.4.3 (San Diego, CA, US)
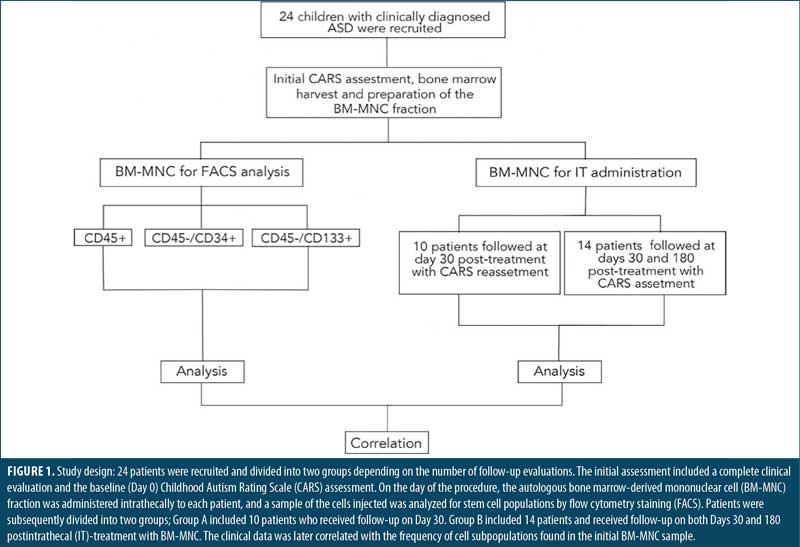
Results
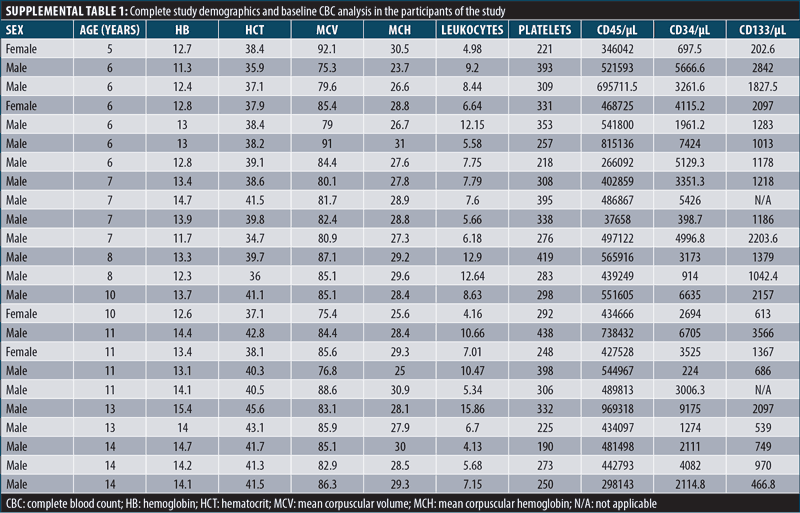
Description of the study population. The study design is depicted in Figure 1. A total of 24 patients, 19 male and five female, with a median age of 12.5±7 years, were recruited for this study (Supplemental Table 1). Patients received an IT injection of autologous BM-MNC and were divided in two groups according to the number of follow-ups with CARS after the IT administration of BM-MNC (Figure 1). Ten patients, nine male and one female, median age 13.5±7 years, were followed only on Day 30. The other 14 patients, 10 male and four female, median age 11.7±7 years, were followed on Days 30 and 180 (Supplemental Table 1). Therefore, all 24 patients were evaluated with CARS on Day 30. Cytometric analyses showed no differences in leukocyte, platelet, or red blood cell count among patients (Supplemental Table 1).
Intrathecal bone marrow-derived mononuclear cell administration. Patients received 10mL of a suspension containing the BM-MNC fraction. On average, a total of 4.73×109±1.83 x109 cells were injected. The most frequently reported side effect was nausea occurring during the first 72 hours, observed in 48.3 percent of patients, followed by vomiting, headache, and fever, which were documented in 27.6 percent of individuals. Side effects were resolved with conventional interventions with no further complications.
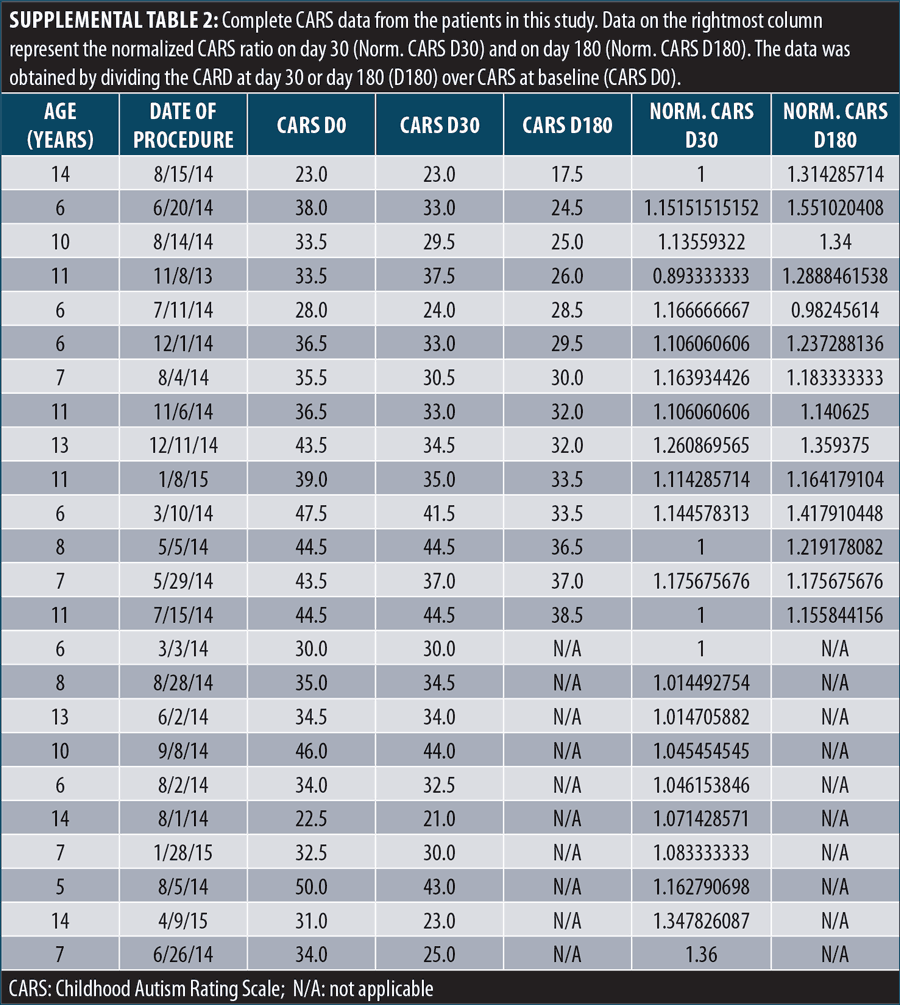
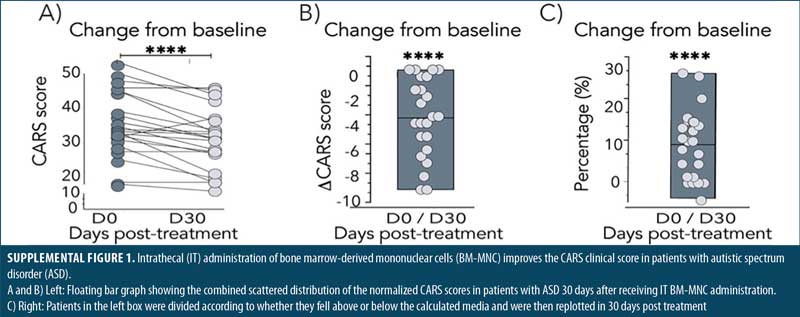
IT BM-MNC improves the clinical outcome of patients with ASD. To evaluate the therapeutic value of an IT BM-MNC intervention in patients with ASD, we determined changes in CARS scores from Day 0 to Day 30 following the intervention. The complete CARS data is presented in Supplemental Table 2. The first analysis involved 10 patients followed until Day 30 and showed a statistically significant reduction (improvement) in CARS scores after the treatment (p=0.0039, Figure 2A). No patient experienced worsening symptoms, and only two showed no changes in CARS scores from Day 0 (20%). The remaining 80 percent experienced a mean improvement of –3.25±3.39 points in their CARS scores, corresponding to 11.3±9.7 percent change, with respect to their baseline levels (Figure 2B).
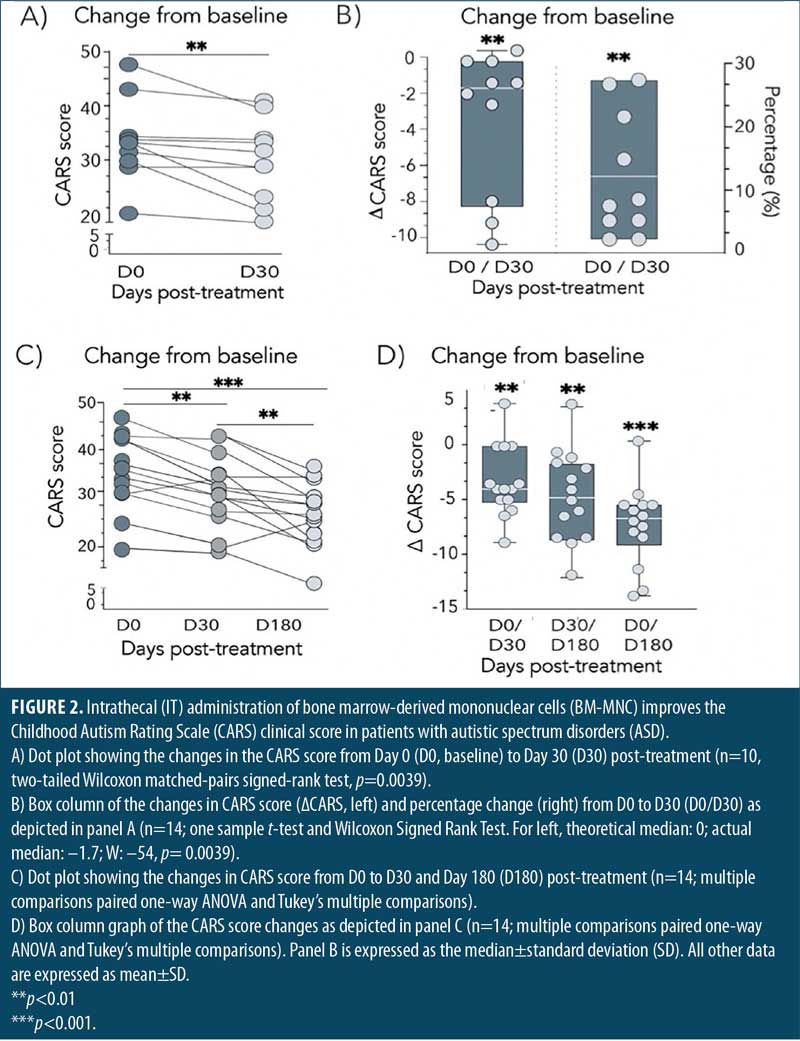
The other 14 patients in the second group were evaluated on Days 30 and 180 post-treatment. As before, IT administration of BM-MNC exerted a statistically significant reduction of patient symptomatology (p=0.0065) by Day 30 post-treatment, with a mean reduction of –3.31±3.32 points (Figure 1C). The combined analysis of all 24 patients on Day 30 post-treatment showed a stronger effect of the IT BM-MNC intervention (p≤0.0001, Supplemental Figure 1), highlighting the early beneficial impact of the treatment in the overall ASD symptomatology.
Improvement from the treatment increased between Days 30 and 180 and was also statistically significant (p=0.009, Figures 2C and 2D). By Day 180, the benefit of the IT BM-MNC treatment on patient symptomatology, according to CARS scores, was more pronounced and statistically significant (p≤0.0001). By Day 180, patients showed, on average, a reduction of –7.61±3.3 points. Together, these results show that IT administration of BM-MNC improves clinical outcomes in patients with ASD.
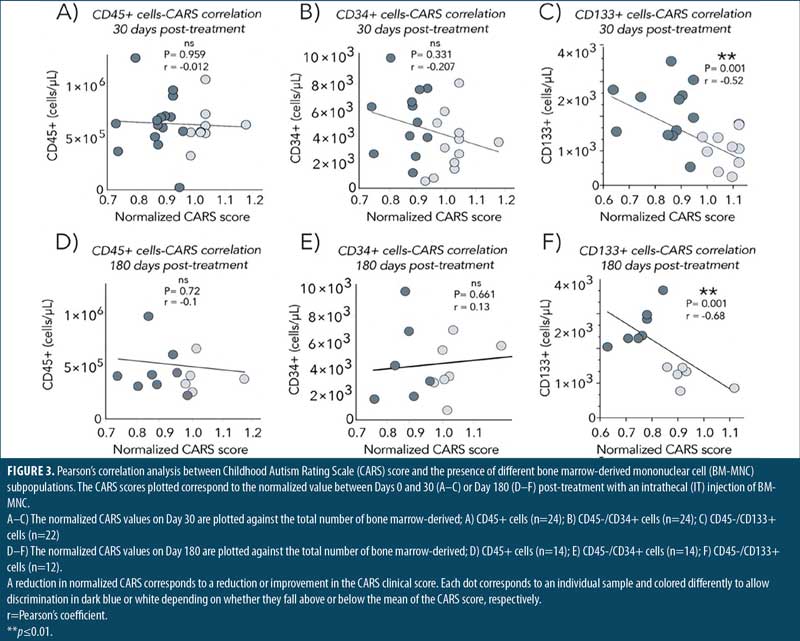
Enrichment of a CD133+ stem cell population correlates with clinical outcome in patients with ASD treated intrathecally with BM. Flow cytometry analysis of the BM-MNC fraction of patients with ASD showed at least four distinctive cell subpopulations, based on the pattern of expression of CD45, CD34, and CD133. Subpopulations included CD45+ (henceforth CD45+), a CD45–/CD34+ (henceforth CD34+) and a CD45–/CD133+ (henceforth CD133+). A CD133+/CD34+ subpopulation was also found but neglected from our analysis since it overlapped with the CD133+ subset, as almost all CD133+ cells expressed CD34 as well.
A correlation was performed by plotting the normalized CARS score (baseline/day of analysis) with the absolute concentration of cells/μL. The computed Pearson’s coefficient analysis showed no correlation between the number of CD45+ (r= –0.012, p=0.92) in the sample and improvement in CARS scores by Day 30 (Figure 3A). We next focused on CD45– cells, where some stem cell subpopulations, including CD34+ and CD133+ hematopoietic stem cells, are found.48,52,54 Although showing a better trend, no correlation was found between the number of CD34+ cells and the improvement in CARS scores observed by Day 30 (r= –0.2, p=0.33; Figure 3B). Strikingly, a subpopulation of cells expressing CD133 but lacking CD45 expression showed a statistically significant negative correlation with change in CARS score at Day 30 (r= –0.52, p=0.001; Figure 3C). A similar tendency remained when the CARS score at Day 180 was correlated with these subpopulations. Specifically, only the CD133+ cells, not the CD45+ or CD34+ cells, showed a significant correlation with the changes in CARS scores by Day 180 (Figures 3D–3G).
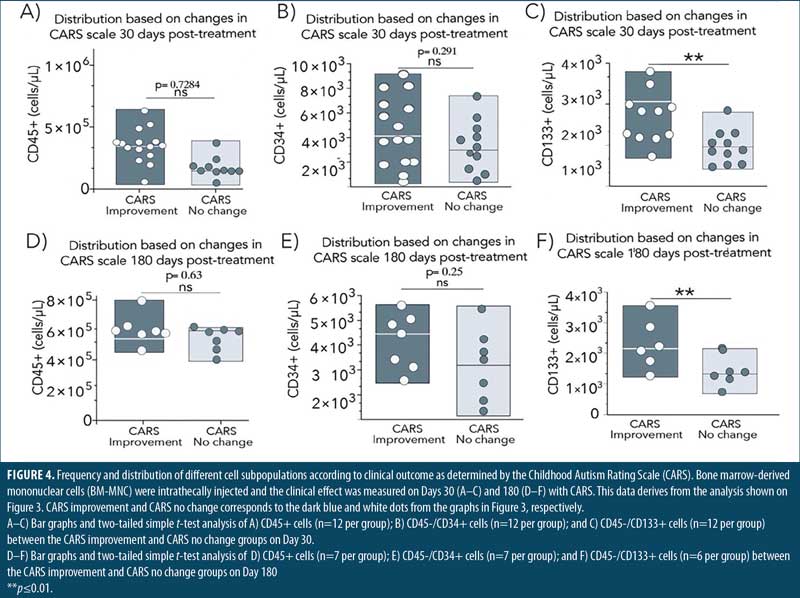
Next, we compared the number of cells present in those patients with low (improvement) and high (no change) CARS scores. T-test analyses showed no difference in the number of CD45+ or CD34+ cells between those patients with improvement or no change in the CARS score on either Day 30 or 180 post-treatment (Figure 4). Importantly, the number of CD133+ cells was significantly higher in patients with lower CARS scores on both Days 30 and 180 post-treatment with BM-MNC, demonstrating that enrichment with CD133+ stem cells correlates with clinical outcome in patients with ASD treated with IT BM-MNC.
Discussion
The development of novel therapies for patients with ASD has been compromised by the slow progress in our understanding of the molecular underpinnings associated with this disease. However, recent evidence pointing to a role for neurotrophic factors, along with the discovery of an immunological derangement component underlying the pathogenesis of the disease,1,2,6,25 has presented an opportunity for the use of stem cell therapies to treat ASD. In this context, an emerging body of literature has supported the therapeutic benefit of a number of cell-based interventions to treat patients with ASD. IT administration of BM preparations, including BM-MNC fractions, has become the standard among these interventions and has consistently been shown to improve the patient symptomatology, as determined with scales such as CARS.20–23 Our work herein adds to this growing body of literature by confirming that IT administration of BM-MNC improves clinical outcome in patients with ASD as early as 30 days post-treatment. Interestingly, our results show that the benefit of this treatment extends beyond Day 30 and increases to reach further improvement of symptoms until Day 180.
While we can only speculate on the mechanism for the long-term improvement observed, the progressive improvement in CARS scores is indicative of a model that involves the sustained and continued release of neurotrophic and immunomodulating factors by the cells injected within the central nervous system (CNS). In this context, the two best known and better characterized stem cells in BM, HSC and MSC, secrete immunomodulatory cytokines, including stromal cell-derived factor 1 (SDF-1) and G-CSF, and can suppress T and B responses.26,27 In addition, MSC can also suppress T cells through cell-cell contact mediated mechanisms, and this effect is documented to be effective even in counteracting states of aggressive immune activation, such as during graft versus host disease.27–30 Considering the underlying inflammatory nature of disorders within the autism spectrum,30–32 reduction of inflammation through one of these mechanisms could cause tissue damage to subside, allowing for neural regeneration through alternative mechanisms.
BM-derived stem cells also have the potential to stimulate neuronal regeneration. For instance, SDF-1 is chemotactic factor for neural stem cells.32–34 Moreover, MSC and HSC secrete neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), neurotrophin-3 (NT3), and brain-derived neurotrophic factor (BDNF),34,35 which modulate the activation of microglial cells by favoring the transition to an M2 immunosuppressive state35,36 and stimulate the proliferation and differentiation of neural stem cells.36–39 Finally, there is compelling evidence showing that HSC and MSC differentiate into neural cells to regenerate brain tissue.39–42 Together, the reduction of inflammation, along with neural regeneration, will result in a slow but progressive clinical improvement, as documented herein.
The role of neurotrophic factors secreted by MSC and their effect in improving autistic behaviors has been document experimentally. Studies in the widely used autistic inbreed BTBR T+tf/J (BTBR) mouse model has shown a reduction in the frequency of stereotypical behaviors following MSC transplantation.42–44 Mechanistically, it was demonstrated that the reduction in autistic behavior after MSC transplantation was accounted by the production of neurotrophic factors by these cells.44,45 Moreover, MSC obtained from the adipose tissue were also found to improve the repetitive behavior in a different autistic mouse model induced with valproic acid administration.45,46 Taken together, the overall evidence supports a potential role of MSC in mediating the benefits of BM-MNC IT injections in patients with ASD.
MSC are identified by the expression of CD105, CD73, CD90, CD45, CD34, CD14, CD116, CD79a, and human leukocyte antigen-DR (HLA-DR).46,47 CD133, the marker expressed in the cell subset identified as the one correlating with the benefits herein, is not typically associated with MSC. CD133, also known as prominin-1, is a 59 KDa surface protein expressed primarily in stem cells, particularly in HSC, and it is considered a marker for long-term repopulating stem cells.47–52 However, several studies have documented the expression of CD133 in a subset of MSC that have neurogenic functions.52,53 This subpopulation of MSC co-expresses adenosine triphosphate (ATP) binding cassette subfamily G member 2 (ABCG2) and C-X-C chemokine receptor type 4 (CXCR4), and differentiate into nestin+, tyrosine hydroxylase+ neural cells, with the potential to repair experimental traumatic brain injury.52,53 HSC are among cells who have CD34+ cells as markers. Several clinical trials have been performed with the use of CD34+ stem cells in autism. Numerous studies have demonstrated that cerebral hypoperfusion is associated with many core symptoms of autism.26,27 Since CD34+ cells induce angiogenesis in areas of cerebral hypoperfusion, some authors have proposed the use of MSC and CD34+ cells for ASD treatment.17 We were not able to find an association between the response of the patients and the amount of CD34+ cells administered. Besides, it is relevant to point out that the reproductive behavior of the cells after transplantation is unknown.
However, CD133+ is expressed in a population of endothelial progenitor cells which can restore the brain hypoperfusion that has also been associated with the pathology of ASD. Notwithstanding that the expression of CD133 fits the phenotype of MSC and of a cell bearing the stemness capacity to restore multiple defects associated with ASD pathogenesis, the identity of the cell correlating with the benefit from our therapy remains to be elucidated.
Limitations. The main limitation of our study is the reduced sample size, which limits the representability of a wider population. The short period of evaluation and the loss of patients to follow-up could be assessed to improve the accuracy of the results. Also, no imaging tests were used as a variable when evaluating our patients.
Conclusion
From the results obtained in the present study, we inferred that CD133 cells are important for the improvement in CARS scores observed in patients with ASD, and that cell preparations enriched for CD133+ stem cells might increase the effect, consistency, and reproducibility of BM-derived cell therapies for patients with ASD. Further studies should be designed to corroborate our findings.
Consent for Publication
Consent for publication was obtained from parents of each patient.
Availability of Data and Materials
The datasets used and analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
LVM participated on the design of the work, draft of the work, acquisition and interpretation of data. LEMG, IPRS, NAV, FGG, SPS, CSS, MGCC, AGB, IDV, DZVM, and DGA participated in the draft of the work and interpretation of data. All authors were involved in the elaboration and approval of the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
References
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20(3):369–376.
- Famitafreshi H, Karimian M. Overview of the recent advances in pathophysiology and treatment for autism. CNS Neurol Disord Drug Targets. 2018;17(8):590–594.
- Mitka M. Rising autism rates still pose a mystery. JAMA. 2010;303(7):602.
- Theoharides TC, Kempuraj D, Redwood L. Autism: an emerging “neuroimmune disorder” in search of therapy. Expert Opin Pharmacother. 2009;10(13):2127–2143.
- Rice CE, Rosanoff M, Dawson G, et al. Evaluating changes in the prevalence of the autism spectrum disorders (ASDs). Public Health Rev. 2012;34(2):1–22.
- Hong S-J, Vos de Wael R, Bethlehem RAI, et al. Atypical functional connectome hierarchy in autism. Nat Commun. 2019;10(1):1022.
- Bhat S, Acharya UR, Adeli H, et al. Autism: cause factors, early diagnosis and therapies. Rev Neurosci. 2014;25(6):841–850.
- Velmeshev D, Schirmer L, Jung D, et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science. 2019;364(6441):685–689.
- Velmeshev D, Magistri M, Mazza EMC, et al. Cell-type-specific analysis of molecular pathology in autism identifies common genes and pathways affected across neocortical regions. Mol Neurobiol. 2020;57(5):2279–2289.
- Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–1219.
- Ramaswami G, Won H, Gandal MJ, et al. Integrative genomics identifies a convergent molecular subtype that links epigenomic with transcriptomic differences in autism. Nat Commun. 2020;11(1):4873.
- Varghese M, Keshav N, Jacot-Descombes S, et al. Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 2017;134(4):537–66.
- Shigemori T, Sakai A, Takumi T, et al. Altered microglia in the amygdala are involved in anxiety-related behaviors of a copy number variation mouse model of autism. J Nippon Med Sch. 2015;82(2):92–99.
- Ellegood J, Crawley JN. Behavioral and neuroanatomical phenotypes in mouse models of autism. Neurotherapeutics. 2015;12(3):521–533.
- Liao GP, Harting MT, Hetz RA, et al. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr Crit Care Med. 2015;16(3):245–255.
- Cox CS Jr, Hetz RA, Liao GP, et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079.
- Balboni G, Tasso A, Muratori F, Cubelli R. The Vineland-II in preschool children with autism spectrum disorders: an item content category analysis. J Autism Dev Disord. 2016;46(1):42–52.
- Randall M, Egberts KJ, Samtani A, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev. 2018;7(7):CD009044.
- Cox CS Jr, Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68(3):588–600.
- Sharma A, Gokulchandran N, Sane H, et al. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013:623875.
- Sharifzadeh N, Ghasemi A, Tavakol Afshari J, et al. Intrathecal autologous bone marrow stem cell therapy in children with autism: a randomized controlled trial. Asia Pac Psychiatry. 2021;13(2):e12445.
- Lv Y-T, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Sharma AK, Gokulchandran N, Kulkarni PP, et al. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: a clinical study. Am J Stem Cells. 2020;9(5):89–100.
- Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694.
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910.
- Pinto D, Delaby E, Merico D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94(5):677–694.
- Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507.
- Zhao L, Chen S, Yang P, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10(1):182.
- Fisher SA, Cutler A, Doree C, et al. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst Rev. 2019;1:CD009768.
- Česen Mazič M, Girandon L, Kneževič M, et al. Treatment of severe steroid-refractory acute-graft-vs.-host disease with mesenchymal stem cells–single center experience. Front Bioeng Biotechnol. 2018;6:93.
- Theoharides TC, Stewart JM, Panagiotidou S, Melamed I. Mast cells, brain inflammation and autism. Eur J Pharmacol. 2016;778:96–102.
- Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018;11(2).
- Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122.
- Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894.
- Hsuan YC-Y, Lin C-H, Chang C-P, Lin M-T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016;6(10):e00526.
- Noh MY, Lim SM, Oh K-W, et al. Mesenchymal stem cells modulate the functional properties of microglia via TGF-β secretion. Stem Cells Transl Med. 2016;5(11):1538–1549.
- Zhang J, Li Y, Chen J, et al. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030(1):19–27.
- Neuhuber B, Timothy Himes B, Shumsky JS, et al. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035(1):73–85.
- Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191(2):344–360.
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716.
- Azizi SA, Stokes D, Augelli BJ, et al. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908–3913.
- Hermann A, Gastl R, Liebau S, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117(Pt 19):4411–4422.
- McTighe SM, Neal SJ, Lin Q, et al. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8(4):e62189.
- McFarlane HG, Kusek GK, Yang M, et al. Autism-like behavioral phenotypes in BTBR T tf/J mice. Genes Brain Behav. 2008;7(2):152–163
- Perets N, Segal-Gavish H, Gothelf Y, et al. Long term beneficial effect of neurotrophic factors-secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav Brain Res. 2017;331:254–260.
- Ha S, Park H, Mahmood U, et al. Human adipose-derived stem cells ameliorate repetitive behavior, social deficit and anxiety in a VPA-induced autism mouse model. Behav Brain Res. 2017;317:479–484.
- Li H, Ghazanfari R, Zacharaki D, et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 2016;1370(1):109–118.
- Lapostolle V, Chevaleyre J, Duchez P, et al. Repopulating hematopoietic stem cells from steady-state blood before and after ex vivo culture are enriched in the CD34+ CD133+ CXCR4low fraction. Haematologica. 2018;103(10):1604–1615.
- Koutna I, Peterkova M, Simara P, et al. Proliferation and differentiation potential of CD133+ and CD34+ populations from the bone marrow and mobilized peripheral blood. Ann Hematol. 2011;90(2):127–137.
- Kobari L, Giarratana MC, Pflumio F, et al. CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res. 2001;10(2):273–281.
- Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107(5):2162–2169.
- Handgretinger R, Kuçi S. CD133-positive hematopoietic stem cells: from biology to medicine. Adv Exp Med Biol. 2013;777:99–111.
- Nichols JE, Niles JA, DeWitt D, et al. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+ CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4(1):3.
- Brunet de la Grange P, Vlaski M, Duchez P, et al. Long-term repopulating hematopoietic stem cells and “side population” in human steady state peripheral blood. Stem Cell Res. 2013;11(1):625–633.


