by Christy Magdalena, MD, MRes; Audrey Clarissa, MD, MRes; and Nathania Sutandi, MD, MRes
All authors are with Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo National Referral Hospital in Jakarta, Indonesia.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the article.
Innov Clin Neurosci. 2022;19(4–6):51–64.
Abstract
Introduction: Neuromyelitis optica spectrum disorder (NMOSD) is a neurological condition consisting of relapse-related disability. Treatment options are limited. This systematic review and meta-analysis aimed to evaluate the effectiveness and tolerability of rituximab (RTX) in comparison to azathioprine (AZT) and mycophenolate mofetil (MMF) for the treatment of NMOSD.
Methods: A systematic search was conducted among electronic databases, including PubMed, Scopus, EBSCO, and Cochrane, for relevant studies. We included randomized, controlled trials and prospective and retrospective studies evaluating the efficacy and safety of RTX compared to AZT and/or MMF in adult and pediatric patients with NMOSD. The Newcastle-Ottawa Scale (NOS) and Cochrane Collaboration tool were used to determine the risk of bias.
Results: Eleven studies involving 1,086 patients were included in our study. Treatment with RTX generally yielded favorable annualized relapse rate (ARR) and Expanded Disability Status Scale (EDSS) results in comparison to AZT and MMF, despite its variable statistical significance. RTX treatment reduced the relapse rate and hazard risk for relapse (HRR). Patients in the RTX group experienced significantly fewer adverse events, among which the most common were allergies, infections, and leukopenia.
Conclusion: In this study, RTX appeared to be superior to AZT and MMF in improving disability and reducing relapse in patients with NMOSD. RTX is also associated with fewer adverse events based on pooled analysis. Future randomized clinical trials are needed to establish the efficacy and safety of RTX in patients with NMOSD.
Keywords: Neuromyelitis optica spectrum disorder, rituximab, azathioprine, mycophenolate mofetil, monoclonal antibody
Neuromyelitis optica spectrum disorder (NMOSD) is a rare, inflammatory, autoimmune disorder affecting the central nervous system (CNS).1,2 Incidence rates in the general population have ranged from 0.053 to 0.4 per 100,000 individuals per year.2 The worldwide prevalence of NMOSD ranges from 0.5 to 4.4 cases per 100,000 individuals.2 Patients usually present with acute optic neuritis, longitudinally extensive transverse myelitis, and area postrema or brain stem syndrome that can cause blindness and paralysis.3,4 Studies have indicated the role of B cells in pathogenesis; thus, depletion of B cells seems to be an appropriate treatment approach for patients. NMOSD is associated with the upregulation of specific autoantibodies against aquaporin-4 (AQP-4) as well.5,6
Patients can have a poor prognosis from frequent and severe relapses. The treatment objective for NMOSD is categorized into acute inflammatory attack treatment and prevention of further relapses. Corticosteroids, plasma exchange, and intravenous immunoglobulin (IVIg) are often used to treat acute NMOSD attacks, with the objective of suppressing CNS inflammation.7 To achieve disease control, immunomodulatory agents and monoclonal antibodies, such as azathioprine (AZT), mycophenolate mofetil (MMF), rituximab (RTX), cyclophosphamide (CTX), and novel drugs, such as sastralizumab, tocilizumab, inebilizumab, and eculizumab, have shown benefits in trials.8 However, recommendations for first-line therapies still revolve around conventional therapies, such as AZT, MMF, and RTX.7 AZT and MMF are purine analogs that inhibit the proliferation of lymphocytes by blocking the synthesis of deoxyribonucleic acid (DNA) and guanosine nucleotides, respectively.7,9,10 RTX is a monoclonal antibody that depletes the circulating number of clusters of differentiation 20+ (CD20+) antigen on B lymphocytes and has also shown promising efficacy.11,12
Limited evidence exists on the effects of RTX against other first-line drugs in patients with NMOSD. We performed a systematic review and meta-analysis with the aim of analyzing the evidence regarding the safety and efficacy of RTX, compared to AZA and MMF, in patients with NMOSD.
Methods
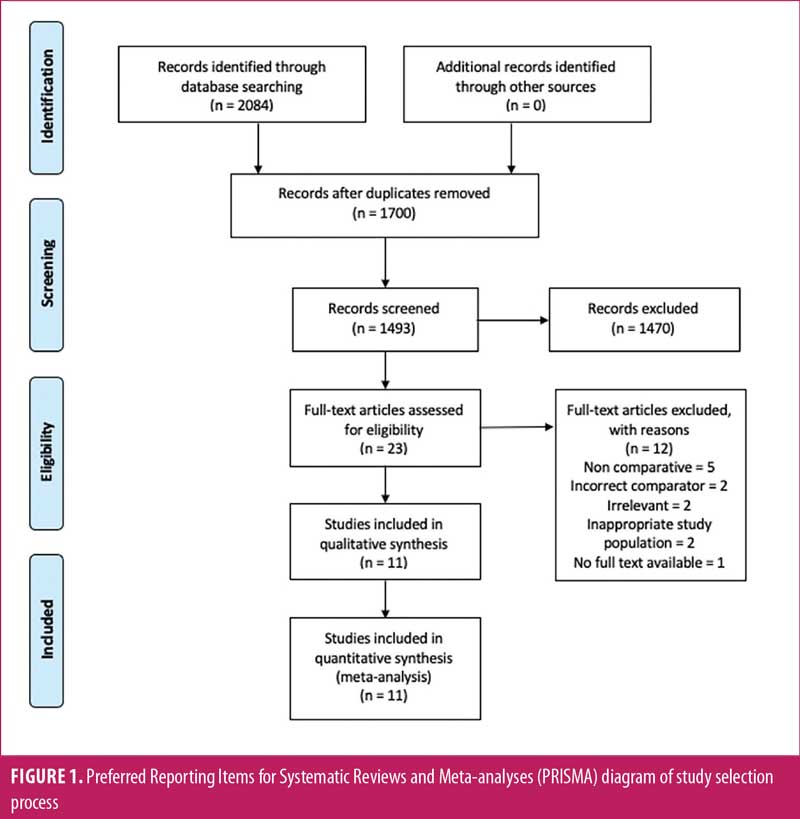
Search strategy and study selection. A comprehensive search of four online databases—PubMed, Scopus, EBSCO, and Cochrane—was conducted on June 29, 2021, by three independent investigators. The following search terms were used: “neuromyelitis optica,” “device,” “NMOSD,” “neuromyelitis optica spectrum disorder,” “azathioprine,” “rituximab,” and “mycophenolate mofetil,” either individually or in combination. After removing duplicates, three investigators independently reviewed the titles, abstracts, and full texts of the studies. A manual search of the reference lists of the eligible studies was also performed. We did not apply any restrictions to the publication date of the study. The details of the study selection were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and flow diagram in Figure 1.13
Inclusion and exclusion criteria. The inclusion criteria were as follows: 1) studies comparing the use of RTX to AZT and/or MMF in adult or pediatric patients diagnosed with NMOSD; 2) studies published in English; and 3) comparative studies, including retrospective or prospective studies and randomized, controlled trials (RCTs). The exclusion criteria were as follows: 1) case reports, conference abstracts, review articles, and noncomparative studies and 2) studies comparing the use of any other agents.
Quality assessment. The Newcastle-Ottawa Scale (NOS)14 was used to assess the quality of the included observational studies. This was done by three independent investigators by considering three domains, with a maximum score of four points for selection, two points for comparibility, and one point for outcome. A higher total score indicates higher quality of each study. Risk of bias assessment for RCTs was performed by using the Cochrane Collaboration Tool,15 which consists of sets of domains with the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each item was appraised by determining the risk of bias as low risk, high risk, or unclear risk.
Data extraction. The following data were extracted from the studies: name of the first author, year of publication, country of origin, type of study, treatment arms compared, total number of patients, sex, age, disease duration before the initiation of first treatment, dose regimen, length of follow-up, number of patients positive for AQP-4 antibody (AQP-4-Ab), other treatments received, outcomes measured, annualized relapse rate (ARR) before treatment, ARR after treatment, ARR changes, Expanded Disability Status Scale (EDSS)16 before treatment, EDSS after treatment, relapse-free rate, hazard risk for relapse (HRR), number of patients who experienced adverse drug events (e.g., total, hepatotoxicity, leukopenia, gastrointestinal intolerance, allergy, infection), and number of patients who discontinued the drugs due to side effects.
Definitions. ARR was defined as the number of relapses divided by the time in years. The EDSS is a scale used to assess disability and neurological impairment in patients with multiple sclerosis (MS). The score ranges from 0 to 10, with 1 to 4.5 referring to patients who are fully ambulatory and 5 to 9.5 referring to patients with mobility difficulties. The relapse rate was defined as the number of patients who experienced at least one recurrence of neurological attack divided by the total number of patients in a treatment arm. HRR was calculated by dividing the hazard risk in the treatment arm by the hazard risk in the control arm.
Statistical analysis. This systematic review and meta-analysis were conducted according to the recommendations of the Cochrane Library and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.17,18 Review Manager (RevMan) Version 5.4 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for statistical analyses. Dichotomous variables were analyzed by using odds ratio (OR) and 95% confidence interval (CI). For HRR, analysis was performed by calculating the logarithm of the hazard ratio (HR) with 95% CI. HR and its variance were calculated from the risk of relapse data in the published manuscripts. Random effect models (REMs) were used for the outcomes. Heterogeneity between studies was determined, with I2 values of less than 25 percent, 25 to 75 percent, and greater than 75 percent considered low, moderate, and high, respectively. To combine the ORs of the variables, the Mantel-Haenszel method17 was used. Peto-OR was chosen in certain cases with low expected frequency. For pooling the continuous outcomes, an inverse variance method was utilized. A p-value of <0.05 was considered statistically significant.
Results
Studies and patient characteristics. Our preliminary search resulted in 2,084 manuscripts. After excluding 384 duplicates, we screened 1,700 titles and abstracts (Figure 1). Twenty-three full-text articles were evaluated for eligibility. We excluded 10 articles for the following reasons: noncomparative (n=5); incorrect comparator (n=2); irrelevant (n=2); inappropriate study population (n=2); and no full text available (n=1). A total of 11 journal articles, consisting of 7 retrospective studies, three prospective studies, and one RCT study, which were published from 2014 to 2020, were included in our study. Summaries of study quality are presented in Supplemental Table S1 and Supplemental Figure S1.
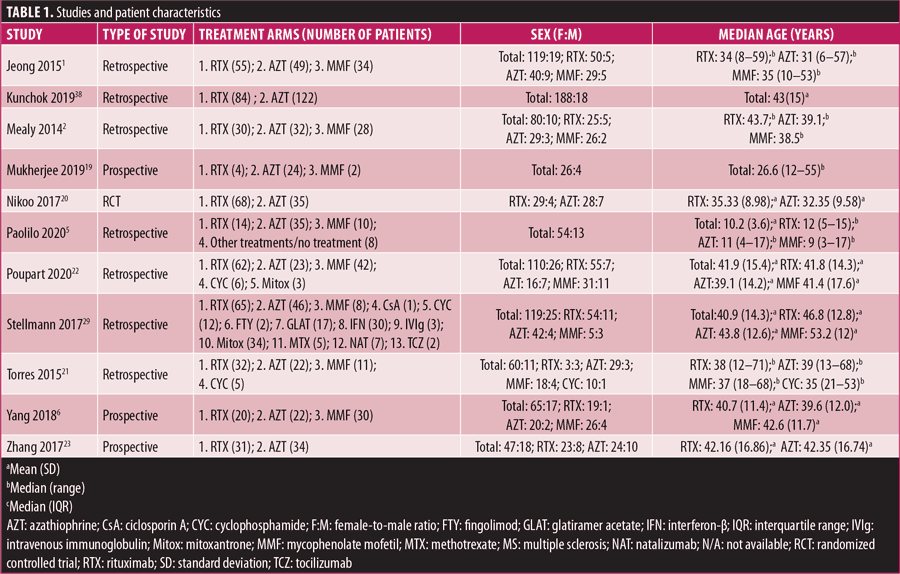
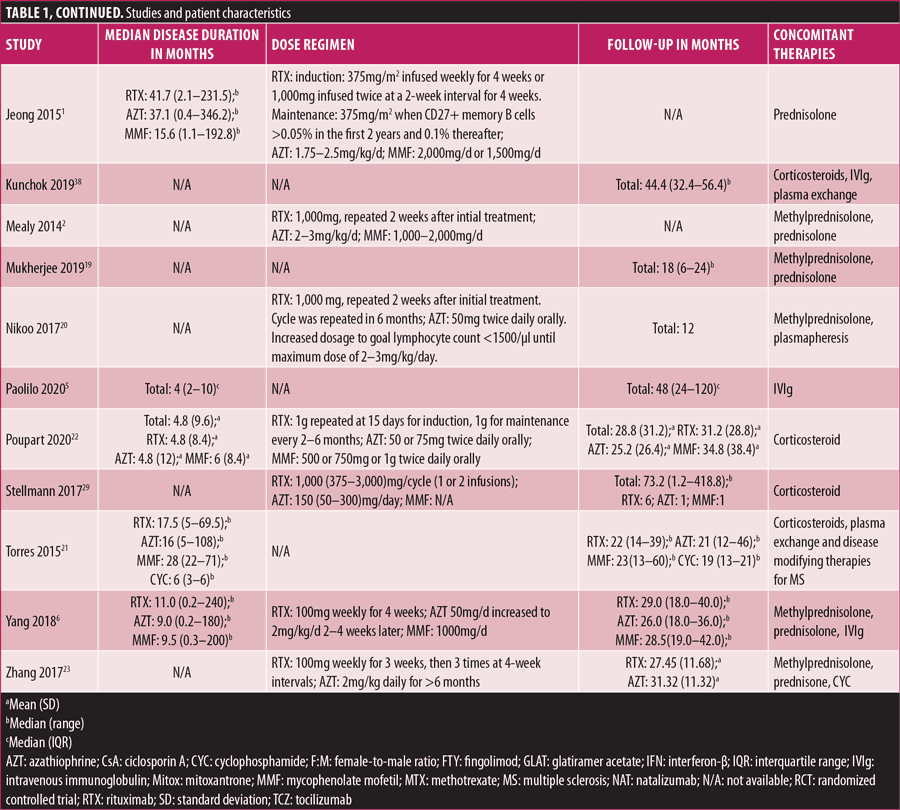
Study and patient characteristics are listed in Table 1. In our analysis, 1,086 patients with NMOSD were included; 1,039 patients received RTX, AZT, and MMF, and the rest received other interventions, such as mitoxantrone, CTX, and methotrexate. Regarding the anti-AQP-4 immunoglobulin G (AQP-4-IgG) status, 844 (81.23%) patients tested positive for the antibody. There were 1,005 (96.72%) female participants, with a female-to-male ratio ranging from 2.61:1 to 8:1. The age of the study participants ranged from 3 to 71 years. In addition to the intervention in each treatment arm, the majority of the patients in these studies received other treatments, such as corticosteroids, IVIg, and plasma exchange, as listed in Table 1. The median follow-up period ranged from 1.2 to 418.8 months.
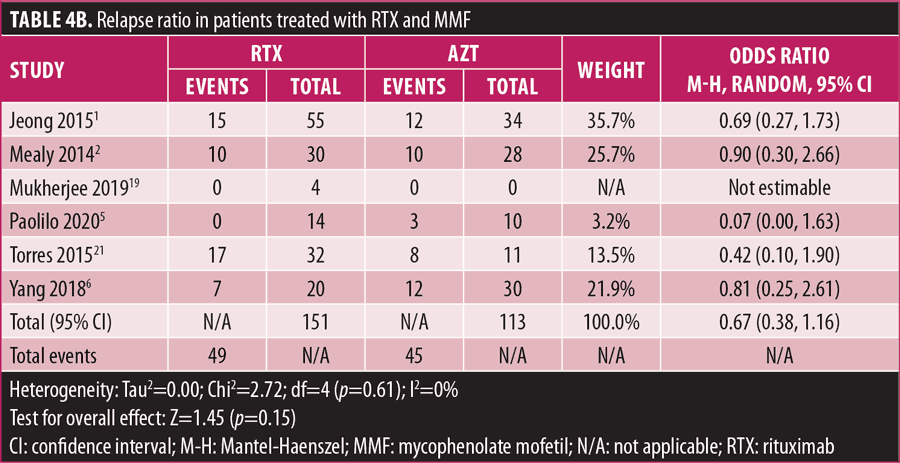
ARR. The pre- and posttreatment ARRs for patients treated with RTX, AZT, and MMF are listed in Tables 2 and 3. Most of the included studies showed significant improvement of ARR after treatment. A study by Mukherjee et al19 showed no significant mean difference in pre- and posttreatment ARR values between the RTX, AZT, and MMF groups (p= 0.82). In contrast, studies by Nikoo et al20 and Torres et al21 demonstrated a significantly greater mean difference in pre- and posttreatment ARR in the RTX group than in the AZT group, with p<0.001 and p=0.021, respectively. However, studies by Poupart et al22 and Yang et al6 found that the differences in the posttreatment ARR values among the RTX, AZT, and MMF groups were not statistically significant, with p=0.11 and p=0.78, respectively.
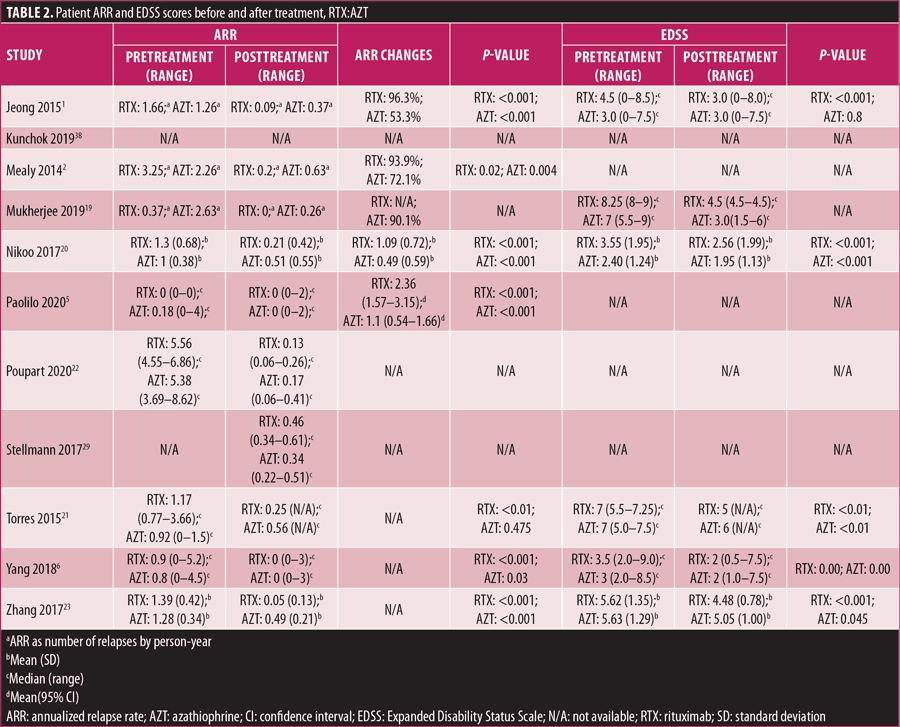
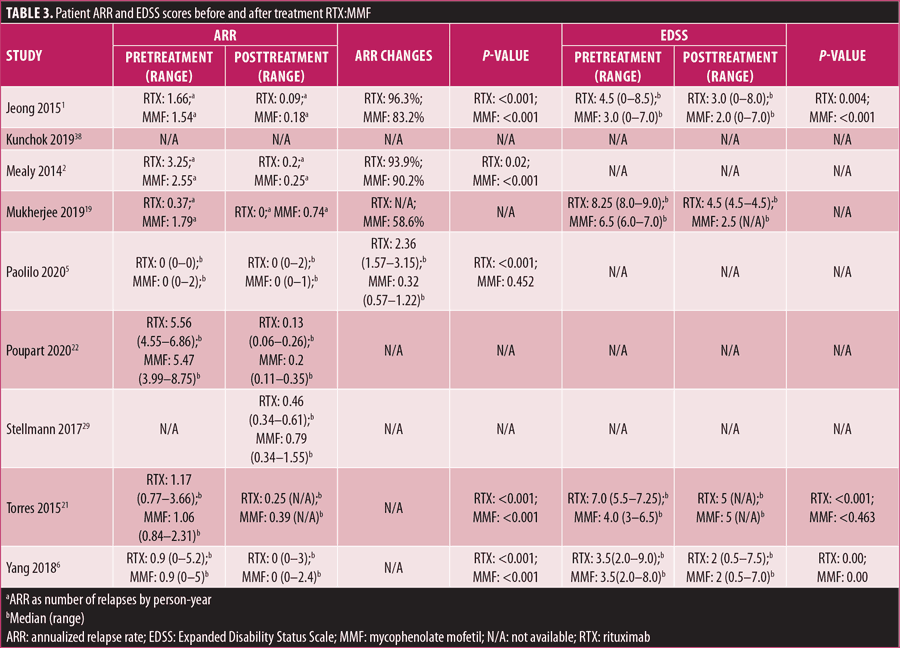
EDSS score. EDSS scores of patients before and after treatment with RTX, AZT, and MMF are presented in Tables 2 and 3. In general, most of the included studies showed a significant decrease in EDSS scores following treatment. Yang et al6 reported that all median posttreatment EDSS scores decreased significantly in patients treated with RTX, AZT, and MMF (p=0.00, p=0.00, p=0.00, respectively). However, direct comparison between the treatments was not statistically significant (p>0.05).6 Torres et al21 reported similar results, as the median posttreatment EDSS scores with RTX (p<0.01) and AZT (p<0.01) decreased significantly, yet a direct comparison of the EDSS score was also not statistically significant (p-value not reported). Nikoo et al20 and Zhang et al23 showed significant mean differences in pre- and posttreatment EDSS scores, with a greater mean difference reported in patients treated with RTX than in those treated with AZT (p<0.001 and p=0.02, respectively).
Conflicting results were found in the EDSS score of patients treated with MMF. The EDSS score decreased in the studies by Jeong et al1 (p< 0.001), Mukherjee et al19 (p-value not reported), and Yang et al6 (p=0.00). In contrast, Torres et al21 showed an increase in the posttreatment median EDSS score (p<0.463).
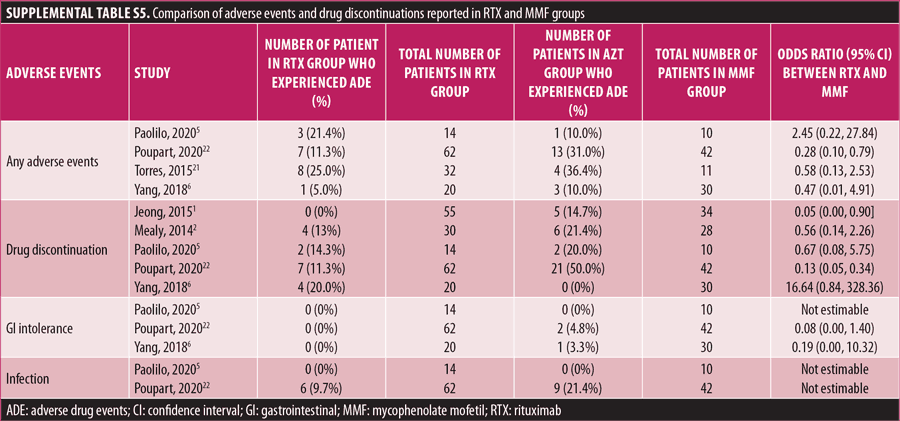
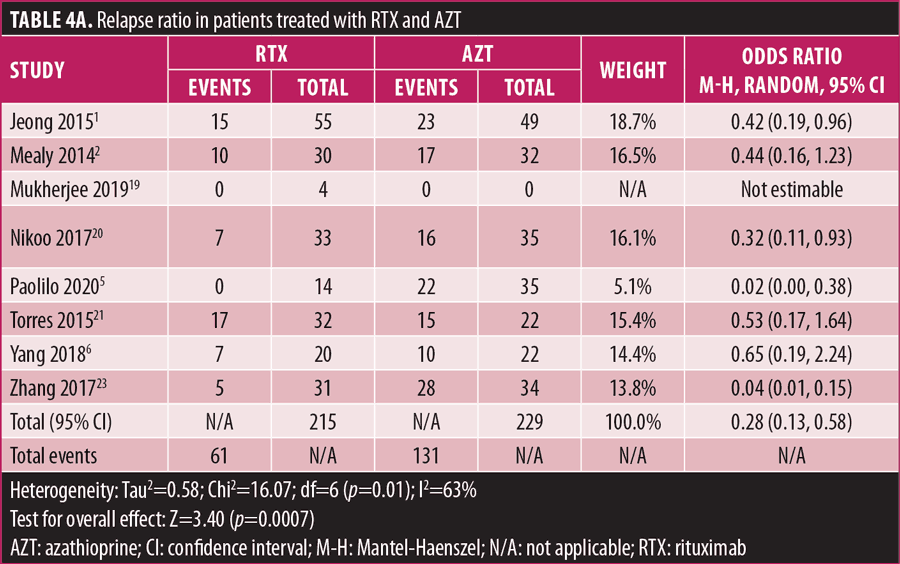
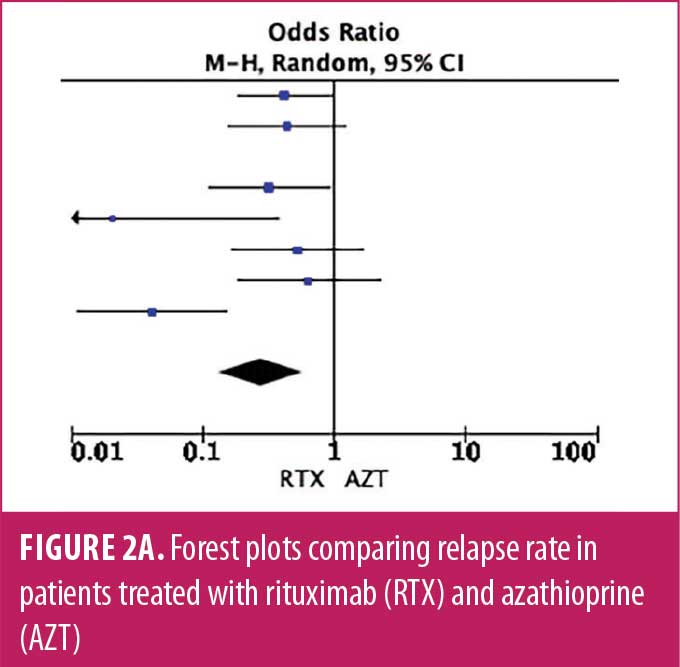
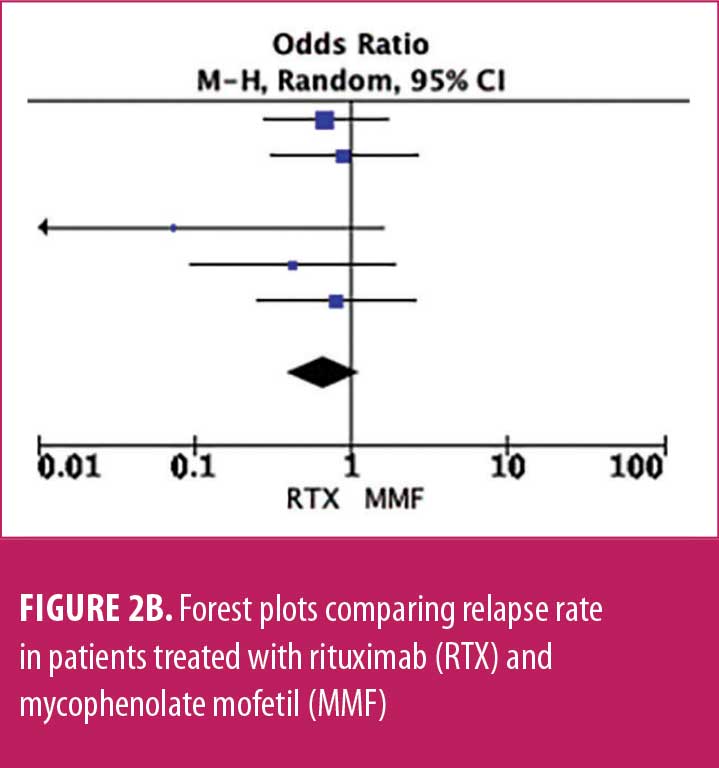
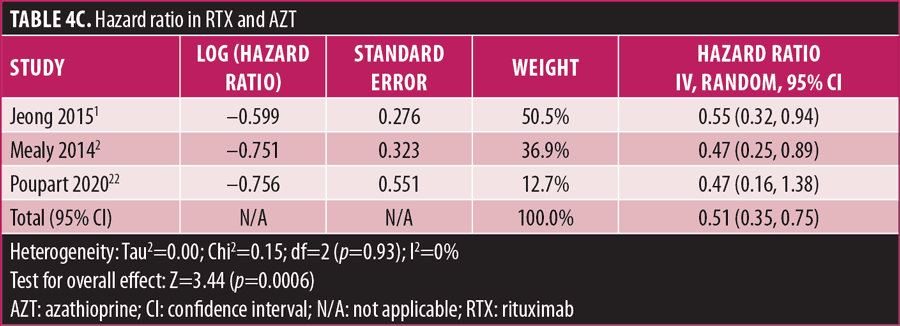
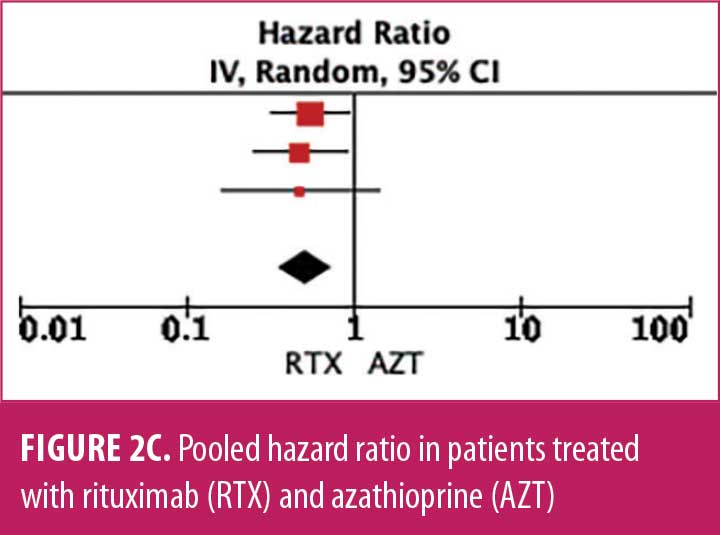
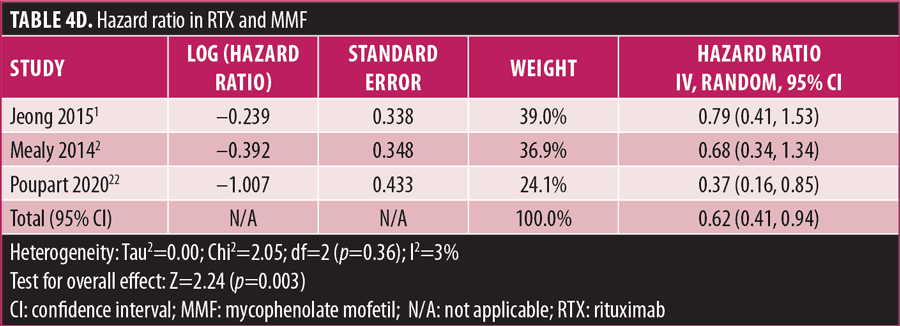
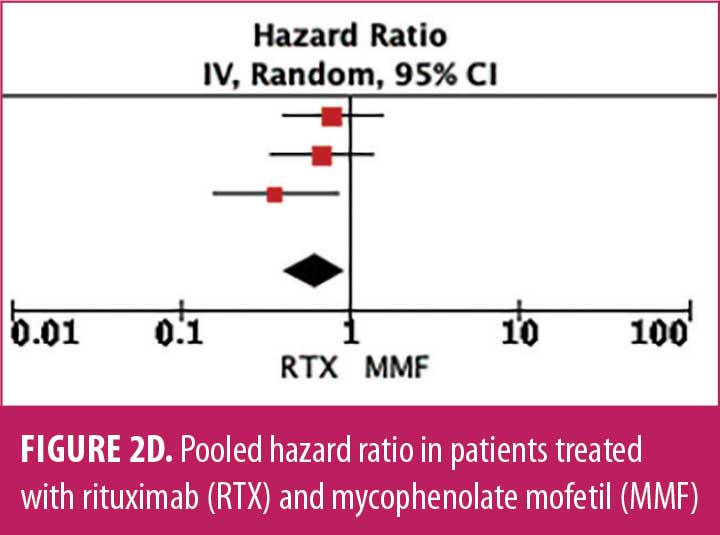
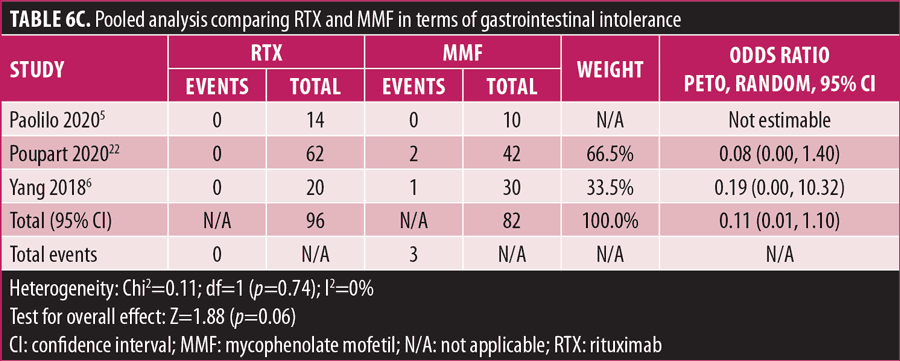
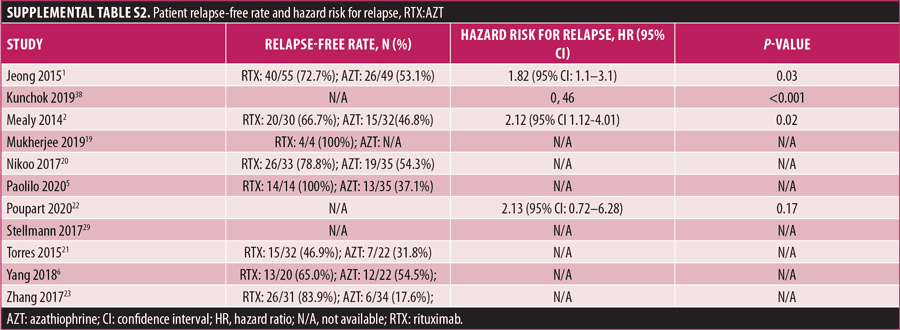
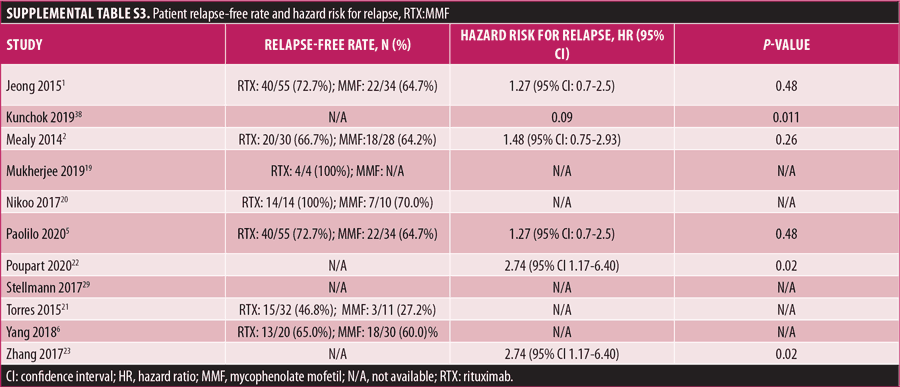
Relapse rate and HRR. A forest plot comparing the relapse rate between RTX and AZT is presented in Figures 2A to 2D, with relapse ratio and HR presented in Tables 4A to 4D. Fewer patients treated with RTX experienced at least one relapse, compared to AZT (8 studies; 444 patients; OR: 0.28 [95% CI: 0.13–0.58]; p=0.0007). Similarly, this phenomenon was observed in the RTX group, compared to the MMF group (6 studies; 264 patients; OR: 0.67, [95% CI: 0.38–1.16]; p=0.15). However, this number did not reach statistical significance. RTX significantly reduced the HRR, compared to AZT (3 studies; 251 patients; HRR: 0.51; [95% CI: 0.35–0.75]; p=0.006). We also recorded a similar trend in the RTX group, compared to the MMF group. (3 studies; 251 patients; HRR: 0.62; [95% CI: 0.41–0.94]; p=0.03). Therefore, RTX was superior to AZT and MMF in preventing relapses. A summary of the relapse rate and HRR comparisons are listed in Supplemental Tables S2 and S3.
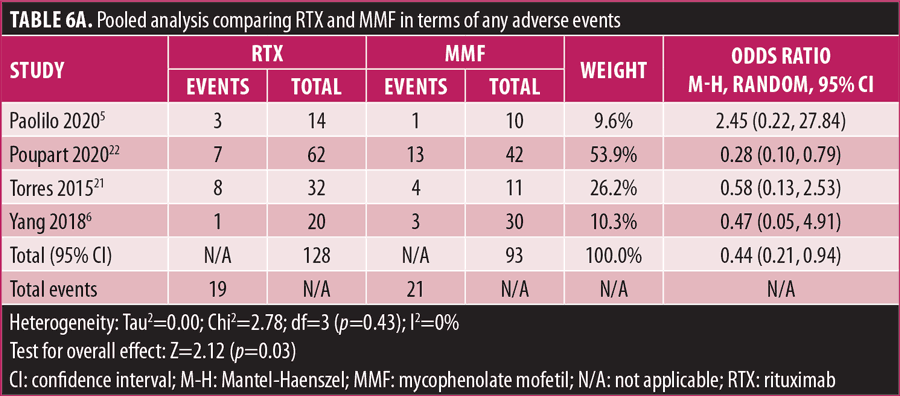
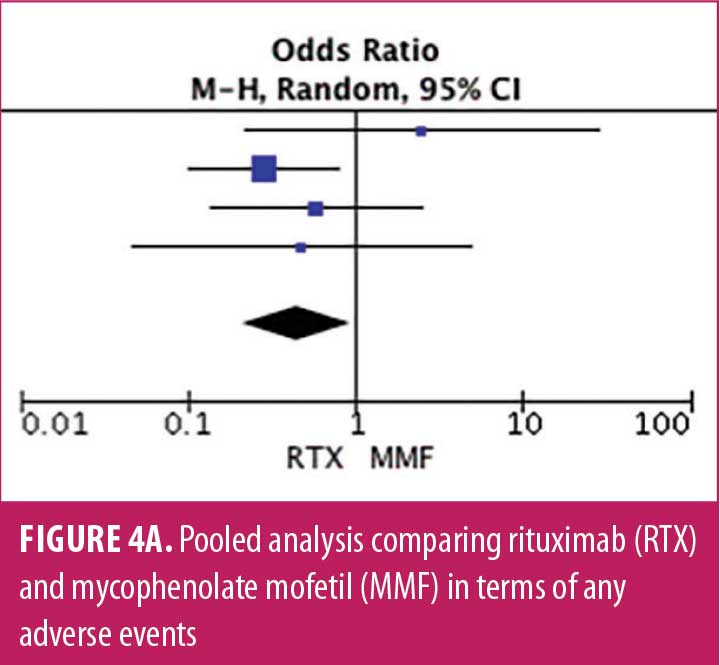
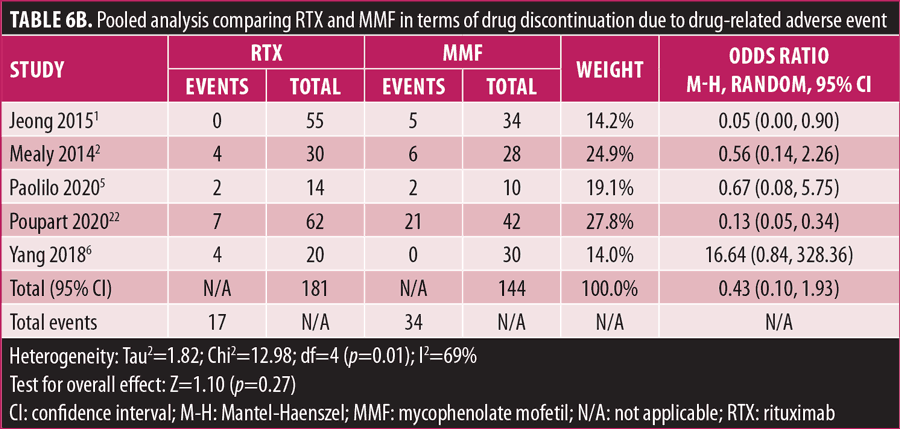
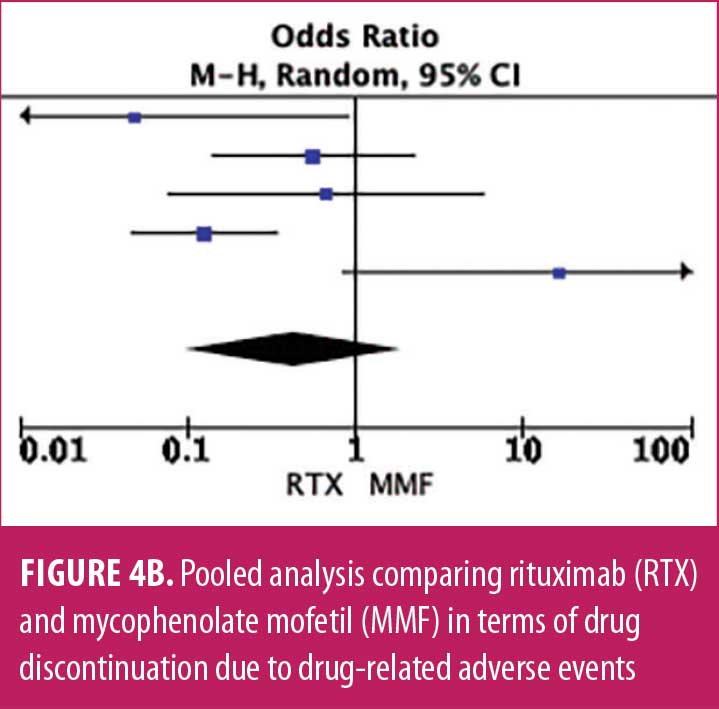
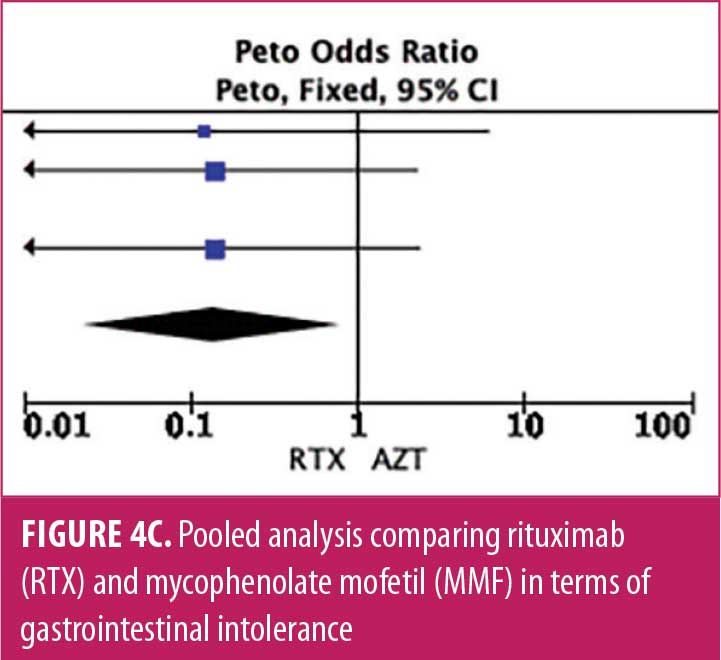
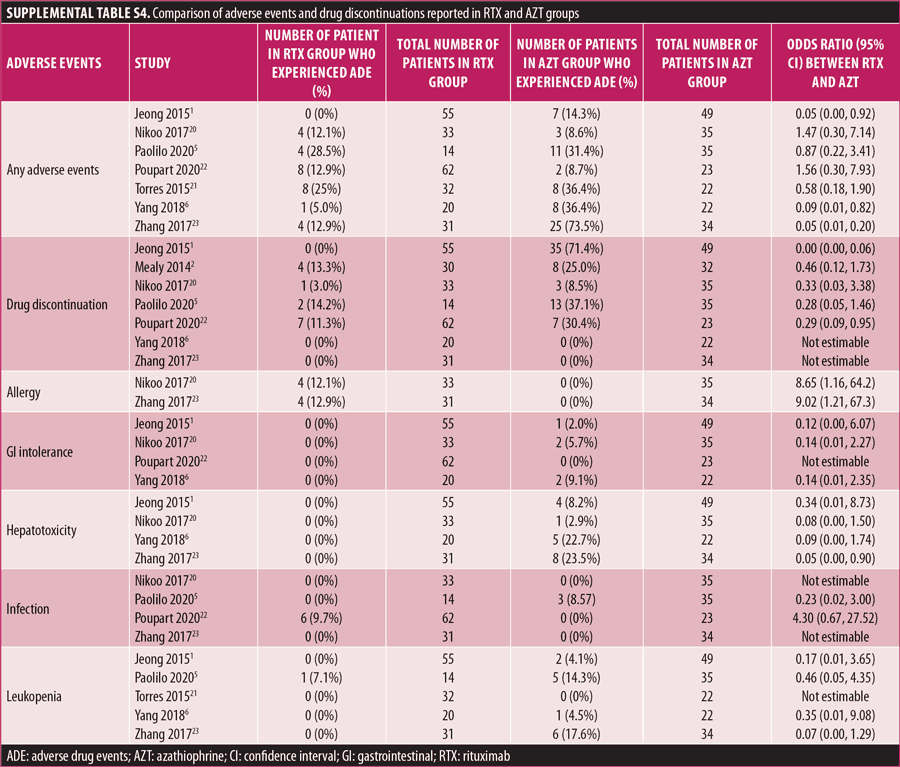
Adverse drug reaction. Comparisons of adverse drug events between RTX and AZT and RTX and MMF are shown in Figures 3A to 3G and 4A to 4C, as well as Tables 5A to 5D and 6A to 6C. The number of patients associated with RTX, AZT, and MMF treatments from each study is listed in Supplemental Tables S4 and S5. In general, patients in the RTX group experienced fewer side effects than the other two groups, ranging from 0 to 28.5 percent. The most common side effects in this group reported were allergic reactions (12.12–12.9%), local and systemic infections (0–9.68%), and leukopenia (0–7.1%). None of the patients in the RTX group experienced gastrointestinal intolerance or hepatotoxicity during the course of the treatments. The proportion of patients who discontinued RTX treatment ranged from 0 to 14.2 percent.
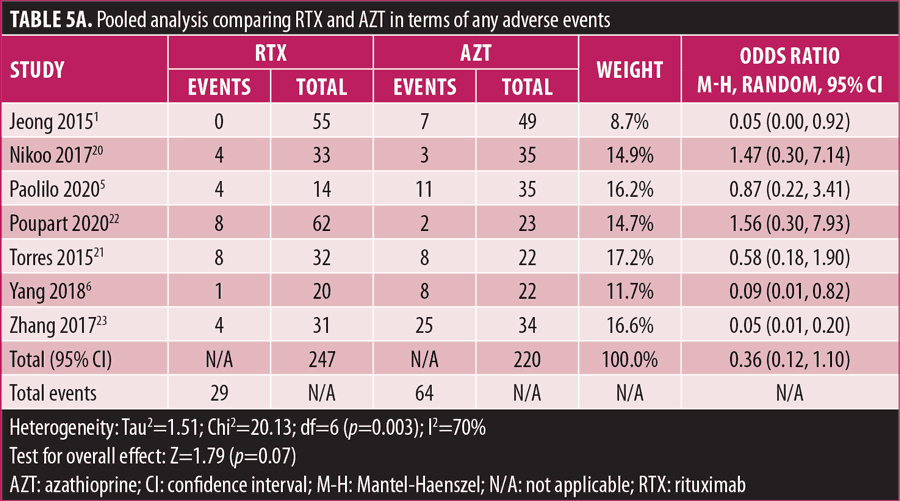
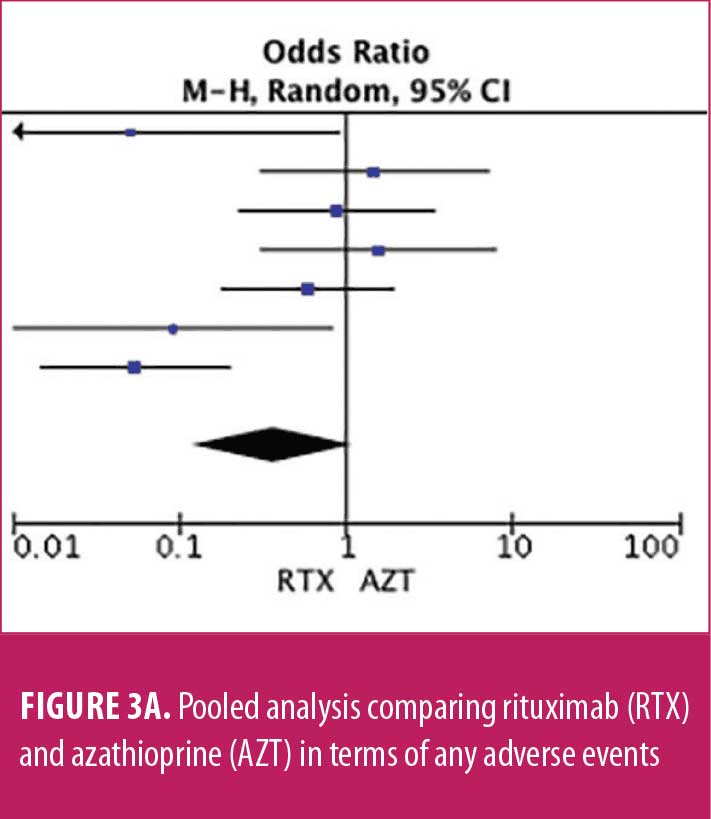
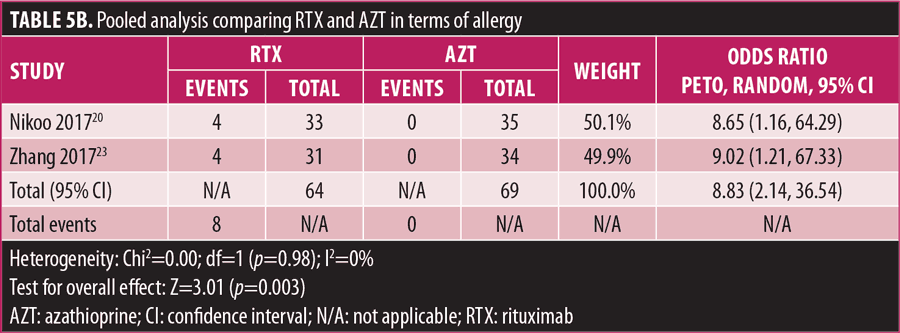
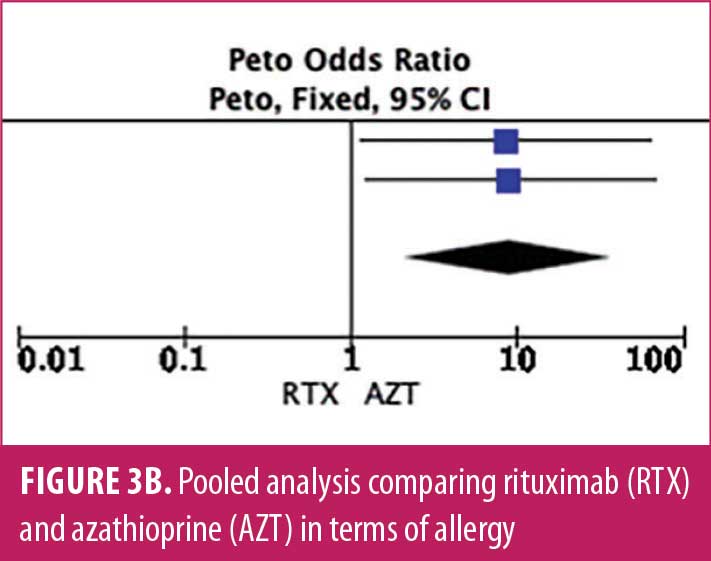
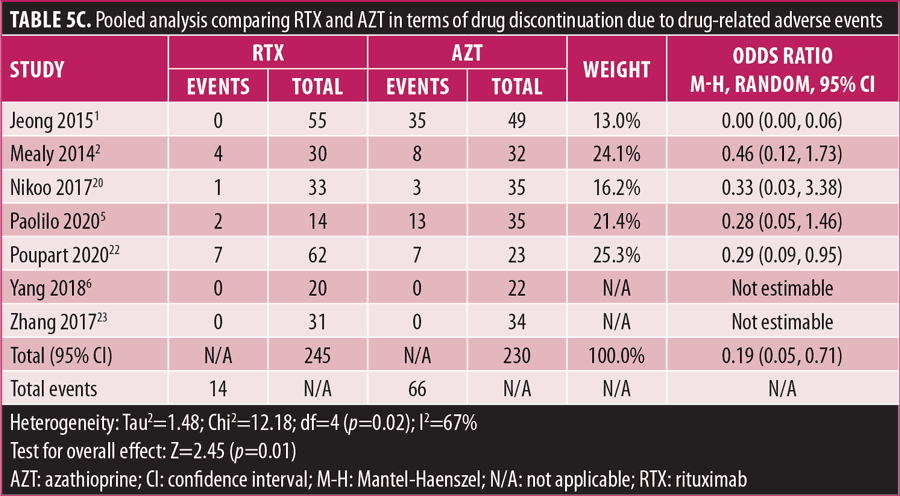
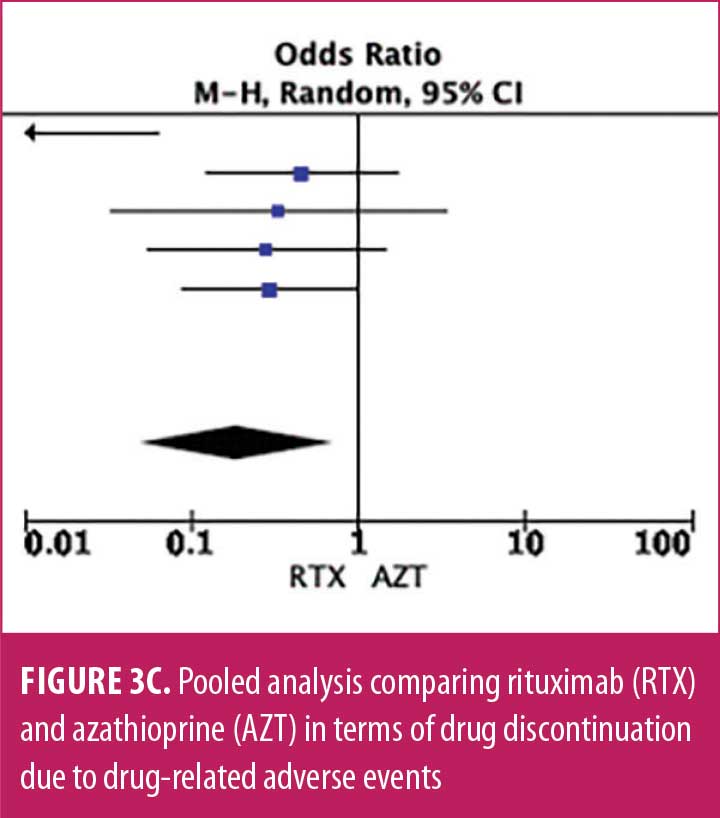
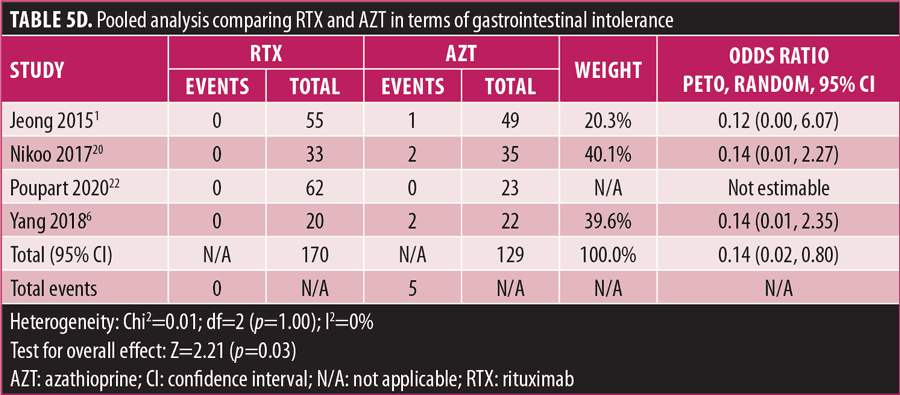
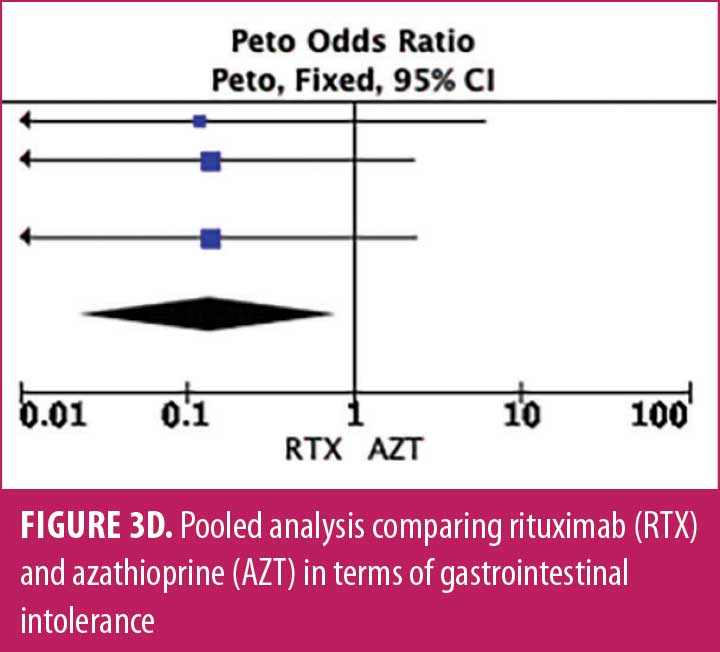
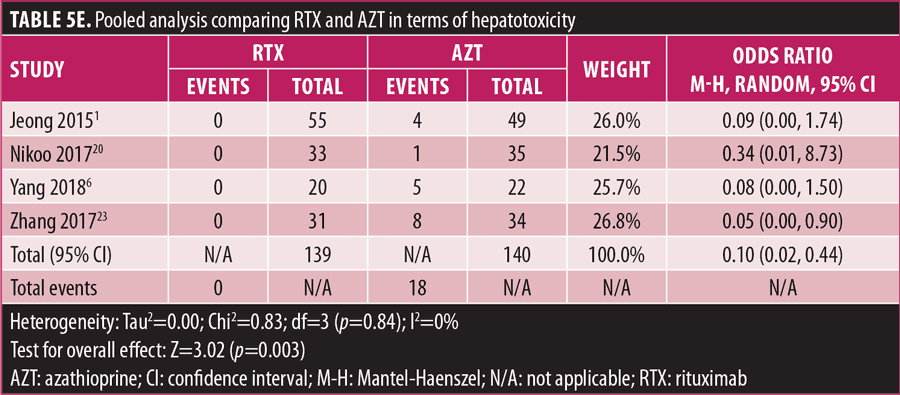
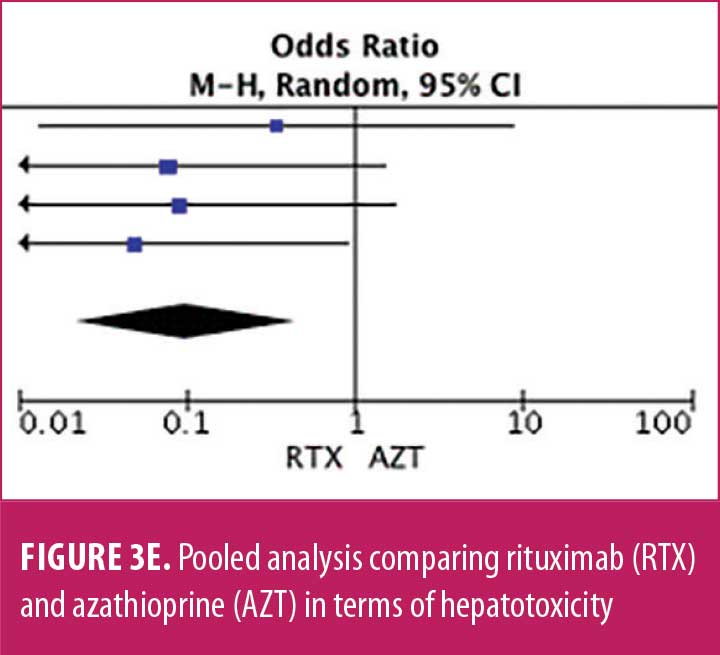
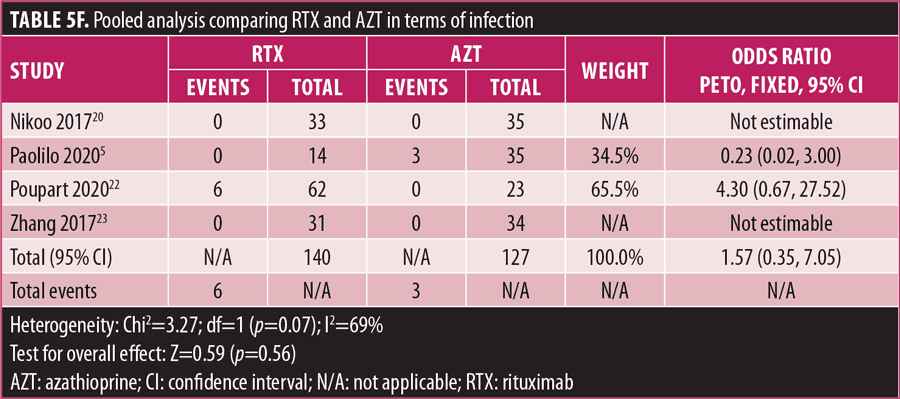
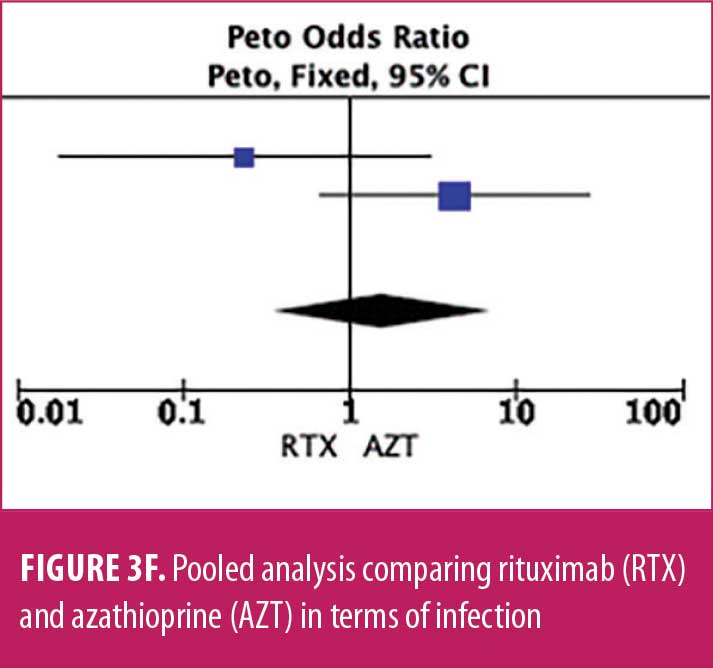
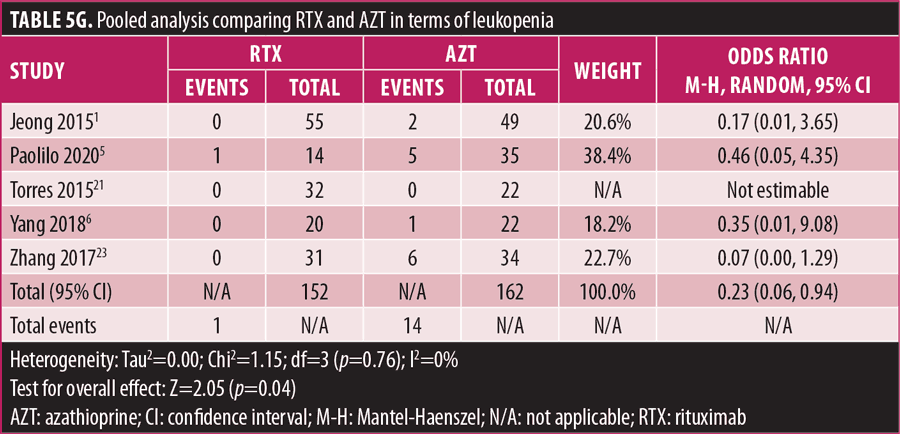
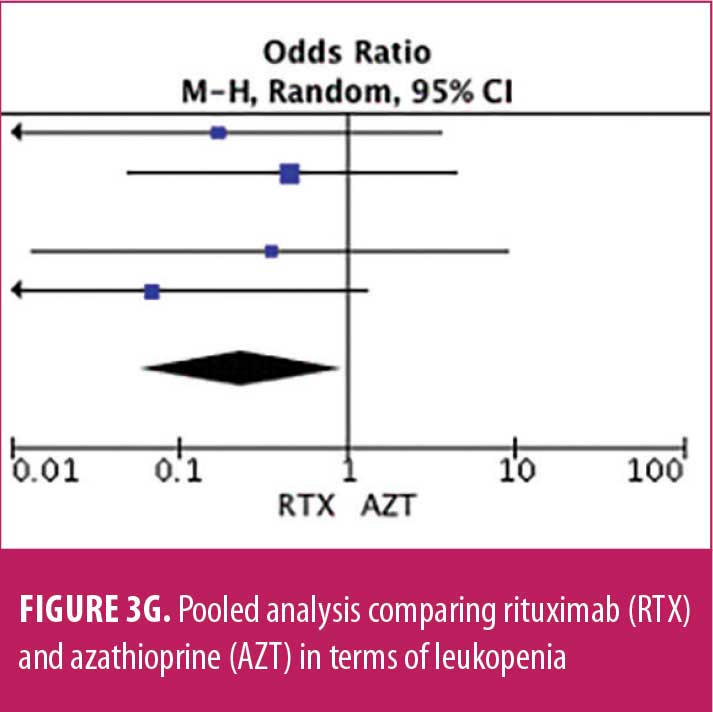
The forest plots showed that significantly fewer patients in the RTX group than in the MMF group had total adverse drug events (4 studies; 221 patients; OR: 0.44 [95% CI: 0.21, 0.94]; p=0.03). In addition, the proportion of patients in the RTX group who experienced gastrointestinal intolerance (4 studies; 299 patients; OR: 0.14 [95% CI: 0.02, 0.80]; p=0.03), hepatotoxicity (4 studies; 279 patients; OR: 0.10 [95% CI: 0.02, 0.44]; p=0.003), and leukopenia (5 studies; 314 patients; OR: 0.23 [95% CI: 0.06, 0.94]; p=0.04) was significantly lower than that in the AZT group. In contrast, there were significantly more patients in the RTX group experiencing allergic reactions than in the AZT group (2 studies; 133 patients; OR: 8.83 [95% CI: 2.14, 36.54]; p=0.003). There was a significant difference in the number of patients who discontinued the drug due to side effects between the RTX and AZT groups (7 studies; 475 patients; OR: 0.19 [95% CI: 0.05, 0.71]; p=0.01). Other pooled analyses of adverse drug events did not reach statistical significance.
Discussion
NMOSD is a rare neurological disorder with high morbidity.24 The goal of NMOSD treatment is to prevent relapses.24,25 Current immunomodulatory therapies in NMOSD are focused on reducing relapse rates and the severity of the attacks. However, most of the available data are from small, randomized and nonrandomized controlled trials, and no guidelines have been established for NMOSD treatment. This systematic review and meta-analysis aims to provide the most extensive data to evaluate the potential efficacy and safety of RTX compared to AZT and MMF as immunomodulatory agents for patients with NMOSD.
In our systematic review, the mean difference in pre- and posttreatment ARR and EDSS scores from all three treatment groups mostly produced favorable results with diverse statistical significance. This might be due to the different study designs, varied follow-up periods, variable drug doses, patient heterogeneity, and wide range of patient baseline characteristics. Additionally, this review included all patients diagnosed with NMOSD according to the previous and newly revised diagnostic criteria for NMOSD. Regarding the knowledge of RTX efficacy in treating patients with NMOSD, many of our previous data were sourced from noncomparative studies. In their meta-analyses, Gao et al26 and Damato et al24 reported that RTX was significantly effective in reducing the ARR and EDSS score.
Prevention of relapse reflects the control of disease activity in NMOSD, which in our analysis was expressed by HRR and relapse rate. We believe these outcomes should be the parameter of interest, as disability is linked to the accumulation of relapses. We found that RTX prevents relapses better than AZT and MMF.
In our study, patients in the RTX group had fewer side effects than the other two groups. RTX has been proven to be well tolerated for patients during long-term follow-up. The most commonly reported side effect of RTX in our patients was an allergic reaction. However, slowing the infusion rate and using preventive medications are shown to be effective in minimizing hypersensitivity reactions.7,27,28 AZT remains the least tolerated drug, with its most common side effects including bone marrow suppression, gastrointestinal intolerance, elevated liver enzymes, leukopenia, and mild hair loss.8,9
The longer treatment interval in RTX might ultimately promote better treatment adherence, compared to the other two drugs, which require daily doses of treatment.12 Unlike AZT and MMF, which are typically prescribed as oral corticosteroids, RTX-treated patients necessitate no immunosuppressive bridge.8 Treatment discontinuation in the AZT group was also observed to be higher, mainly due to severe side effects associated with steroid use.
Consensus on RTX induction strategy is still lacking. Two well-known induction strategies are 375mg/m2/week for four weeks and 1,000mg twice at a two-week interval.1,2,20,22,29–33 Previous studies found a lower relapse frequency in the latter regimen.34,35 Meanwhile, the two most widely discussed reinfusion approaches are fixed time points every six months and monitoring parameter-driven reinfusion. Novi et al34 demonstrated a lower relapse in the latter group, which led to longer infusion intervals, reduced doses, fewer side effects, and potentially lower costs, without compromising the efficacy.34,36 Therefore, personalized monitoring and maintenance strategies should be adopted.
Two trials in our analysis also investigated the efficacy of low-dose RTX. Low-dose RTX decreased the ARR and EDSS scores, which indicates that the efficacy of low-dose RTX is not different from that of high-dose RTX.4,37
Limitations. There were several limitations in our study. Long-term side effects of treatment are still undetermined, as our included studies had variable lengths of follow-up. The severity of relapse and time to first severe relapse were not assessed in the majority of the studies included, although these two factors were substantial in deciding the course of treatment.1 Due to the overall limitations, future RCTs are needed to determine with certainty the safety, efficacy, and cost-effectiveness of these immunomodulatory agents.
Conclusion
In summary, RTX may be used as first-line therapy in patients with NMOSD. Limited evidence suggests that RTX is superior to AZT and MMF in preventing relapses and improving neurological disabilities. Based on pooled analysis, patients in the RTX group also experienced fewer side effects. Low-dosing strategies appear to be as effective as conventional approaches. Future RCTs are needed to evaluate the safety and efficacy of RTX in patients with NMOSD.
References
- Jeong IH, Park B, Kim S-H, et al. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler. 2016;22(3):329–339.
- Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3):324–330.
- Fujihara K, Cook LJ. Neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease: current topics. Curr Opin Neurol. 2020;33(3):300–308.
- Lin J, Li X, Xue B, et al. Low-dosage of rituximab in Chinese patients with neuromyelitis optica spectrum disorder. J Neuroimmunol. 2018;317:1–4.
- Paolilo RB, Hacohen Y, Yazbeck E, et al. Treatment and outcome of aquaporin-4 antibody-positive NMOSD: a multinational pediatric study. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e837.
- Yang Y, Wang CJ, Wang BJ, et al. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and lower dosages of rituximab among patients with neuromyelitis optica spectrum disorder. J Neurol Sci. 2018;385:192–197.
- Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
- Huang W, Wang L, Zhang B, et al. Effectiveness and tolerability of immunosuppressants and monoclonal antibodies in preventive treatment of neuromyelitis optica spectrum disorders: a systematic review and network meta-analysis. Mult Scler Relat Disord. 2019;35:246–252.
- Espiritu AI, Pasco PMD. Efficacy and tolerability of azathioprine for neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;33:22–32.
- Songwisit S, Kosiyakul P, Jitprapaikulsan J, et al. Efficacy and safety of mycophenolate mofetil therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. Sci Rep. 2020;10(1):16727.
- Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019–1032.
- Wingerchuk DM. Immune-mediated myelopathies. Continuum (Minneap Minn). 2018;24(2, Spinal Cord Disorders):497–522.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 4 Jun 2021.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33(11):1444–1452.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions, version 6.1. Updated Feb 2021. https://training.cochrane.org/handbook. Accessed 10 Jun 2021.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012.
- Mukherjee S, Guha G, Roy M, et al. A study on patients with neuromyelitis optica spectrum disorder from Eastern India. Neurol Psychiatry Brain Res. 2020;35:22–28.
- Nikoo Z, Badihian S, Shaygannejad V, et al. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: a randomized clinical trial. J Neurol. 2017;264(9):2003–2009.
- Torres J, Pruitt A, Balcer L, et al. Analysis of the treatment of neuromyelitis optica. J Neurol Sci. 2015;351(1–2):31–35.
- Poupart J, Giovannelli J, Deschamps R, et al. Evaluation of efficacy and tolerability of first-line therapies in NMOSD. Neurology. 2020;94(15):e1645–e1656.
- Zhang M, Zhang C, Bai P, et al. Effectiveness of low dose of rituximab compared with azathioprine in Chinese patients with neuromyelitis optica: an over 2-year follow-up study. Acta Neurol Belg. 2017;117(3):695–702.
- Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1342–1348.
- Ashtari F, Mehdipour R, Shaygannejad V, Mansourian M. Disease course, progression and activity of neuromyelitis optica (NMOSD) in patients who were treated with rituximab, 6 and 12 months after receiving the first dose of drug, in Isfahan city. Mult Scler Relat Disord. 2019;34:77–82.
- Gao F, Chai B, Gu C, et al. Effectiveness of rituximab in neuromyelitis optica: a meta-analysis. BMC Neurol. 2019;19(1):36.
- Lu Q, Luo J, Hao H, et al. A long-term follow-up of rituximab treatment in 20 Chinese patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2020;40:101933.
- Shaygannejad V, Fayyazi E, Badihian S, et al. Long-term tolerability, safety and efficacy of rituximab in neuromyelitis optica spectrum disorder: a prospective study. J Neurol. 2019;266(3):642–650.
- Stellmann JP, Krumbholz M, Friede T, et al. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J Neurol Neurosurg Psychiatry. 2017;88(8):639–647.
- Cree BA, Lamb S, Morgan K, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64(7):1270–1272.
- Kim S-H, Huh S-Y, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurology. 2013;70(9):1110–1117.
- Yang C-S, Yang L, Li T, et al. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology. 2013;81(8):710–713.
- Rommer PS, Dörner T, Freivogel K, et al. Safety and clinical outcomes of rituximab treatment in patients with multiple sclerosis and neuromyelitis optica: experience from a national online registry (GRAID). J Neuroimmune Pharmacol. 2016;11(1):1–8.
- Novi G, Bovis F, Capobianco M, et al. Efficacy of different rituximab therapeutic strategies in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2019;36:101430.
- Annovazzi P, Capobianco M, Moiola L, et al. Rituximab in the treatment of Neuromyelitis optica: a multicentre Italian observational study. J Neurol. 2016;263(9):1727–1735.
- Cohen JC, Lewis RA. Rituximab for chronic inflammatory demyelinating polyneuropathy—a potential therapeutic option. Muscle Nerve. 2020;61(5):549–551.
- Xiao H, Zeng W, Li L, et al. Retrospective observation of low-dose rituximab treatment in Chinese patients with neuromyelitis optica spectrum disorders in a real-world setting. Front Neurol. 2020;11:642.
- Kunchok A, Malpas C, Nytrova P, et al. Clinical and therapeutic predictors of disease outcomes in AQP4-IgG+ neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2020;38:101868.