by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
Dr. Nakamura is with the Department of Psychiatric Internal Medicine, Kosekai-Kusatsu Hospital in Hiroshima, Japan. Dr. Nagamine is with the Department of Psychiatric Internal Medicine, Sunlight Brain Research Center in Yamaguchi, Japan.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(4–6):70–77.
Abstract
Objective: Lurasidone is a second-generation antipsychotic (SGA) that contributes an antipsychotic and antidepressant effect, with low incidences of metabolic-related diseases and hyperprolactinemia for the treatment of psychological disorders. However, evidence on lurasidone is limited in psychiatric clinical settings. This study aimed to investigate the effect of short-term lurasidone treatment on metabolic effects and prolactin (PRL) levels, in relation to the differences of psychiatric disorders, lurasidone dosages, and introducing methods, in 35 female and 12 male Japanese inpatients with psychiatric disorders.
Methods: Subjects were placed into six subgroups divided by three categories (schizophrenia/schizoaffective disorder or bipolar disorder, 20mg/day or 40mg/day, adding or switching). Sequential changes in 10 items of metabolic parameters, including estimated insulin resistance and PRL levels at one month, were evaluated. The variations of metabolic parameters that were significantly changed from baseline were analyzed against sample characteristics and other metabolic parameter variations.
Results: In the 40mg/day and switching introduction method groups, lurasidone significantly reduced body weight, body mass index (BMI), levels of alanine amiotransaminase, and levels of fasting blood glucose. PRL levels seemed to increase when lurasidone was added and decrease when lurasidone was switched to from other antipsychotics. Switching introduction method and higher dosage correlated with weight loss and lowering fasting blood glucose levels, respectively.
Conclusion: Lurasidone administration offered the potential for weight loss, lowered serum blood glucose levels, and converging serum PRL concentrations. Moreover, switching introduction method with higher dosages might alleviate basal metabolism and glucose homeostasis. Further prospective studies combining measurements of serum insulin and psychometric evaluation will help to confirm our conclusions.
Keywords: dosage, lurasidone, metabolic effect, prolactin, psychiatric diagnosis, switching
Second-generation antipsychotics (SGAs) are prescribed medications to treat schizophrenia, bipolar disorders and other psychotic disorders. However, they generally cause metabolic syndrome, in terms of weight gain, high blood pressure, dyslipidemia, Type 2 diabetes, and hyperprolactinemia, which are responsible for reduced life expectancy and poor adherence.1,2
Lurasidone is an SGA that was approved for the treatment of schizophrenia and bipolar depression in Japan on March 25, 2020, based mainly on the results of multinational Phase III studies3,4 and a long-term extension study5 in patients with schizophrenia and those of a multinational Phase III study6 in patients with bipolar I depression.
In-vitro receptor binding studies and preclinical behavioral studies indicate that lurasidone is an antagonist with high affinity at dopamine-2 (D2), serotonin 5-hydroxytryptamine-2A (5-HT2A), and 5-HT7 receptors; moderate affinity at human adrenergic α2C receptors; and weak affinity at adrenergic α2A receptors. Also, lurasidone acts as a partial agonist at 5-HT1A receptors, with moderate-to-high affinity. Unlike some other SGAs, lurasidone has either no or minimal affinity for the 5-HT2C receptors, histamine H1 receptors, and muscarinic M1 receptors, which are thought to contribute to side effects, such as metabolic syndrome, sedation, and worsening of cognitive deficits.7 While lurasidone is reported to induce modest dose-dependent prolactin (PRL) elevations, especially at the beginning of treatment,8 in many patients, it seems to be associated with no clinically meaningful PRL alterations that are comparable to olanzapine.9 On the basis of these pharmacological profiles, lurasidone is hypothesized to contribute to antipsychotic and antidepressant effects, with low incidence of metabolic-related diseases and hyperprolactinemia, for the treatment of psychological disorders.
A recent meta-analysis of placebo-controlled and head-to-head randomized controlled trials compared 32 different antipsychotics in terms of efficacy and adverse effects. Subtle differences were found in efficacy, but marked differences were found in adverse events.10 Much research has been conducted on the older SGAs (e.g., aripiprazole, clozapine, olanzapine, risperidone); however, minimal research have been conducted on the metabolic effects of new SGAs (e.g., asenapine, brexpiprazole, lurasidone).11 We have previously reported that the relationships between the potency of 5-HT2C blockade and hypertriglyceridemia and the potency of the M3 blockade and glucose intolerance in patients who switched from olanzapine to clozapine or asenapine,12 and that brexpiprazol treatment displayed a low propensity to influence metabolic parameters and minimal impact on PRL levels,13 which were estimated from receptor-binging profiles and dopaminergic inhibitory effect on PRL secretion.
The purpose of this study was to investigate the relationships between the short-term lurasidone treatment and metabolic effects and PRL levels, in relation to the differences of psychiatric disorders, lurasidone dosages, and introduction methods in Japanese inpatients with psychiatric disorders.
Subjects and Methods
Subjects. We recruited inpatients who were treated with lurasidone from June 2020 to July 2021 at Kusatsu Hospital in Hiroshima, Japan. At registration, subjects were excluded if they had current use of lurasidone, concomitant use of other antipsychotics, or were diagnosed with a psychiatric disorder other than schizophrenia or bipolar disorder. Throughout the observation period, the exclusion criteria included discontinuation of lurasidone because of ineffectiveness, side effects, refusal to intake, introduction of other antipsychotics, medical treatment that directly affected the patient’s metabolic systems, and leaving the hospital for less than one month. Use of antidepressants, mood stabilizers, or hypnotics was permitted. Under these criteria, a total of 47 subjects, consisting of 35 female and 12 male patients, were selected and studied retrospectively (Figure 1).
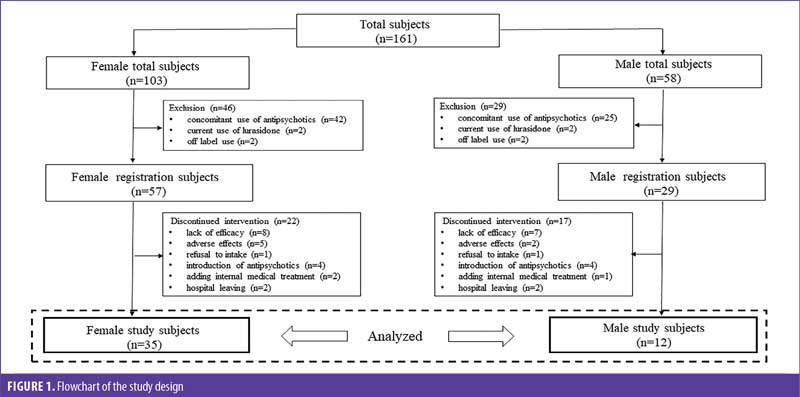
Outcome measures. Basic demographic data included age at the start of lurasidone treatment, diagnosis of psychiatric disorder, according to the International Statistical Classification of Disease and Related Health Problems 10th Revision (ICD-10), current dose of lurasidone at one month, and introduction method (switching to or adding lurasidone), in addition to breakdown of previous antipsychotics.
Body weight (BW), body mass index (BMI), and fasting plasma samples of aspartate aminotransferase (AST), alanine amiotransaminase (ALT), uric acid (UA), triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glucose (FBS), and PRL were collected and measured at baseline and one month after lurasidione treatment. The ratio of triglyceride to HDL-C values (triglyceride/HDL-C) reflecting insulin resistance were assessed together with these values.14
Statistical methods. Differences in age between female and male subjects, baseline differences of the levels of 10 metabolic markers (BW, BMI, AST, ALT, UA, triglyceride, HDL-C, LDL-C, FBS, and triglyceride/HDL-C) and the levels of PRL, which were divided by three categories (ICD-10, lurasidone dosage, and introduction method) into two subgroups, were evaluated using the unpaired Mann-Whitney U test and considered statistically significant at p<0.05.
Categorical variables, including the distribution into three categories (ICD-10, lurasidone dosages, and introduction methods) and the breakdown of previous antipsychotic use between female and male subjects were analyzed using the Chi-squared test and considered statistically significant at p<0.05.
Within the total patient population and assigned subgroups, comparisons of the measured values at baseline and one month were performed using the Wilcoxon signed-rank test and considered statistically significant at p<0.05.
After we calculated the variations in levels of metabolic markers from baseline to one month, the data were analyzed to see whether there was any significant correlation between the variations in levels of metabolic markers that were significantly changed from baseline in total subjects and the five sample characteristics (sex, age, ICD-10, lurasidone dosage, and introduction method) and nine metabolic markers (BW, AST, ALT, UA, triglyceride, HDL-C, LDL-C, FBS, and triglyceride/HDL-C) using the Spearman’s correlation coefficients method and considered statistically significant at p<0.05.
Ethical approval. Considering the retrospective nature of this study, no informed consent was obtained. However, all patients have the right to refuse participation in medical research through an opt-out form provided via an in-hospital bulletin board. The ethics committee of Kusatsu Hospital approved this study to analyze the stacked data for publication. Personally identifiable information could not be specified throughout a research investigation.
Results
There were no differences between male and female patients in the age at start of lurasidone, distribution in the three categories, and breakdown of previous antipsychotics (Table 1).
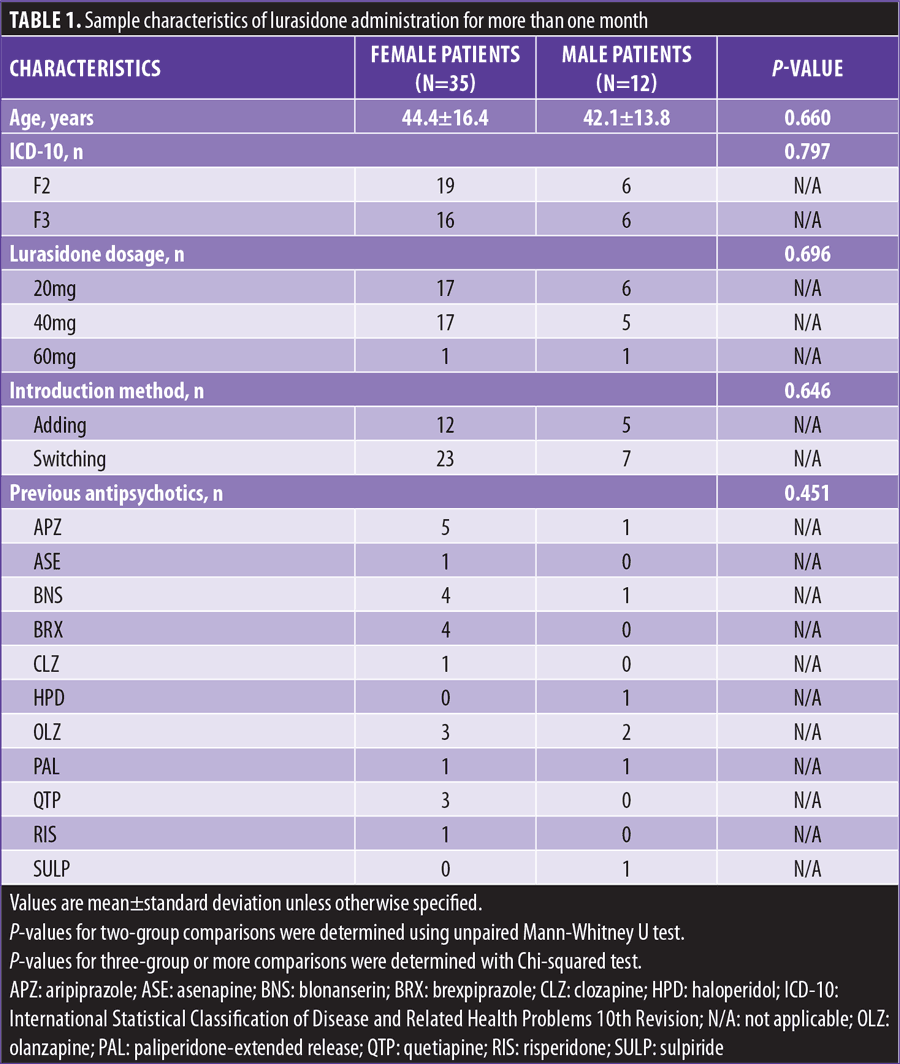
At baseline, LDL-C levels in subjects prescribed 40mg lurasidone were significantly higher than those prescribed 20mg lurasidone, and PRL levels in female switching subjects were significantly higher than those in adding subjects. Comparisons of subjects prescribed 60mg lurasidone versus subjects prescribed 40 or 20mg lurasidone were not performed because of the very small number of subjects prescribed 60mg lurasidone. No other baseline differences were found between the assigned subgroups.
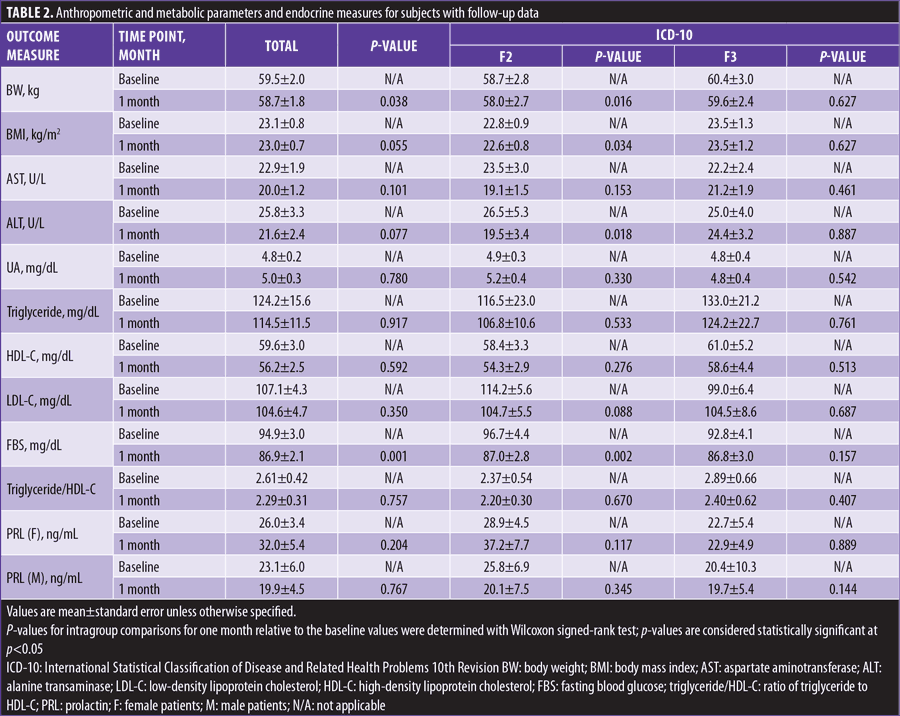
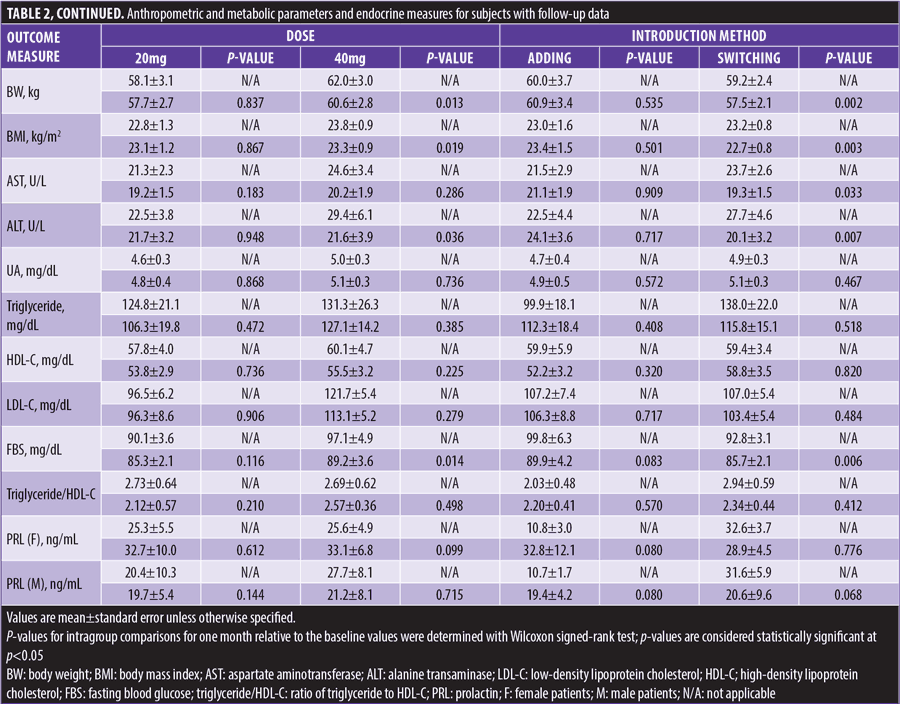
At one month, BW significantly decreased from baseline in total, ICD-10 F2 (Schizophrenia, Schizotypal and Delusional Disorders), 40mg prescribed, and switching subjects. AST levels significantly decreased from baseline in switching subjects. ALT levels significantly decreased from baseline in ICD-10 F2, 40mg prescribed, and switching subjects. FBS levels significantly decreased from baseline in total, ICD-10 F2, 40mg prescribed, and switching subjects (Table 2 and Figure 2).
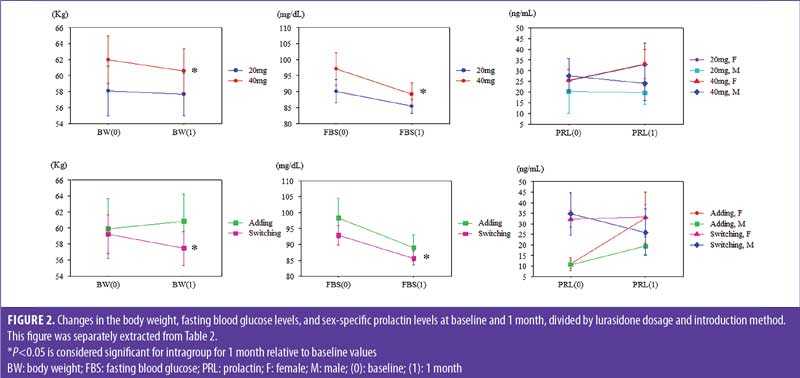
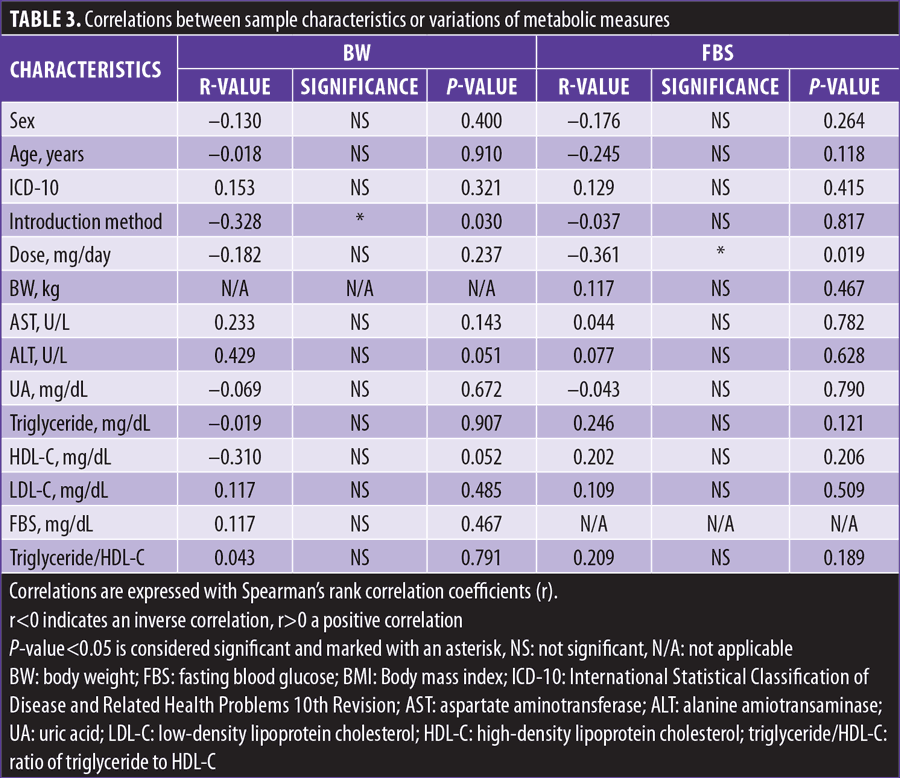
BW changes from baseline to one month were significantly negatively correlated with the switching group but not the adding group, and FBS changes from baseline to one month were significantly negatively correlated with lurasidone dosage (Table 3).
Discussion
The results of the present study are summarized as follows: 1) lurasidone reduced BW, BMI, levels of ALT, and levels of FBS under higher dosage and switching introduction method; 2) PRL levels seemed to increase when lurasidone was added and decrease when lurasidone was switched to from other antipsychotics; and 3) switching introduction method and higher dosage correlated with weight loss and lowered blood glucose levels, respectively, after lurasidone administration.
A real-world analysis of weight changes associated with lurasidone monotherapy is limited. Pochiero et al15 reported that the risk of clinically relevant weight gain was numerically higher with all antipsychotics versus lurasidone, and the likelihood of seven percent weight loss or greater was significantly higher with lurasidone versus all antipsychotics, except ziprasidone, in adult patients with schizophrenia over 12 months. Meyer et al16 reported lurasidone was associated with a mean reduction in weight of 0.77kg during a follow-up period of 12 months, and the average weight change after lurasidone treatment in subgroups with or without prior use of SGAs were –1.50 and –0.29kg, respectively. Stahl et al17 demonstrated that patients previously treated with olanzapine experienced reduction in weight, whereas patients previously treated with lurasidone or placebo experienced minimal changes in BW in six-month, open-label extension trials. Similarly, McEvoy et al18 demonstrated that patients switching to lurasidone from other antipsychotics lost an average of 0.2kg at six weeks, and more patients had clinically significant (≥7%) weight loss (1.8%) than weight gain (0.9%) in a six-week lurasidone switch trial for patients with schizophrenia or schizoaffective disorder. Consistent with these findings, we observed significant weight loss in subjects who switched to lurasidone from previous antipsychotics, most of which contribute to weight gain. Moreover, lurasidone has a likelihood of dose-dependent weight loss, because significant weight loss was found in subjects receiving 40mg lurasidone. These results do not mean that lurasidone has an effect on reducing BW, because adding subjects did not show weight loss. Also, we observed significant weight loss in patients with schizophrenia/schizoaffective disorder; however, most of these patients were treated with lurasidone at higher dosages and using the switching method, so there might be no differences in relation to BW and BMI changes attributed to differences of psychiatric diagnosis.
Increased levels of ALT are a biomarker for metabolic syndrome, but this relationship remains unproven in patients with schizophrenia. Kim et al19 reported a prospective association between ALT levels and metabolic syndrome, highlighting the value of ALT levels, even mild ALT elevations within the normal range, as a predictor of metabolic syndrome risk in male patients with schizophrenia. Lee et al20 reported elevated levels of serum ALT that were still in the normal range were associated with the presence of metabolic syndrome, which suggests the possibility of using serum ALT level as an early indicator for metabolic syndrome in patients treated with clozapine. Baeza et al21 reported significant increases in ALT levels were observed in children and adolescents on antipsychotics during six-month follow-up, and patients with metabolic syndrome showed higher ALT concentrations than patients without metabolic syndrome. Therefore, it was more likely that ALT levels, as well as BW, decreased under switching method and higher dosages of lurasidone, because lurasidone is unlikely to cause metabolic abnormalities among SGAs.
A systematic review and network meta-analysis revealed that, compared with placebo, glucose concentrations reduced with lurasidone use and increased with olanzapine and clozapine use among 18 antipsychotics in a median treatment duration of six weeks.22 This research supported our results that levels of FBS significantly decreased in switching subjects and tended to decrease in adding subjects. This is because lurasidone has a potential lowest affinity for muscarinic M3 receptors, which control cholinergic-dependent insulin release and have a propensity to promote glucose dysregulation. However, we found that the levels of triglycerides, which have relationships between the 5-HT2C receptors, and ratios of triglyceride/HDL-C reflecting insulin resistance, did not significantly decrease, even in switching subjects. Considering another mechanism of glucose concentration reduction with lurasidone, we came up with one possible explanation: in addition to serotonin, histamine, and muscarinic activity, dopaminergic signaling might play a role in defining the metabolic profiles, especially glucose outcomes associated with dopamine receptor antagonists.23
Catecholamines have marked metabolic effects, particularly on glucose metabolism. Barth et al24 discussed that in animal experiments, infusing catecholamine is associated with enhanced rates of aerobic glycolysis, glucose release, and inhibition of insulin-mediated glycogenesis under physiologic conditions. Hong et al25 found that in mice, intrathecal injection with a D2 receptor agonist caused an elevation of the blood glucose level in a dose-dependent manner, and this increase of blood glucose was significantly attenuated by a D2 receptor antagonist. We have reported that hyperglycemia during the acute phase might be the result of excitation, such as increased sympathetic tone, and glucose levels decreased when an improvement in Excited Component of the Positive and Negative Syndrome Scale score was achieved.26 Moreover, we observed a strong association between norepinephrine administration and psychosis in patients with schizophrenia.27 Therefore, we speculate that variations of circulating monoamines are closely associated with psychiatric symptom and glucose metabolism simultaneously. As our study subjects were continuously treated with lurasidone for more than one month, without exacerbation of psychiatric symptoms, normalization of dopaminergic signaling after lurasidone administration might result in glucose concentration reduction relative to baseline in total and all subgroup subjects.
Product labeling notes that short-term, placebo-controlled trials found small dose- and sex-related effects on PRL levels, with a median change from baseline to endpoint of 0.4ng/mL for all lurasidone-treated patients with schizophrenia, compared to –1.9ng/mL for placebo. There was no indication of clinically relevant changes in PRL in published short-term studies on lurasidone. McEvoy et al28 reported mean changes from baseline to last-observation-carried-forward (LOCF) endpoint (lurasidone 40–120mg/day) in PRL concentrations, which were –0.5 and 2.0ng/mL for male and female patients, respectively, in a randomized, six-week, open-label study. Nasrallah et al29 showed modest increases in PRL following treatment with lurasidone (40, 80, or 120mg/day), compared to placebo; changes were greater among female than male patients, particularly in those receiving lurasidone 120mg/day, in a six-week, randomized, placebo-controlled study. Ogasa et al30 found median PRL levels at the Week 6 LOCF endpoint were modestly increased relative to baseline in the lurasidone 40 and 120mg/day groups, but not in the placebo group; a sex difference was observed, with lurasidone producing greater increases in PRL in female than male patients. These short-term studies might support our findings that PRL levels tended to increase when lurasidone 40mg/day was added and used, particularly in female subjects, although used dosage in our study was under 60mg/day and the observation period was limited to four weeks.
In a recently published network meta-analysis of placebo-controlled and head-to-head randomized, controlled trials comparing the efficacy and tolerability of 32 antipsychotics, 90 studies with 21,569 participants reported usable results for PRL.10 Aripiprazole and quetiapine did not cause PRL increases, compared to placebo. Asenapine, brexpiprazole, olanzapine, lurasidone, amisulpride, and haloperidol were associated with significant elevation of PRL levels. Risperidone and paliperidone were associated with significantly greater PRL increases than all other antipsychotics. Considering these rankings in order of PRL increase and breakdown of previously used antipsychotics, as shown in Table 1, it was more likely that PRL levels tended to decrease after switching to lurasidone, particularly in male subjects.
It was interesting to note that the switching introduction method, but not the adding introduction method, and higher dosage were correlated with weight loss and lowered blood glucose, respectively, and differences in psychiatric diagnosis (schizophrenia or schizoaffective disorder versus bipolar disorder) were not correlated with weight loss or lowered blood glucose in this study, though there might be some confounding factors between sample characteristics and variations of metabolic measures.
Limitations. The present study has some limitations. First, it was a retrospective, observational study with a small sample size, lacking control subjects treated with antipsychotics other than lurasidone. Second, it would be difficult to fit these results into outpatients, though we assumed that the closed-ward setting and the short-term observation would minimize interindividual differences in metabolic parameters. Third, we did not measure plasma concentration of insulin, which regulates serum glucose levels. Finally, psychometric evaluations, which might be associated with metabolic effects and PRL concentrations, were not performed.
Conclusion
Despite these limitations, this is the first report, to our knowledge, investigating the effects of lurasidone treatment on metabolic parameters and PRL levels, based on differences of psychiatric diagnosis, dosage, and introduction methods in psychiatry.
SGAs are recommended in various guidelines as first-line therapy due to their greater efficacy on negative, cognitive, and depressive symptoms and superior neurological tolerability. However, metabolic and cardiovascular adverse effects and hyperprolactinemia are still a major concern of SGAs, which form a heterogeneous group in terms of efficacy and adverse event profiles.
Lurasidone administration in our study subjects offered the potential for weight loss, lowered serum blood glucose levels, and converging serum PRL concentrations. Moreover, switching to lurasidone with a higher dosage from other SGAs might alleviate metabolic outcomes, such as BW changes and glucose homeostasis.
Further prospective studies combining measurement of serum insulin and psychometric evaluation will help to confirm our conclusions.
References
- Penninx BWJH, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20(1):63–73.
- Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421–425.
- Higuchi T, Ishigooka J, Iyo M, et al. Lurasidone in the treatment of schizophrenia: results of a double-blind, placebo-controlled trial in Asian patients. Asia Pac Psychiatry. 2019;11(2):e12352.
- Iyo M, Ishigooka J, Nakamura M, et al. Efficacy and safety of lurasidone in acutely psychotic patients with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2021;75(7):227–235.
- Iyo M, Ishigooka J, Nakamura M, et al. Safety and effectiveness of lurasidone in patients with schizophrenia: a 12-week, open-label extension study. Neuropsychiatr Dis Treat. 2021;17:2683–2695.
- Kato T, Ishigooka J, Miyajima M, et al. Double-blind, placebo-controlled study of lurasidone monotherapy for the treatment of bipolar I depression. Psychiatry Clin Neurosci. 2020;74(12):635–644.
- Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334(1):171–181.
- Samalin L, Garnier M, Llorca PM. Clinical potential of lurasidone in the management of schizophrenia. Ther Clin Risk Manag. 2011;7:239–250.
- Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27(3):165–176.
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951.
- Greger J, Aladeen T, Lewandowski E, et al. Comparison of the metabolic characteristics of newer second generation antipsychotics: brexpiprazole, lurasidone, asenapine, cariprazine, and iloperidone with olanzapine as a comparator. J Clin Psychopharmacol. 2021;41(1):5–12.
- Nakamura M, Nagamine T. Metabolic changes in patients with schizophrenia switched from olanzapine to asenapine or clozapine. Innov Clin Neurosci. 2018;15(7–8):11–12.
- Nakamura M, Nagamine T. Brexpiprazole as a new serotonin-dopamine receptor modulator: considering the clinical relevance for metabolic parameters and prolactin levels. Innov Clin Neurosci. 2019;16(9–10):30–32.
- von Bibra H, Saha S, Hapfelmeier A, et al. Impact of the triglyceride/high-density lipoprotein cholesterol ratio and the hypertriglyceremic-waist phenotype to predict the metabolic syndrome and insulin resistance. Horm Metab Res. 2017;49(7):542–549.
- Pochiero I, Calisti F, Comandini A, et al. Impact of lurasidone and other antipsychotics on body weight: real-world, retrospective, comparative study of 15,323 adults with schizophrenia. Int J Gen Med. 2021;14:4081–4094.
- Meyer JM, Ng-Mak DS, Chuang CC, et al. Weight changes before and after lurasidone treatment: a real-world analysis using electronic health records. Ann Gen Psychiatry. 2017;16:36.
- Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507–515.
- McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–179.
- Kim EY, Kim SH, Lee NY, et al. Aminotransferase levels as a prospective predictor for the development of metabolic syndrome in patients with schizophrenia. Psychopharmacology (Berl). 2014;231(23):4479–4487.
- Lee NY, Roh MS, Kim SH, et al. The prevalence of metabolic syndrome and its association with alanine aminotransferase in clozapine-treated Korean patients with schizophrenia. Int Clin Psychopharmacol. 2013;28(2):71–79.
- Baeza I, de la Serna E, Calvo-Escalona R, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28(7):463–473.
- Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77.
- Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704.
- Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(Suppl 9):S508–S518.
- Hong J, Feng J, Lee J, et al. The role of spinally located dopamine D 2 receptors in the regulation of the blood glucose level in mice. Pharmacol Rep. 2020;72(6):1666–1675.
- Nagamine T. Abnormal laboratory values during the acute and recovery phases in schizophrenic patients: a retrospective study. Neuropsychiatr Dis Treat. 2010;6:281–288.
- Nagamine T. Role of norepinephrine in schizophrenia: an old theory applied to a new case in emergency medicine. Innov Clin Neurosci. 2020;17(7–9):8–9.
- McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–179.
- Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–677.
- Ogasa M, Kimura T, Nakamura M, Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225(3):519–530.


