
by Georgia Mitsi, PhD; Todd Grinnell, BA; Suzanne Giordano, PhD; Thomas Goodin, Phd; Shahin Sanjar, PhD; Elizabeth Marble, BS; and Andrei Pikalov, MD, PhD
All authors are with Sunovion Pharmaceuticals Inc. in Marlborough, Massachusetts.
Funding: The studies described herein and medical writing support were supported by funding from Sunovion Pharmaceuticals Inc.
Disclosures: All authors are employees of Sunovion Pharmaceuticals Inc.
Innov Clin Neurosci. 2022;19(4–6):65–69.
Abstract
Multiple digital health technologies have been evaluated across clinical development programs, including external, wearable, implantable, and ingestible devices and sensors, along with digital mobile health applications (apps) that are accessible via users’ personal electronic devices (e.g., smartphones, tablets, and computers). Several of these technologies have been incorporated into our ongoing neurology and respiratory clinical development programs. Based on our experience, one of the greatest potential benefits of digital health technologies is the ability to collect objective and/or biological data continuously or at regular intervals outside of office visits during a patient’s normal daily activities to provide additional efficacy and safety information, versus data capture from traditional episodic, time point-based office visits. Many challenges encountered with digital health technologies can be successfully addressed by providing the appropriate training to staff and patients, ensuring availability of appropriate infrastructure support, and conducting pilot studies before scaling up to larger trials. Overall, our experience with digital health technologies demonstrated their potential to increase the amount of objective data collected in clinical trials, expand patient access to trials, and facilitate further improvement of clinical outcomes.
Keywords: Clinical trials, digital health technologies, machine learning, mobile applications, wearables
Evolution of digital health solutions and wearable technology has launched new possibilities for biopharmaceutical research and development. These technologies cover products such as external, wearable, implantable, and ingestible devices or sensors, along with digital mobile health applications (apps) that are accessible via users’ personal electronic devices (e.g., smartphones, tablets, and computers).1,2 As technologies evolve, these products and their capabilities can mature and expand, creating greater opportunities for implementation in the healthcare delivery setting. In particular, incorporation of digital technologies into clinical trials holds great promise for transforming the traditional approach to drug research and development at different stages of a product’s lifecycle.
Several pharmaceutical companies, academic institutions, technology start-up companies, government agencies, and others are investigating the utility of integrating different digital platforms into clinical trials. A search of ClinicalTrials.gov yielded almost 900 studies of wearable technologies or virtual trials evaluating pain perception, gait parameters, cardiac outputs, obesity, sun exposure, urinary incontinence, and depression, among others.3 Collectively, these digital health technologies have a broad spectrum of potential applications.
Current Experience
We incorporated several digital health technologies into ongoing clinical development programs in the neurology and respiratory therapeutic areas based on a thorough evaluation using several criteria, including United States (US) Food and Drug Administration (FDA) clearance, data availability, publications, and prior clinical trial experience, among others (Table 1). Valuable insights have been gained using these technologies. Here, we report on the lessons learned from each program.
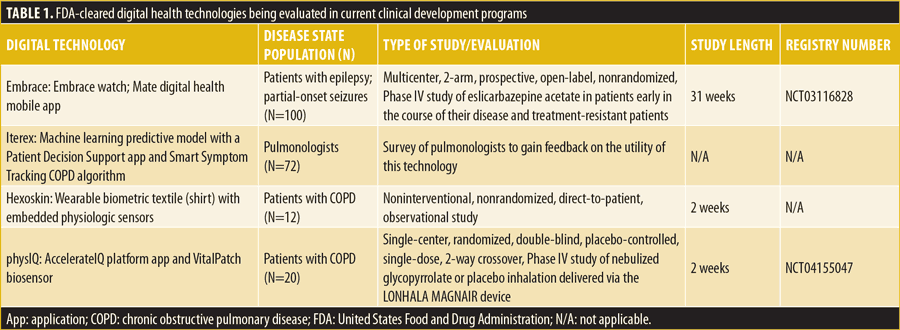
Embrace watch and Mate digital health mobile app. One of the unmet needs in the treatment of epilepsy is the ability to track and record seizures objectively on a 24/7 basis. Patients typically use paper or electronic diaries to record their seizures; however, these tools rely on patient and caregiver recognition of the signs and symptoms of a seizure, which might be overlooked in some circumstances (e.g., when seizures are subtle, occur during sleep, or when there is no immediate access to the diary to record the event). The system we implemented consists of a wearable watch device (Embrace, Empatica Inc.; Boston, MA, US) paired to an electronic seizure diary in the form of a smartphone app (Mate digital health mobile app, Empatica Inc.; Boston, MA, US). The watch continuously measures physiological data (via 3-axis accelerometer, gyroscope, electrodermal activity sensor, and peripheral temperature sensor), runs pattern analysis, and can issue alerts about seizure occurrence to healthcare providers. The app can be used on a smartphone or other handheld devices, such as an iPod, that use an iOS or Android operating system. When the watch recognizes an event as a seizure, a signal is sent to the app to record the event, thereby providing a passive and objective recording of an event that has been algorithmically determined to be a seizure.
A 31-week, multicenter, prospective, open-label, nonrandomized, Phase IV study (NCT03116828; approved by the local ethics committee [Copernicus Group Independent Review Board (IRB); Cary, NC, US] and conducted in accordance with the Declaration of Helsinki [IRB #QUI1-17-115]) investigated the effects of eslicarbazepine acetate as adjunctive therapy in patients with partial-onset seizures. The Embrace digital system was used as an exploratory endpoint to assess multiple parameters, including concordance between daily seizure frequency recorded by the patient and the device. A total of 102 participants were enrolled, 72 percent of whom completed the study. There were three main data sources: 1) a paper diary (primary data source; subjective); 2) the electronic Mate app as an electronic diary (alternative data source; subjective); and 3) the Embrace watch device (exploratory data source; objective). The mean (95% confidence interval [CI]) proportion of concordance in the measurement of daily seizure frequency between the app and the paper seizure diaries was high at 93.0 percent (88.6%, 97.5%) and 88.2 percent (82.6%, 93.7%) for the two study arms (arm 1: eslicarbazepine acetate as first add-on; arm 2: eslicarbazepine acetate as later add-on), respectively.
The device manufacturer provided training on watch operations, iPod pairing, and portal functionality to both the study coordinators and participants. Study coordinators were responsible for pairing the watch with the iPod and providing technical support to participants. Participants received a detailed training manual, prepared jointly by the study sponsor and the device manufacturer.
In general, technology was well accepted, and participants were interested in understanding the potential of the watch to monitor seizures objectively and by the potential of the software to improve the self-management of their seizures. Some participants, who were generally uncomfortable with technology, experienced initial reluctance. Training was deemed successful as both site staff and participants felt comfortable with all study procedures, and most participants who completed the study provided watch-based data, suggesting appropriate use of the technology.
The watch used in this study had a battery life of about 24 hours, which required that participants use two different watches (to ensure 24 hours of data collection), both of which needed to be paired to a single app to track seizure activity across a full 24-hour period. Changing from one watch to the other resulted in a small gap in the continuous 24-hour data collection. During the study, an unexpected iPod manufacturer firmware update caused a software crash that led to a recall of devices deployed to the field, necessitating reinstallation of the software and testing. A help desk had been established as part of the study; however, due to the uniqueness of the device and the innovative study design of the interventional clinical trial, both the study sponsor and manufacturer faced a steep learning curve in successfully addressing many technical and operational challenges, which further highlights the importance of proper training when a technology is included in a trial.
Upon completion of the trial, this technology confirmed its ability to detect seizures and was as reliable as patients’ diaries in recording the frequency and duration of partial onset seizures.
Iterex. Chronic obstructive pulmonary disease (COPD) is a progressive, chronic, inflammatory lung disease characterized by persistent airflow limitation. Acute exacerbations of COPD symptoms negatively affect health status, increase hospitalization and readmission rates, and contribute to disease progression. Patients often have difficulty detecting impending exacerbations and making informed decisions about the level of medical attention they might need.4 Currently, patients use paper or online app checklists (e.g., The COPD Pocket Consultant Guide Mobile App5) personalized by healthcare professionals for self-management of exacerbations and symptom management; however, these action plans are associated with several limitations, including a lack of ability by patients and caregivers to recognize an exacerbation and poor adherence to the plan.6
Iterex (Iterex Therapeutics; New York, NY, US) is a machine learning predictive model that uses a customizable, interactive Patient Decision Support app, with cloud-based access to healthcare providers that serves as an at-home triage option for the management of COPD.7 The algorithm was developed using a comprehensive review of patient cases, assessments by trained pulmonologists, and a thorough literature review.7 First, patients create their individual, COPD-related medical profile on the app. Then, by answering questions on the app, either during routine monitoring or while experiencing a change in symptoms, patients are provided recommendations on a specific course of action (i.e., continue treatment, contact their physician, or go to the emergency department, if needed).
The system was presented to a group of pulmonologists (N=72) to understand their perceptions toward digital health technologies designed for patients with COPD. Of the 44 physicians who responded, 75 percent reported they would likely encourage their patients to use the app. The reasons given for not wanting to recommend the app included a variety of technology, logistical, and patient-related factors. For example, some physicians were unsure if older patients had access to a smartphone. However, research has shown that more than half of people over 65 years of age own a smartphone.8 Another barrier to adoption for physicians was time constraints associated with training for themselves and their patients, as well as reviewing and interpreting the data (data overload). Respondents suggested the use of plain language, suitable graphics, easy-to-read fonts, and easy navigation, modified appropriately for a specific patient population. In addition, clinicians asked for scientific data to justify the use of this digital technology in routine medical practice. At the current stage of development, the system remains a research tool with potential for clinical use, pending additional validation and adaptation of data interpretation interface for use by healthcare providers.
Hexoskin. A wearable biometric shirt (Hexoskin, Carré Technologies, Inc.; Montreal, Canada) with embedded physiologic sensors was investigated for the continuous and passive monitoring of patients with COPD in a real-world setting. In collaboration with the COPD Foundation, a pilot study (approved by the local ethics committee [WCG; Durham, NC, US] and conducted in accordance with the Declaration of Helsinki [IRB #20182924]) was initiated to assess the feasibility of using the shirt to collect overnight activity data (i.e., cardiac, respiratory, and sleep data) from patients with COPD. The objective of the study was to evaluate patients’ experiences with the wearable, which was a prerequisite to understand the suitability of the technology for this specific patient population before including it in a larger trial. Patients with COPD (N=12) were asked to wear the biometric shirt for at least three nights during a seven-night period.
After recruitment and screening, patients participated in an in-person group training session where they were fitted for the shirt by a patient advocate or investigator and trained on how to use the wearable, including attaching and charging the recording device and uploading the data. All but one participant had technical issues with data uploading, which did not compromise data collection. However, we recommend that sample size calculations for future studies should account for potential technology failures. Four participants needed assistance putting on the shirt some or all of the time, four needed assistance applying the conductive cream for the heart sensors some or all of the time, and one needed assistance removing the shirt every time. The variety of different-sized wearable samples was limited, and customized fittings took longer than expected. Nevertheless, most participants stated that they were willing to wear the shirt again in another study. The activity sensor and recording device had to be charged between uses, and the charging station also collected and uploaded the data. Four participants found charging and uploading the data “difficult” or “very difficult,” and five were not confident that they charged the device and uploaded the data correctly.
Overall, the technology was found to hold promise in the continuous monitoring of health metrics related to COPD. In addition, the training and support offered by the manufacturer was timely and helpful; however, several limitations for use in a clinical trial setting and in this specific patient population were identified. Conducting a pilot study, as was done here, allowed us to identify certain limitations and plan accordingly before integrating this technology into a larger-scale clinical trial. The physiological data from the study are being analyzed and will be published separately. Hexoskin remains a research technology tool, which enables collection of continuously reported physiological data for analysis and better understanding of disease pathophysiology and impact of various treatments.
PhysIQ (accelerateIQ™ platform and VitalPatch® biosensor). PhysIQ (Chicago, IL, US) is a wireless, remote patient monitoring system consisting of the accelerateIQ platform, which includes a smartphone app for data transmission, cloud-based information technology infrastructure, physiology analytics modules, and healthcare provider user interface (physIQ; Chicago, IL, US), and wearable biosensors, such as the VitalPatch biosensor (VitalConnect; San Jose, CA, US), a patch with integrated biosensors and a wireless transceiver. This technology represents an additional option for continuous monitoring of activity and vital signs in patients with COPD. The feasibility of using the patient monitoring system for continuous 24/7 data collection was evaluated as an exploratory objective in a single-site, Phase IV study (NCT04155047; approved by the local ethics committee [Advarra, Inc.; Columbia, MD, US] and conducted in accordance with the Declaration of Helsinki [IRB #Pro00039173]) in patients with COPD (N=20) receiving nebulized glycopyrrolate inhalation solution delivered via the LONHALA® MAGNAIR® nebulizer device (Sunovion Pharmaceuticals Inc.; Marlborough, MA, US). The biosensor was worn on the torso throughout the study period (22 days) and replaced every five days. It recorded electrocardiogram data, heart rate variability, R-R intervals, respiratory rate, skin temperature, activity (including step count), and posture. Data were wirelessly transmitted from the sensor to the app for storage and analysis via machine learning analytics. Digital data were supplemented by clinical collection of vital signs for comparison.
The instructions and real-time support from the patient monitoring system for both the study sites and participants greatly assisted in implementing the technology, and the portal was easy to understand. In addition, the biosensor in the program appeared to be patient-friendly (disposable, waterproof, easy to apply, comfortable to wear, and long battery life [up to 5 days]). The physiological and activity data will be published separately.
This patient monitoring system could be seen as the analog of Holter monitoring for COPD. The latter technology existed for years and has developed analytical and reporting tools. This system is in its early stages of development and holds promise for use in healthcare settings upon further development of the interface and compatibility and electronic medical record (EMR) systems.
Summary
Our experience has shown that patient input and feasibility efforts are important in identifying the potential for using digital health technologies as an endpoint in clinical trials. Learnings from these early studies should facilitate the successful incorporation of digital technologies into future studies. Technological challenges should be anticipated, and risk management protocols should be established before a study launches. The uniqueness of these technologies might require the development and adaptation of targeted risk management principles to meet the specific criteria of the individual technology under investigation.
One of the greatest potential benefits of these technologies is the opportunity to collect new types of data previously unavailable to collect in a remote manner. Relevant physiological data can now be collected continuously or at regular intervals during patients’ normal daily activities, outside of the clinical setting.2,9 Ordinarily, to capture vital signs and other physiological data, patients were required to go to a clinical trial site. However, this setting provides only a snapshot view of patient conditions, results in limited trial participation due to travel requirements,10 and might misrepresent outcomes on account of the artificial nature of the environment (e.g., whitecoat hypertension)11,12 In addition, patients often need to enter their experiences into paper or electronic diaries, which are dependent on patient recall.13 It is also common for patients to delay entering the information or miss recording entries entirely, which could impact data quality.14 The density and continuous nature of the data collected, particularly during early drug development, could support earlier detection of adverse events and allow for more optimized dose titration outside of office visits.2 Furthermore, continuous data output creates an opportunity for development of novel endpoints that were previously inaccessible via traditional clinical trials; however, validation of these endpoints might be necessary before widespread acceptance.2 Of significant promise is the passive overnight collection of data, which incorporates the natural circadian changes associated with many diseases and allows for monitoring of nighttime pharmacodynamic drug effects.2,15,16
While the potential benefits of digital health technologies are evident, challenges remain to be addressed before widespread adoption can be realized. Timing and volume of data collection should be carefully considered to ensure that the appropriate technology infrastructure and personnel are in place to house and analyze the newly developed data sets and troubleshoot any technical issues.2 Whenever possible, pilot studies should be conducted first to maximize protocol adherence, ensure uninterrupted data capture and transmission, and establish healthcare provider and patient acceptability and usability before incorporation of novel digital technology into larger-scale clinical trials.2 Pilot studies also help establish baseline parameters in continuously monitored data, so that pattern recognition analysis of in-study physiological changes can distinguish between normal variability or improvement from an undesirable change.2,17 Some participants in our trials expressed concerns regarding data confidentiality. Therefore, data protection and privacy safeguards need to be in place and should be communicated to clinical trial participants to ensure comfort with these novel approaches.2,17 In-depth due diligence of the right technology partner during the selection process could minimize many of these challenges, yet risk management protocols should be established to prospectively address any emerging human and/or technological issues that could impact data integrity.
As the technology sector grows, so too do the choices for digital health approaches and associated partners. We chose devices with FDA 501(k) clearance or those with strong evidence indicating that FDA clearance would be issued (e.g., Embrace at the time of the study was not FDA-cleared) to minimize regulatory hurdles and increase the chances for quality of data. The FDA Center for Devices and Radiological Health has established the Digital Health program,1,18 which provides regulatory advice for the application of digital health technology and acts as a resource for biopharmaceutical companies that implement these types of initiatives.18,19 Outside of considerations regarding the actual device, it is important to consider selecting a technology provider that is experienced in collaborating on biopharmaceutical projects and possesses the appropriate infrastructure to support clinical trial requirements. For our projects, data were stored and analyzed by the technology provider under our guidance, but internal (sponsor) data storage might be another option if the capabilities are present.
In our experience, the greatest predictor of patient acceptance was the complexity of the digital health technology rather than any specific patient factors, such as age or disease state. However, patients were less receptive to try something new if procedures and tools for managing their disease were already firmly established. For example, patients with epilepsy are well versed in using paper and electronic diaries, and these participants were less enthusiastic about investigating a novel digital option. Thus, conducting pilot studies and soliciting patient input during protocol development are particularly important to address potential concerns before full study initiation.20 Across all studies, participants were interested in learning about the potential of digital technology as an approach to decrease frequency of office visits and assist in disease self-management.
Varying degrees of acceptance among physicians should also be carefully considered. We found that physicians who were already comfortable with technology in general were more open to experimenting with wearable devices. Several physicians questioned the usefulness of the technologies over current practices and the potential of adding complications to their everyday workflow, along with the relative lack of evidence supporting the utility for assessing the exploratory endpoints; thus, some physicians were hesitant to recommend them to their patients. This confirms earlier findings that the strongest influence for physicians and patients on the use of new technology is its perceived usefulness, and a barrier to use is the lack of evidence of clinical efficacy.21–23 Physicians in our studies and others have voiced the concern of data overload and lack of confidence in interpreting novel data from continuous monitoring platforms.23 In a survey of providers who treated patients using digital health technologies, the most common complaint was the lack of time needed to review patient-collected data.24
In summary, payers, healthcare systems, and regulatory bodies are increasingly relying on real-world evidence in making healthcare decisions.25 Digital health solutions and wearable technologies are uniquely positioned to meet this need by applying validated analytical approaches to extract clinically meaningful information from newly acquired data sets. In the clinical trial setting, these technologies are not only practical to implement, but generalizable to disease management in real-world clinical practice as well. Although challenges associated with implementing these technologies remain, continued implementation of digital health solutions has the potential for designing patient-centric studies with improved and inclusive access and developing a better understanding of the patient population and a drug’s effectiveness through continuous objective data. Our experience with several digital health technologies in the neurology and respiratory fields has identified the need for specific internal analytic expertise to handle the newly acquired data sets. Furthermore, the appropriate and frequent training of clinical trial staff, healthcare providers, and patients on the use of these technologies, along with software/hardware testing, are prerequisites to success.
Acknowledgments
The authors wish to thank the participants in the studies included in this report and the COPD Foundation for their collaboration on the Hexoskin pilot study in patients with COPD. Medical writing and/or editorial assistance was provided by Meredith Rogers, MS, CMPP, and Payal N. Gandhi, PhD, CMPP, of the Lockwood Group, Stamford, CT.
Author Contributions
All authors participated in drafting the manuscript, provided critical feedback, and have read and approved the final version. All authors had full access to all of the data cited in the manuscript and take responsibility for the integrity of the information, the relevance of the methodologies, and the accuracy of the content.
References
- Byrom B, Watson C, Doll H, et al. Selection of and evidentiary considerations for wearable devices and their measurements for use in regulatory decision making: recommendations from the ePRO Consortium. Value Health. 2018;21(6):631–639.
- Izmailova ES, Wagner JA, Perakslis ED. Wearable devices in clinical trials: hype and hypothesis. Clin Pharmacol Ther. 2018;104(1):42–52.
- Digital Medicine Society (DiMe). DiME’s library of digital endpoints. https://www.dimesociety.org/index.php/knowledge-center/library-of-digital-endpoints. Accessed 13 Aug 2020.
- Korpershoek Y, Vervoort S, Nijssen L, et al. Factors influencing exacerbation-related self-management in patients with COPD: a qualitative study. Int J Chron Obstruct Pulmon Dis. 2016;11:2977–2990.
- COPD Foundation. The COPD pocket consultant guide mobile app. https://www.copdfoundation.org/Learn-More/The-COPD-Pocket-Consultant-Guide/Patient-Caregiver-Track.aspx. Accessed 21 May 2020.
- Jalota L, Jain VV. Action plans for COPD: strategies to manage exacerbations and improve outcomes. Int J Chron Obstruct Pulmon Dis. 2016;11:1179–1188.
- Swaminathan S, Qirko K, Smith T, et al. A machine learning approach to triaging patients with chronic obstructive pulmonary disease. PLoS One. 2017;12(11):e0188532.
- Pew Research Center. Mobile fact sheet. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed 14 Dec 2019.
- Godfrey A, Hetherington V, Shum H, et al. From A to Z: wearable technology explained. Maturitas. 2018;113:40–47.
- Nipp RD, Powell E, Chabner B, Moy B. Recognizing the financial burden of cancer patients in clinical trials. Oncologist. 2015;20(6):572–575.
- Pickering TG, James GD, Boddie C, et al. How common is white coat hypertension? JAMA. 1988;259(2):225–228.
- Papi E, Murtagh GM, McGregor AH. Wearable technologies in osteoarthritis: a qualitative study of clinicians’ preferences. BMJ Open. 2016;6(1):e009544.
- Gater A, Coon CD, Nelsen LM, Girman C. Unique challenges in development, psychometric evaluation, and interpretation of daily and event diaries as endpoints in clinical trials. Ther Innov Regul Sci. 2015;49(6):813–821.
- Erb MK, Karlin DR, Ho BK, et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit Med. 2020;3:6.
- Gharibans AA, Smarr BL, Kunkel DC, et al. Artifact rejection methodology enables continuous, noninvasive measurement of gastric myoelectric activity in ambulatory subjects. Sci Rep. 2018;8(1):5019.
- Bhidayasiri R, Sringean J, Anan C, et al. Quantitative demonstration of the efficacy of night-time apomorphine infusion to treat nocturnal hypokinesia in Parkinson’s disease using wearable sensors. Parkinsonism Relat Disord. 2016;33(Suppl 1):S36–S41.
- Perry B, Geoghegan C, Lin L, et al. Patient preferences for using mobile technologies in clinical trials. Contemp Clin Trials Commun. 2019;15:100399.
- United States Food and Drug Administration. Digital health. https://www.fda.gov/medical-devices/digital-health. Accessed 1 Nov 2019.
- United States Food and Drug Administration. Medical device overview. https://www.fda.gov/industry/regulated-products/medical-device-overview. Accessed 1 Nov 2019.
- Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340–349.
- Nasir S, Yurder Y. Consumers’ and physicians’ perceptions about high tech wearable health products. Procedia Soc Behav Sci. 2015;195(3):1261–1267.
- Hu PJ, Chau PY, Sheng ORL, Tam KY. Examining the technology acceptance model using physician acceptance of telemedicine technology. J Manag Inf Syst. 1999;16(2):91–112.
- Runkle J, Sugg M, Boase D, et al. Use of wearable sensors for pregnancy health and environmental monitoring: descriptive findings from the perspective of patients and providers. Digit Health. 2019;5:2055207619828220.
- Chung CF, Cook J, Bales E, et al. More than telemonitoring: health provider use and nonuse of life-log data in irritable bowel syndrome and weight management. J Med Internet Res. 2015;17(8):e203.
- Hampson G, Towse A, Dreitlein WB, et al. Real-world evidence for coverage decisions: opportunities and challenges. J Comp Eff Res. 2018;7(12):1133–1143.


