 by Emanuele Lo Voi, PhD; Giorgio Carmelo Basile, MD; Alessia Bramanti, PhD; Giuseppe Paladina, Annalisa Militi, MD; Daniele Bruschetta, MD; Angelo Alito,MD; Filippo Cavallaro; Salvatore Bertino, MD; and Demetrio Milardi, MD, PhD
by Emanuele Lo Voi, PhD; Giorgio Carmelo Basile, MD; Alessia Bramanti, PhD; Giuseppe Paladina, Annalisa Militi, MD; Daniele Bruschetta, MD; Angelo Alito,MD; Filippo Cavallaro; Salvatore Bertino, MD; and Demetrio Milardi, MD, PhD
Drs. Lo Voi, Bramanti, and Paladina are with the IRCCS Centro Neurolesi Bonino Pulejo in Messina, Italy. Drs. Basile, Militi, Bertino, and Milardi are with the Department of Biomedical, Dental Sciences, and Morphological and Functional Images at the University of Messina, Messina, Italy. Dr. Alito, Dr. Bruschetta, and Mr. Cavallaro are with U.O.C of Physical and Rehabilitation Medicine and Sports Medicine, Policlinico Universitario G. Martino in Messina, Italy.
FUNDING: No funding was provided for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Cerebellar involvement in primary Sjögren’s syndrome (pSS) is an uncommon condition, with only a limited number of cases described worldwide. A 43-year-old woman affected by cerebellar atrophy associated with pSS was referred to our center to undergo a cycle of physical rehabilitation therapy. Although motor symptoms started when the patient was 23 years of age, the underlying disease remained undiagnosed for several years. Neurological examination before rehabilitation revealed ataxic gait, dysmetria, nystagmus, and hypermetric saccades; the patients complained about unsteadiness while standing or walking. To improve balance and gait abilities, a 20-session cycle of balance rehabilitation, based on a combination of conventional physical therapy and virtual reality exergames, was prescribed. The outcomes of rehabilitation were evaluated with balance tests and three-dimensional (3D) gait analysis. To our knowledge, this is the first case describing the diagnostic workout for cerebellar atrophy associated with pSS and the subsequent motor rehabilitation. This work highlights the importance of early diagnosis and rehabilitation in patients with central nervous system involvement in pSS.
Keywords: Primary Sjögren’s syndrome; cerebellar ataxia; virtual reality rehabilitation; 3D gait analysis; cerebellar rehabilitation
Innov Clin Neurosci. 2021;18(7–9):11-17
Primary Sjögren’s syndrome (pSS) is a systemic auto-immune disease with heterogeneous manifestations, characterized by chronic inflammation of lacrimal and salivary glands, with xerophtalmia and xerostomia. The central nervous system (CNS) can be involved with an highly variable prevalence, estimated to be between 0.3 and 60 percent.1,2 Among CNS manifestations, cerebellar involvement is relatively uncommon; to the best of our knowledge only 15 cases have been described worldwide before.3–12 According to Yang et al,12 who reviewed 13 cases, gait ataxia (100% of reviewed cases)—sometimes accompanied by dysarthria, limb tremors, and nystagmus—magnetic resonance imaging (MRI) finding of cerebellar atrophy (84.6%), and oligoclonal bands in cerebrospinal fluid (CSF) (62.5%), are the most frequent manifestations of cerebellar involvement in pSS.
Physical rehabilitation can improve motor symptoms of cerebellar ataxia but is considered particularly challenging in patients suffering from diffuse cerebellar damage.13,14 In focal lesions (e.g., stroke, trauma), intact regions can support the defective parts, while in degenerative conditions, any part of the cerebellum is potentially involved, leading to a reduction of supporting structures. This is thought to determine an impairment of motor relearning abilities.13,15
Recently, virtual reality (VR) has been proposed as an effective tool for physical rehabilitation of balance and gait. VR can be defined as an user-computer interface that is able to generate perception of an interactive environment, object or activity.16 It has been suggested that VR based on center of pressure (COP) biofeedback could result in a better exercise technique in patients suffering from neurological or musculoskeletal conditions, helping in the restoration of functional balance and gait abilities.17,18 In addition, VR might have positive effects on patients’ motivation, engagement, and body awareness.18,19
In the present report, we describe the case of a 43-year-old woman affected by pSS, who suffered from cerebellar ataxia as first clinical sign, with onset several years before diagnosis. Furthermore, we describe a personalized rehabilitation approach based on a combination of conventional physiotherapy and biofeedback-based VR training.
Case Report
Clinical history. A 43-year-old woman was admitted to the Scientific Institute for Research, Hospitalization and Health Care (IRCCS) Neurolesi Bonino-Pulejo to undergo a cycle of physical rehabilitation. Her clinical history revealed over 20 years of disease burden.
The patient experienced mild symptoms of impaired gait, disequilibrium, and discoordination for the first time when she was 23 years of age. She also suffered from arthralgia, especially in the morning. No additional relevant symptoms or comorbidities were reported. Subsequently, the patient visited the Rheumatology Department of the Universitary Policlinic G. Martino (Messina, Italy).
She was first diagnosed with rheumatoid arthritis and treated with leflunomide (10mg/day) and celecoxib (200mg/as needed) for about two years until the complete remission of symptoms.
At 26 years of age, the patient reported a relapse, complaining of unsteadiness while standing or walking, functional impairment of fine upper limb movements, and clumsiness while writing, eating, and playing piano. Moreover, she started suffering from intermittent diplopia and dry mouth. Unsteadiness, clumsiness, diplopia, and xerophthalmia started as mild symptoms but slowly worsened over time.
At 26 years of age, the symptoms were progressive and became worse, which required a new hospitalization. On this occasion, she was administered the following tests for auto-immune disease:
- Immunofluorescence for antinuclear antibodies (ANA)
- Enzyme-Linked ImmunoSorbent Assay (ELISA) for Sjögren’s-syndrome-related antigen A autoantibodies (Ro/SSA)
- Anti SSB
- Anti-Smith
- Anti-nuclear ribonucleoprotein (RNP)
- Anti Scleroderma 70 kD (Scl70)
- Anti endomysial antibodies
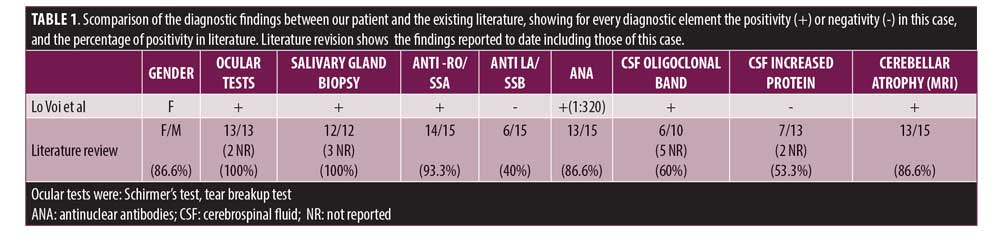
Positivity of ANA (1:320) and Ro/SSA antibodies lead to the suspected diagnosis of pSS, which was then confirmed by Schirmer’s test. In this test, a strip of filter paper is placed on the lower eyelid margin, and, after five minutes, the amount of wetting is used to measure tear production. The patient also underwent salivary gland biopsy (Table 1), which was consistent with the criteria proposed by the American College of Rheumatology/European League Against Rheumatism (ACR/EuLAR).20
Complete blood count revealed a mild normocytic anemia (hemoglobin: 11.3g/dL) with moderate elevation of erythrocyte segmentation rate (38mm/h) and normal C-reactive protein (5mg/L).
Subsequently, the following tests were performed to exclude other conditions:
- Thyroid function test
- Anti-hepatitis C virus
- Venereal Disease Research Laboratory test
- Serum C3, C4, and CH50
- aAnti-ds-DNA
- Lupus anticoagulant
- Anti-cardiolipin antibody
- Cytoplasmic antineutrophil cytoplasmic antibodies
- Perinuclear antineutrophil cytoplasmic antibodies
- Serum folate
- Vitamin B12
- Antihuman immunodeficiency virus (HIV) antibody
- HIV antigen
- Paraneoplastic antibodies (anti-Hu, anti-Ri, anti-Yo, and anti-CRMP5)
All were all executed with negative results.
Genetic tests were carried out to exclude spinocerebellar ataxias Types 1 and 2 and ataxia with vitamin E deficiency. Both had negative results. Gluten related ataxia was excluded due to negative duodenal biopsy. CSF analysis revealed the presence of oligoclonal bands (<1 cell mm3, 28mg/dL of total proteins). MRI showed a reduced cerebellar volume, suggesting the diagnosis of cerebellar atrophy associated with pSS.
The patient was treated with metylprednisolone (0.5/mg/kg/day), intravenous immunoglobulins (IvIG) (1g/kg/month on 5 days), and celecoxib (200mg/as needed). Initially, this treatment, administered for three months, determined a significant reduction of the symptoms. Subsquently, the symptoms reached stability the treatment was suspended.
When she was 32 years of age, she had her first pregnancy, during which the symptoms temporarily worsened. The last documented visit before the rehabilitation was in 2010 when the patient was 33 years of age. This visit confirmed the clinical stability of the patient.
In 2012, the second pregnancy was accompanied by a transient worsening of the symptoms, similar as the first one. Since 2012, she did not report other relevant health issues or comorbidities.
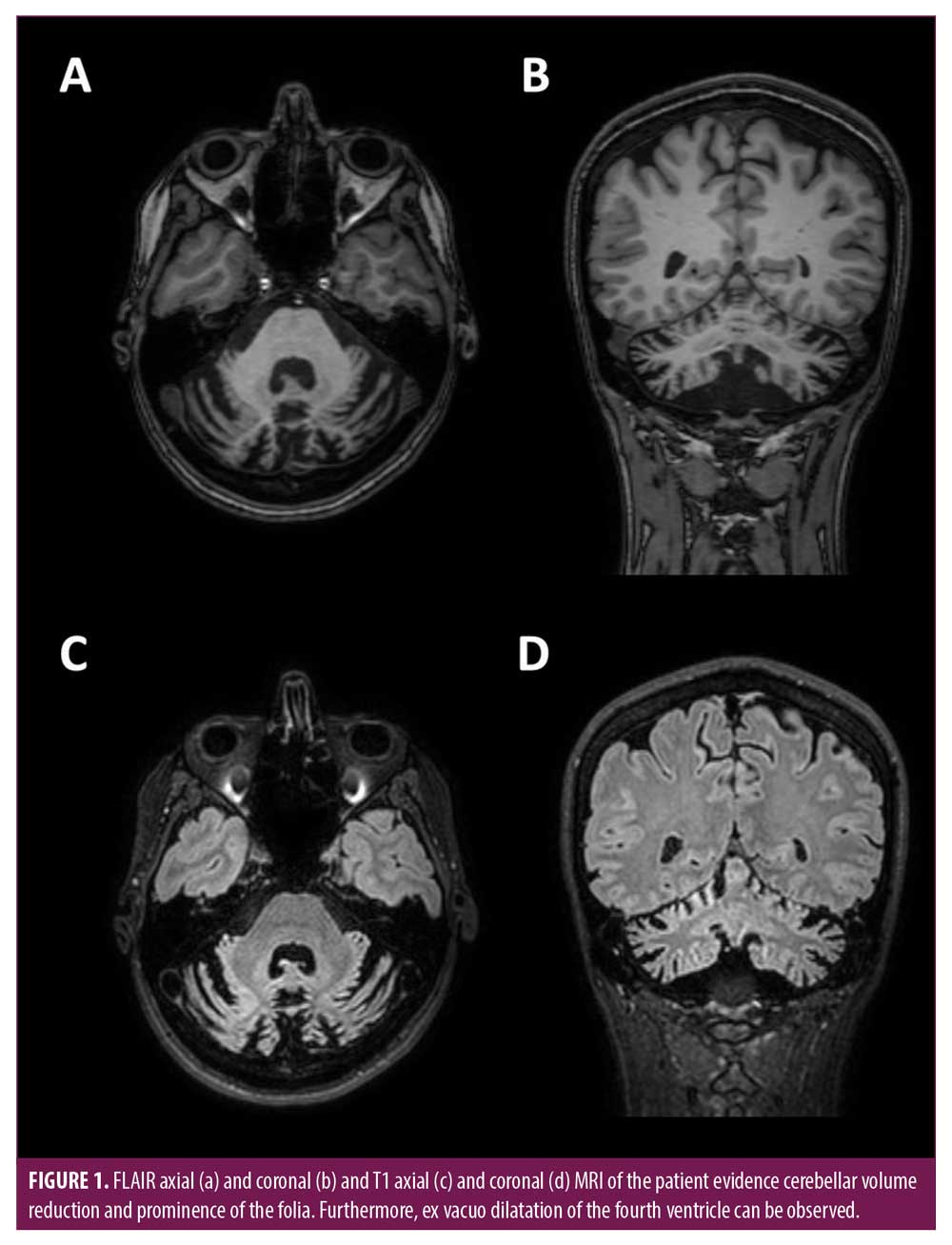
In 2019 when the patient was 43 years of age, she was admitted to our facility to undergo a cycle of physical rehabilitation. The patient reported treatment for xerophthalmia (artificial tears in form of eye drops). Xerostomia was mild, with no treatment received. In the context of the preliminary neurological examination, the observation of the gait revealed dyssynergy, decomposition of multijoint movements, and incoordination of trunk and arm swing, which resulted in reduced speed and cadence, increased step width, irregular step length, and unsteadiness during sudden changes of direction. The Romberg’s test was negative. The patient also showed marked difficulties during tandem gait. Clinical examination revealed a moderate dysmetria, revealed by finger to nose and heel to shin tests. The motor sequences of the two tests were executed by the patient with considerable effort. Ocular examination revealed gaze-evoked horizontal nystagmus on both sides (more markedly on the left) and hypermetric saccades. The clinical signs of the neurological examination were considered consistent with ataxia of cerebellar origin. A 3T clinical MRI (T1, T2, FLAIR) showed moderate cerebellar volume reduction and ex vacuo dilatation of the fourth ventricle and subarachnoid spaces (Figure 1).
Assessment. After clinical examination, the patient underwent baseline balance and gait evaluation.
For the assessment of balance skills, an experienced physiotherapist administered Berg Balance Scale (BBS), a commonly used and well validated measure of functional balance.21,22 In addition, two stabilometric tests were administered: the patient had to maintain balance while standing on a footboard with eyes open (bipodalic balance open eyes, BBOE) or closed (bipodalic balance closed eyes, BBCE). During these tests, COP sway (i.e., the ratio between the displacement of the COP from the center of the footboard and the dimensions of the footboard), COP path length, and COP velocity were measured.
These stabilometric tests and the related measurements, together with the following rehabilitation, were performed using Riablo (CoRehab, Trento, Italy), a device based on a stabilometric/proprioceptive footboard integrated with a VR display. In addition, a baseline gait analysis was performed using a 3D gait analysis tool (GAITLAB system, BTS Bioengineering, Milan, Italy).
Twenty-two reflective spherical markers were placed according to the Davis-Heel protocol.23 During walking acquisition, the subject walked straight at self-paced speed in the room over five meters. First and last strides were excluded from the analysis. Tracking of the markers and detection of gait events (heel strike, toe off) was automatically performed using the integrated software for data analysis (BTS Smart Analyzer). The results were checked and manually corrected, if needed, by an experienced bioengineer. Outcomes of gait analysis were temporal parameters (e.g., gait cycle time, single support phase %, double support phase %, gait speed, cadence), spatial parameters (e.g., step length, step width), and Gait Profile Score (GPS), which is considered an overall measure of the kinematic gait profile of a subject. GPS is based on Gait Variable Scores (GVS), described as the root mean square average differences between patient’s time-normalized kinematic variables and the values of the same variables of a reference healthy population. GVS are the following:
- Pelvic tilt
- Obliquity
- Rotation from the pelvis and hip flexion
- Abduction
- Internal rotation
- Knee flexion
- Dorsiflexion
Foot progression both from left and right foot24,25
GPS summarizes the quality of the gait profile and is responsive to change: due to these characteristics, it is considered a good single index to evaluate rehabilitation outcomes.24–26
GVS and GPS can be combined to form a movement analysis profile (MAP), a graphical representation showing immediately which variable contributes more to the GPS, and, thus, helping the observer to individuate elements of abnormality.24,25
Rehabilitation. After baseline assessment, the patient started a 20-session rehabilitation program based on a first part of traditional physical therapy and a second part consisting of VR exergames on Riablo. This program ran for 10 weeks, with each week having two 60-minute sessions.
The first part of the session, which lasted 20 to 25 minutes, started with five minutes of warmup on the treadmill. This was followed by passive stretching and mobilization for the following 5 to 10 minutes. The next 10 minutes consisted of coordinative exercises such as standing balance (i.e., before on two and one leg), stair climbing, standing heel-to-toe balance, kneeling, hip adduction-abduction, hip intra-extrarotation, and sitting to standing.
The second part of the session, which lasted between 30 and 35 minutes, included the following:
- Weight transfer (knee bent)
- Weight sagittal and lateral transfer
- Trunk flexion
- Squats
- Standing hip abduction
- Lateral hip abduction
- Standing hip flexion
- Standing hip extension
In this case, these exercises were based on interactive exergames, while standing on the footboard, the patient’s COP movement determined the correspondent movement of a cursor, which had to be moved into a 3D target visualized on a screen. The images and acoustic signals, provided when an exercise was executed correctly or incorrectly, reacted in real time to variations of the patient’s position. Feedback of the patient’s position was obtained by the analysis of the body COP and by the use of a well-validated inertial sensors system.27
All the sessions were supervised by an experienced physiotherapist, that checked for the correct execution of the exercises and the general conditions of the patient, providing verbal instructions to help the patient to maintain correct posture during walking, physical, and VR exercise.
Outcomes. At the last evaluation session, the patient underwent the same tests of the baseline (BBS, BBOE, BBCE, gait analysis), which showed an improvement in gait and balance skills.
BBS showed an improvement of +18 percent compared to the baseline (T0=44; T1=:52).
During BBOE, we observed a reduction of 61 percent in sway score (T0=6.81; T1=2.18)
COP path length and COP velocity increased of 18 percent (COP path length: T0=105mm, T1=124mm; Velocity: T0=17.4mm/s; T1=20.06mm/s).
During BBCE, sway score increased of 10 percent (T0=3.86; T1 4.27) as well as COP path length (+155%) and COP velocity (+158%) (COP path length T0=62mm, T1=158mm; velocity: T0=10.03mm/s; T1=26.2mm/s).
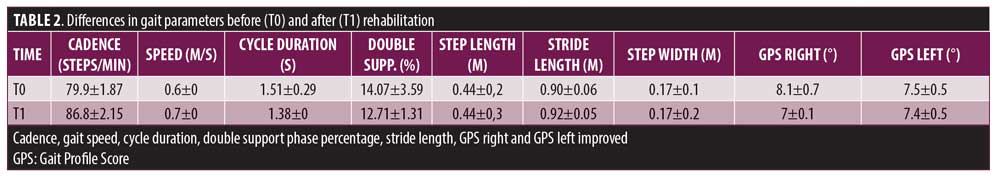
Gait analysis parameters showed a +8.6 percent improvement in cadence, +16.6 percent in gait speed, a +8.6 percent gait cycle duration and improvement in left and right GPS (Table 1).
Discussion
Cerebellum plays a central role in motor control and takes part in cognitive and emotional processes. From an anatomical standpoint, the medial and intermediate cerebellum are the areas involved in stance control, upright balance, and gait control, while part of the intermediate cerebellar cortex, the dentate and the interpositus nucleus, take part in the motor control of upper and lower limbs. The posterior lobe (posterolateral cerebellum) and cerebellar nuclei (mainly parts of dentate nuclei) are thought to be involved in cognitive operations, while speech coordination is controlled by the superior paravermal region, the intermediate cerebellar cortex, and the dentate nucleus.
Finally, parts of the vermis (lobules VI and VII), fastigius nucleus, flocculus and paraflocculus, uvula, and nodulus are the main structures determining vestibulo-ocular reflex, controlling in eye stability, and even the processing of visual fearful stimuli. For example, floccular lesions are associated with impairments in vestibulo-ocular reflex adaptation or suppression.28,29
Consequently, symptoms and signs of cerebellar syndromes are often apparently heterogenous, including balance and postural impairment (e.g., dizziness and widened foot stance), ocular symptoms (e.g., nystagmus, saccadic intrusions, and impaired smooth ocular pursuit), and dysarthria. Often, motor control of limbs can be impaired, with subsequent hypotonia, dys- or a-diadochokinesia, dysmetria, intentional tremor, and deficits of fine motor operations of upper limb, such as grasping. Furthermore, cognitive and affective abnormalities can occur, with alteration of the executive functions, visual-spatial processing, and affective regulation (e.g., flattening of affect, disinhibition, appearance of obsessive–compulsive traits).30 The disruption of cortico-ponto-cerebellar and cerebello-thalamo-cortical loops, which physiologically establish connections between the cerebellum and supratentorial structures, such as basal ganglia, motor, paralimbic and associative cortices, is associated with cerebellar symptoms.30–33
In the present case, diagnosis of cerebellar ataxia associated with pSS was based on four major elements:
- Diagnosis of pSS according to the ACR/EuLAR criteria
- MRI evidence of cerebellar degeneration
- Oligoclonal bands in CSF analysis
- Exclusion of other causes of acute or subacute ataxia
Although there are not gold standard diagnostic criteria for cerebellar involvement in pSS, these diagnostic elements are in line with those reported in existing literature since this date (Table 1). It is worth noting that among the 15 patients described in the literature, only four (26.6%) developed ataxia after the diagnosis of pSS.9,12 In the most of cases, motor impairment anticipated or manifested together with typical pSS symptoms, such as xerostomia, xerophtalmia (sicca syndrome), or arthralgia.4,8,11 Often, screening for specific antibodies was administered after the worsening of these symptoms or the appearance of new rheumatological manifestations. It is not unusual for physicians to initially consider neurological and rheumatological symptoms as unrelated, leading to a relevant delay in diagnosis. Such a delay could have a critical impact on short- and long-term disability, as neurological symptoms can benefit from early corticosteroid treatment.34,35 Cerebellar involvement can become evident with either an acute or subacute/progressive onset.8,12 In some cases, early administration of therapy based on corticosteroids, IvIG, and immunosuppressants could lead to remission, or, more often, clinical stabilization.8,11,12 In this patient, the recognition of a cerebellar ataxia of unknown origin led to a complete research of the background etiology. Nevertheless, the identification of clinical elements suggestive of cerebellar disfunction goes beyond the mere research of typical motor signs of cerebellar ataxia.36 From an anatomical standpoint, it is well known that neurons of the lateral cerebellar nucleus provide inputs to the central lateral nucleus of thalamus, which projects to motor cortex and striatum; it is thought that the interplay of these structures is crucial not only for motor, but also for cognitive and emotional processing.31 Even if our patient did not show disfunction of cognitive-affective domains, it is necessary to reiterate that cerebellar degeneration could often manifest itself with impairment of executive functions, alteration of spatial orientation, mood, behavior, and even speech disturbances. Furthermore, psychiatric symptoms (e.g., depression, anxiety, psychosis) should be investigated when suspecting a cerebellar syndrome.36
When accompanied by Sjögren’s syndrome or arthralgia, the administration of antibody screening for pSS in patients with new onset signs and symptoms of cerebellar disfunction could help the physician in the correct diagnosis.12,34,35,37 As well as for cerebellar involvement, other CNS manifestations of pSS should be considered in differential diagnosis, in presence of a suggestive clinical picture. In any case, correct diagnosis and immediate treatment could lead to an early stabilization of the symptoms, allowing the patient to undergo rehabilitation.
Currently, CNS involvement in pSS should be treated with the use of immunosuppressive and biologic agents. Empirically, high-dose corticosteroids, as well as other immune suppressants, have been suggested to treat myelitis. Other drugs that can be used for CNS manifestations include rituximab, plasma exchange, azathioprine, methotrexate and mycophenolate mofetil, they’re used according to the severity of the condition.20,38,39
Notably, early rehabilitation is a crucial outcome predictor for patient with cerebellar ataxia.13 Otherwise, this patient underwent physical rehabilitation several years after diagnosis and stabilization of the symptoms. We hypothesize that rehabilitation could have led to better outcomes if started earlier.
Rehabilitation is one of the cornerstones in the treatment of ataxias and should be started once neurological symptoms are stabilized. In this report, despite negative predictors for rehabilitation (e.g., diffuse cerebellar damage, the absence of early intervention), the patient benefited from the combination of coordinative training and the use of exergames. Synofzik et al40 outlined how exergaming, when assisted by a physiotherapist, can effectively complement physiotherapy in rehabilitation of patients with degenerative ataxia.13
After the treatment, we observed an increase in COP path length and COP velocity. These measures are related to the variety of postural adjustments performed to maintain stability while standing on the footboard and is usually considered a sign of cerebellar dysfunction. On the other hand, measures of functional balance, like BBS and body sway during BBOE, showed marked improvements. This could suggest that the increase in postural adjustments may have resulted in an improvement in static and dynamic balance. Our patient in particular showed an improvement of eight points in BBS. Minimal clinically important difference for this scale has been estimated to be between thre to four points for different types of patients.41,42 Our patient showed a marked improvement of open-eye sway after rehabilitation, whereas closed-eye sway showed a less evident difference. Speculatively, these results could be interpreted as deriving from an emphasized reliance on visual inputs; as a compensation based on visual, acoustic, and proprioceptive perception is considered a potential mechanism of balance improvement in cerebellar ataxia.13,43
In addition, we found improvements in temporal and kinematic parameters of gait analysis. Among temporal parameters, the patient showed a positive change in cadence, gait speed, and cycle duration. Gait speed showed a clinically important improvement of 16.6 percent (0.1m/s).44 The change in gait speed is associated with an 8.6 percent increase in cadence and a reduction in double support phase percentage, which became more similar to that of healthy subjects (approximately around 10%).45 In contrast, spatial parameters remained similar before and after the treatment.
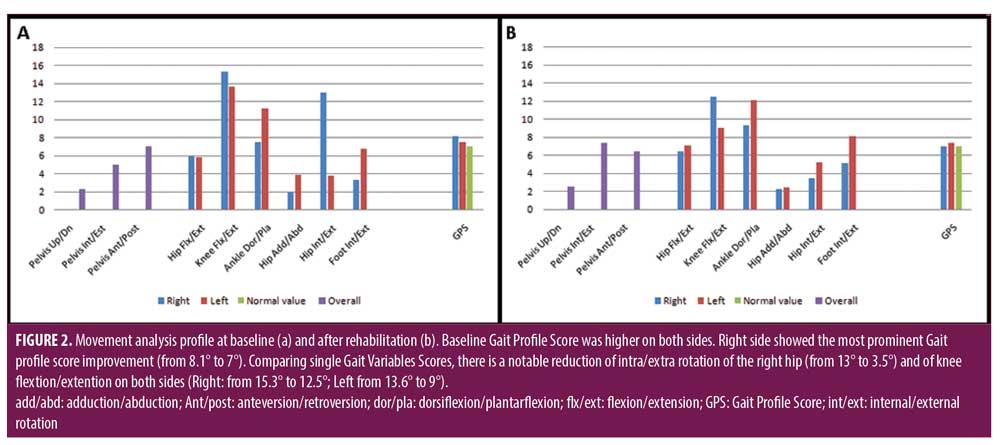
Among kinematic parameters, rehabilitation led to a reduced GPS on both sides. The improvement is more clear on the right side, where GPS reached a borderline physiological value (7°).46 This case provides an example of how MAP in addition to visual evaluation of gait can be useful to represent the kinematic profile and to distinguish which variable is influencing more the GPS outcome.25,46
At baseline assessment, one of the most prominent abnormalities of gait was an abnormal internal rotation of right hip (Figure 2A). The physiotherapist tried to improve this aspect by the administration of specific exercises of stretching, conventional physiotherapy (hip adduction-abduction, intra-extra rotation), and by verbal instructions. At the end of the rehabilitation, GVS for hip rotation showed a reduction (Figure 2B). To sum up, results of our VR biofeedback-based rehabilitation are consistent with previous literature, suggesting that patients with diffuse cerebellar involvement could benefit from an intensive neurorehabilitation cycle balance to enhance multi-joint coordination, gait speed and functional balance.47
Limitations
The first limitation of this study is that results of this rehabilitation cannot be generalized to other patients. Any individual factor—like physical condition, psychological approach and engagement—is crucial in rehabilitation, which can influence the results of each patient.48 Furthermore, we did not have the opportunity to do any follow up to assess to what degree outcomes of rehabilitation persisted over time.
Conclusion
Although cerebellar ataxia associated with pSS is an uncommon condition, early diagnosis is crucial because it could positively impact the clinical course and lead to a reduction of the time between the onset of symptoms, pharmacological therapy, and rehabilitation. Therefore, we suggest administering tests for antibodies to rule out this condition in patients with cerebellar ataxia and signs/symptoms of pSS. When the symptoms are stable, patients could benefit from balance rehabilitation to improve motor function. We suggest that VR could be an effective complement to traditional physiotherapy during the rehabilitation cycle.
References
- Massara A, Bonazza S, Castellino G, et al. Central nervous system involvement in Sjögren’s syndrome: unusual, but not unremarkable—clinical, serological characteristics and outcomes in a large cohort of Italian patients. Rheumatology (Oxford). 2010;49(8):1540–1549.
- Carvajal Alegria G, Guellec D, Mariette X, et al. Epidemiology of neurological manifestations in Sjögren’s syndrome: data from the French ASSESS Cohort. RMD Open. 2016;2(1):e000179.
- Owada K, Uchihara T, Ishida K, et al. Motor weakness and cerebellar ataxia in Sjögren syndrome–identification of antineuronal antibody: a case report. J Neurol Sci. 2002;197(1-2):79–84.
- Farhat E, Zouari M, Abdelaziz IB, et al. Progressive cerebellar degeneration revealing primary Sjögren syndrome: a case report. Cerebellum Ataxias. 2016;3:18.
- Kim MJ, Lee MC, Lee JH, Chung SJ. Cerebellar degeneration associated with Sjögren’s syndrome. J Clin Neurol. 2012; 8(2):155–159.
- Terao Y, Sakai K, Kato S, et al. Antineuronal antibody in Sjögren’s syndrome masquerading as paraneoplastic cerebellar degeneration. Lancet. 1994;343(8900):790
- Collison K, Rees J. Asymmetric cerebellar ataxia and limbic encephalitis as a presenting feature of primary Sjögren’s syndrome. J Neurol. 2007;254(11):1609–1611.
- Wong S, Pollock AN, Burnham JM, et al. Acute cerebellar ataxia due to Sjögren syndrome. Neurology. 2004;62(12):2332–2333.
- Milic V, Ostojic P. Cerebellar ataxia in a patient with primary Sjögren’s syndrome after treatment with chloroquine. Rheumatol Int. 2008;28(12):1295–1296.
- Chen YW, Lee KC, Chang IW, et al. Sjogren’s syndrome with acute cerebellar ataxia and massive lymphadenopathy : a case report. Acta Neurol Taiwan. 2013;22(2):81–86.
- Maciel R, Camargos S, Cardoso F. Subacute cerebellar degeneration as the first manifestation of Sjögren’s Syndrome. Mov Disord Clin Pract. 2017;4(4):637–638.
- Yang H, Sun Y, Zhao L, et al. Cerebellar involvement in patients withprimary Sjögren’s syndrome: diagnosis and treatment. Clin Rheumatol. 2018;37(5):1207–1213.
- Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25(3):195–216.
- Mitoma H, Manto M. The physiological basis of therapies for cerebellar ataxias. Ther Adv Neurol Disord. 2016;9(5):396–413.
- Ilg W, Bastian AJ, Boesch S, et al. Consensus paper: management of degenerative cerebellar disorders. Cerebellum. 2014;13(2):248–268.
- Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: a review. NeuroRehabilitation. 2009;25(1):29–44.
- Giggins OM, Persson UM, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil. 2013;10:60.
- Calabrò RS, Cacciola A, Bertè F, et al. Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now?. Neurol Sci. 2016;37(4):503–514.
- Im SJ, Kim YH, Kim KH, et al. The effect of a task-specific locomotor training strategy on gait stability in patients with cerebellar disease: a feasibility study. Disabil Rehabil. 2017;39(10):1002–1008.
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(1):35-45.
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 Suppl 2:S7–S11.
- Pérennou D, Decavel P, Manckoundia P, et al. [Evaluation of balance in neurologic and geriatric disorders]. Ann Readapt Med Phys. 2005;48(6):317–335.
- Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10(5):575–587.
- Speciali DS, Oliveira EM, Cardoso JR, et al. Gait profile score and movement analysis profile in patients with Parkinson’s disease during concurrent cognitive load. Braz J Phys Ther. 2014;18(4):315–322.
- Baker R, McGinley JL, Schwartz MH, et al. The gait profile score and movement analysis profile. Gait Posture. 2009;30(3):265–269.
- Kark L, Vickers D, McIntosh A, Simmons A. Use of gait summary measures with lower limb amputees. Gait Posture. 2012;35(2):238–243.
- Leardini A, Lullini G, Giannini S, et al. Validation of the angular measurements of a new inertial-measurement-unit based rehabilitation system: comparison with state-of-the-art gait analysis. J Neuroeng Rehabil. 2014;11:136.
- Manto M, Bower JM, Conforto AB, et al. Consensus paper: roles of the cerebellum in motor control–the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–487.
- Cacciola A, Bertino S, Basile GA, et al. Mapping the structural connectivity between the periaqueductal gray and the cerebellum in humans. Brain Struct Funct. 2019;224(6):2153–2165.
- Bodranghien F, Bastian A, Casali C, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15(3):369–391.
- Milardi D, Quartarone A, Bramanti A, et al. The cortico-basal ganglia-cerebellar network: past, present and future perspectives. Front Syst Neurosci. 2019;13:61.
- Cacciola A, Milardi D, Basile GA, et al. The cortico-rubral and cerebello-rubral pathways are topographically organized within the human red nucleus. Sci Rep. 2019;9(1).
- Basile GA, Quartu M, Bertino S, et al. Red nucleus structure and function: from anatomy to clinical neurosciences. Brain Struct Funct. 2021;226(Suppl 1):1–23.
- Alhomoud IA, Bohlega SA, Alkawi MZ, et al. Primary Sjögren’s syndrome with central nervous system involvement. Saudi Med J. 2009;30(8):1067–1072.
- Santosa A, Lim AY, Vasoo S, et al. Neurosjögren: early therapy is associated with successful outcomes. J Clin Rheumatol. 2012;18(8):389–392.
- Liszewski CM, O’Hearn E, Leroi I, et al. Cognitive impairment and psychiatric symptoms in 133 patients with diseases associated with cerebellar degeneration. J Neuropsychiatry Clin Neurosci. 2004;16(1):109-112.
- Alexander EL, Malinow K, Lejewski JE, et al. Primary Sjögren’s syndrome with central nervous system disease mimicking multiple sclerosis. Ann Intern Med. 1986;104(3):323–330.
- Alunno A, Carubbi F, Bartoloni E, et al. The kaleidoscope of neurological manifestations in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2019;37 Suppl 118(3):192–198.
- Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3).
- Synofzik M, Ilg W. Motor training in degenerative spinocerebellar disease: ataxia-specific improvements by intensive physiotherapy and exergames. Biomed Res Int. 2014;2014:583507.
- Winser SJ, Smith CM, Hale LA, et al. Core set of measures of balance for people with multiple sclerosis and cerebellar ataxia. Mov Disord. 2016; 31(Suppl 2):S337.
- Gervasoni E, Jonsdottir J, Montesano A, Cattaneo D. Minimal clinically important difference of Berg Balance Scale in people with multiple sclerosis. Arch Phys Med Rehabil. 2017;98(2):337–340.e2.
- Horak FB. Postural compensation for vestibular loss. In: Annals of the New York Academy of Sciences. 2009.
- Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract. 2014;20(4):295–300.
- Schmeltzpfenning T, Brauner T. Foot biomechanics and gait. In: Handbook of Footwear Design and Manufacture. 2013.
- Thomason P, Yu X, Baker R, Graham HK. P061 Evaluating the outcome of single event multilevel surgery: find the way use the MAP (movement analysis profile). Gait Posture. 2008.
- Ilg W, Synofzik M, Brötz D, et al. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73(22):1823–1830.
- Covassin T, Beidler E, Ostrowski J, Wallace J. Psychosocial aspects of rehabilitation in sports. Clin Sports Med. 2015;34(2):199–212.


