
by Leslie Citrome, MD, MPH; Marko A. Mychaskiw, BSRPh, MS, PhD; Alma Cortez, BA; Mark Opler, MPH, PhD; Liza Sopina, MPH, PhD; and Sameer Kotak, MS, MBA
Dr. Citrome is with Department of Psychiatry and Behavioral Sciences, New York Medical College in Valhalla, New York. Dr. Mychaskiw and Ms. Cortez are with Global Health Economics and Outcomes Research, Teva Branded Pharmaceutical Products R&D, Inc., in West Chester, Pennsylvania. Dr. Opler is with WCG MedAvante-ProPhase, Inc., in New York City, New York, and The PANSS Institute in Monroe, New York. Dr. Sopina is an Independent Consultant in Odense, Denmark. Mr. Kotak is with Yorker Health in Glen Rock, New Jersey.
Funding: This research was supported by Teva Branded Pharmaceutical Products R&D, Inc.
Disclosures: Dr. Citrome is a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Marvin, Merck, Mitsubishi-Tanabe Pharma, Neurocrine, Neurelis, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, and University of Arizona, and provides one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; is a speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies, such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; holds stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer, purchased over 10 years ago, with stock options in Reviva; receives royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–present), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through the end of 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). Dr. Mychaskiw and Ms. Cortez are employed by and hold stock in Teva Branded Pharmaceutical Products R&D, Inc. Dr. Opler is employed by WCG Clinical, Inc., and has a leadership role/board member/executive director at The PANSS Institute. Dr. Sopina is consultant for Yorker Health. Mr. Kotak is a consultant for Teva Branded Pharmaceutical Products R&D, Inc.
Innov Clin Neurosci. 2023;20(4–6):14–33.
Abstract
Objective: The complexity inherent in the treatment of schizophrenia results in a multitude of outcome assessments being employed when conducting clinical trials. Subjective outcome assessments and minimal clinically important differences (MCIDs) to evaluate clinical meaningfulness have gained traction; however, the extent of application in evaluation of treatments for schizophrenia is unknown. A scoping review was conducted to assess the availability of published psychometric evaluations, including MCIDs, for clinical outcome assessments used to evaluate treatments for schizophrenia.
Method of Research: Key databases (PubMed®, Embase®, APA PsycINFO®, International Society for Pharmacoeconomics and Outcomes Research) were searched for studies on schizophrenia published from 2010 to 2020. Secondary sources (ClinicalTrials.gov, PROLABELS™, FDA.gov) were also reviewed. Clinical outcome assessments were organized by type (patient-reported outcomes [PROs], clinician-reported outcomes [ClinROs], observer-reported outcomes [ObsROs]) and further classified by intended use (generic, mental health, schizophrenia). Reliability and internal consistency were evaluated using Cronbach’s α. External validity was evaluated by intraclass correlation coefficient (ICC).
Results: Across 140 studies, 66 clinical outcome assessments were identified. MCIDs were reported for eight of the 66 studies. Of these, two were PROs (generic) and six were ClinROs/ObsROs (three mental health-specific, three schizophrenia-specific). Reliability was good across generic, mental health-specific, and schizophrenia-specific categories, whereas external validity was strong mainly for schizophrenia-specific PROs. Overall, ClinROs/ObsROs that focused on mental health had good reliability and strong external validity.
Conclusions: This review provides a comprehensive overview of the clinical outcome assessments used in schizophrenia research during the past ten years. Results highlight the heterogeneity of existing outcomes and a growing interest in PROs for schizophrenia.
Keywords: Minimal clinically important differences, patient-reported outcomes, clinician-reported outcomes, psychometric evaluation, quality of life, schizophrenia
The current state of the management of schizophrenia typically involves a combination of pharmacologic and psychosocial interventions;1 however, because of the complexity of the disease, establishing the effectiveness of any treatment is challenging.2 Traditional approaches have focused on measuring psychopathology, including positive symptoms (e.g., delusions, hallucinations, and disorganized speech or behavior) and negative symptoms (e.g., affective flattening, alogia, and avolition), as well as quantifying cognitive impairment.3,4 Other outcomes based on assessment of functioning are also of great interest. However, in contrast to studies on interventions in mood disorders, definitions of response and remission in studies on schizophrenia lack universally accepted or otherwise simple-to-understand definitions; this lack of consensus complicates the process of interpreting the clinical relevance of study results.5,6 Thus, the implementation of standardized, validated clinical outcome assessments can allow researchers and clinicians to make sound inferences regarding the effectiveness of interventions,2 provided that what is meaningful or not can be determined.
Clinical outcome assessments can be broadly divided into clinician- (ClinROs), observer- (ObsROs), or patient-reported outcomes (PROs). There is a rich tradition of utilizing clinician and observer outcomes; however, the self-assessment of patients/subjects can provide different and actionable information. A PRO is “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”7,8 The potential value of PROs over ClinROs has become more evident relatively recently. The United States (US) Food and Drug Administration (FDA) recommends the use of PROs in pivotal clinical trials.9 Although patients with schizophrenia and their assessments of disease and treatment outcomes may be impacted by anosognosia,10 PROs are still seen as essential to understanding patient preferences for treatments and facilitating comparisons between different therapies.7,8
The inclusion of PROs in clinical trials is now advised and supported by various guidelines, including some that focus on schizophrenia.11–13 However, selecting a meaningful assessment that reflects the breadth of experiences and symptoms that patients with schizophrenia have and the impact of treatments may be challenging.11–13 A potential solution to this obstacle would be the selection of a PRO with a reported minimal clinically important difference (MCID), which is defined as the minimal amount of change in an outcome assessment that would be important to a patient.14 MCIDs can help guide both clinicians in the assessment of treatment effectiveness and researchers in trial design by providing a rationale for the selection of the most suitable outcome assessment.
In this scoping review, a database and literature search was performed to identify existing outcome assessments used in clinical studies, assess the availability of MCIDs for these assessments used to evaluate patients with schizophrenia, and propose recommendations for furthering MCID research.
Methods
Scoping review design. This scoping review was performed in accordance with the methodology outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA ScR) checklist and the Joanna Briggs Institute guidelines for performing scoping reviews.15,16 The key objective of this review was to identify the existing and relevant clinical outcomes for the assessment of schizophrenia, rather than to assess the quality of studies utilizing these outcomes.
Literature search strategy. Four key databases (PubMed®, Embase®, APA PsycINFO®, and International Society for Pharmacoeconomics and Outcomes Research [ISPOR; through Value in Health]) were used for this scoping review to assess a large variety of studies of interest. Searches were conducted between April 24, 2020, and April 27, 2020, to reduce the risk of missing newly published studies. EndNote™ X7 was used to import and screen the articles.
Initially, a search utilizing keywords and medical subject headings (MeSH) related to schizophrenia, clinical outcome assessments, and quality of life (QoL) was performed using the PubMed® database. Because Embase® and APA PsycINFO® do not use MeSH terms, keyword-only searches were performed for these databases; where appropriate, wildcard searches were performed to complement keyword-only searches. The ISPOR database does not permit wildcard searches; therefore, a single-word search (“schizophrenia”) was conducted on article titles, abstracts, and keywords. To verify the robustness of this search, an additional search was performed using alternative terms (“schizoaffective”), which yielded results already included in the first search. A summary of the search terms used can be found in Supplementary Table 1.
Studies were included in this analysis if they met the following criteria: published during or after 2010; included patients with existing schizophrenia or schizoaffective disorder diagnosis; and reported on or used a patient-level outcome (patient-reported, observer/clinician-reported) for measuring disease progression, severity, and/or treatment effectiveness in schizophrenia. Any outcomes published only prior to 2010 were not considered for the purposes of this review.
An identified study was excluded from this analysis if the study included patients without schizophrenia or other relevant diagnosis; reported the outcomes of caregivers, partners, or relatives of patients with schizophrenia, but not patient outcomes; reported outcomes in “natural units” (e.g., biologic- or chemical-level, imaging results, life expectancy, hospitalization rate) rather than from an assessment tool or scale; measured the effects of secondary health conditions (e.g., smoking cessation, treatment-related diabetes, or high blood pressure); solely assessed medication adherence; reported utilities (e.g., for calculating quality adjusted life-years) from secondary data; evaluated costs or economic burden/cost minimization; was written as an editorial on clinical outcome assessments in schizophrenia; or not published in English.
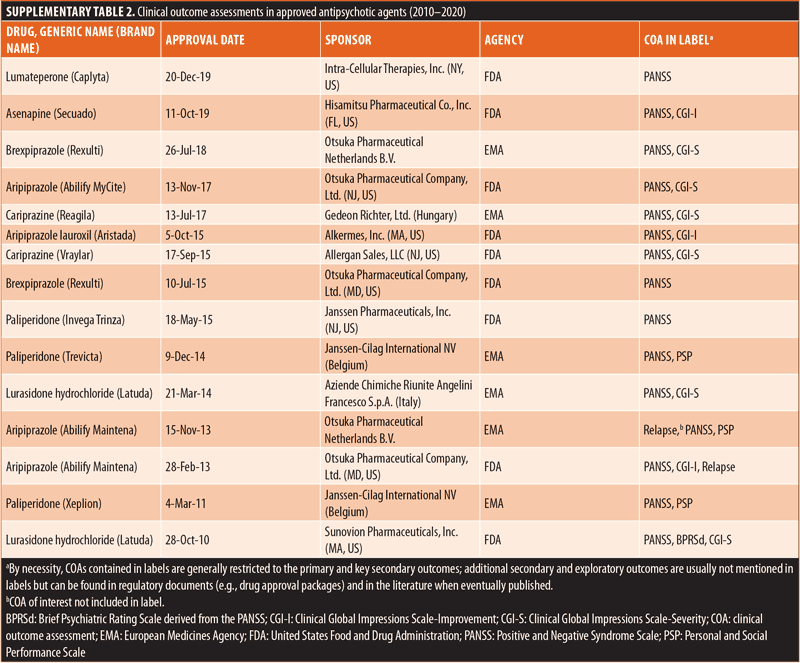
Additional secondary sources were reviewed to support the primary literature search. ClinicalTrials.gov was examined for information concerning ongoing or completed schizophrenia-related, interventional, Phase III trials to identify clinical outcome assessments not already identified by the primary literature search. PROLABELS™ (through ePROVIDE™) was searched for the labels of medications approved within the last ten years (2010–2020) by the FDA and European Medicines Agency (EMA) for the treatment of schizophrenia. The identified labels were searched for additional clinical outcome assessments that could be included in this review. The FDA’s website (FDA.gov) was also reviewed for schizophrenia-related submissions to the FDA Clinical Outcome Assessment (COA) Qualification Program. Studies that were known to investigators prior to the literature search were included in this scoping review if they met the inclusion and exclusion criteria.
Data synthesis plan. Clinical outcome assessments that were identified in the scoping review were categorized according to three factors. Each category was divided into various subcategories to appropriately describe the nature of each outcome assessment. The three factors were: 1) who reported the outcome assessment (type), divided into ClinROs, ObsROs, and PROs; 2) focus or intended use of the outcome assessments (focus), divided into generic, mental health, and schizophrenia; and 3) domain of the outcome (domain), divided into QoL/health-related QoL (HRQoL), treatment-related, emotional/psychological wellbeing, symptomatic, cognition, and need for care (Figure 1).
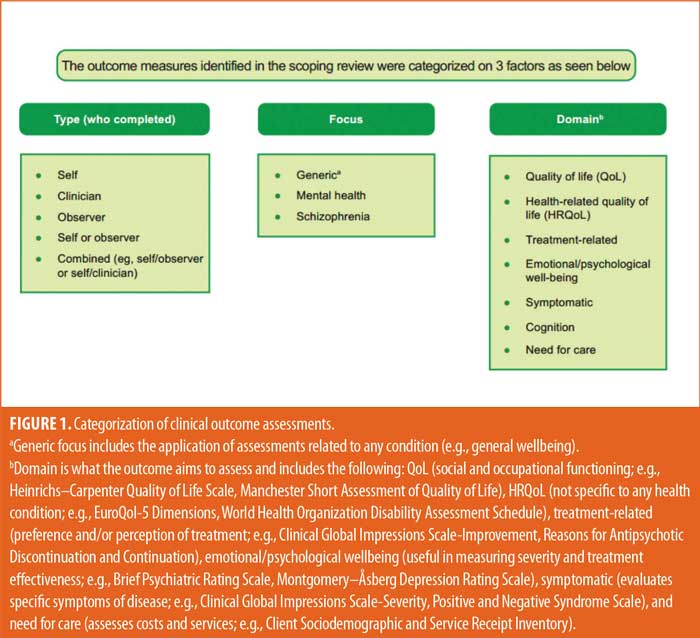
The psychometric properties for all identified clinical outcome assessments were identified to the extent that the literature allowed. The original development and original validation or schizophrenia-specific validation studies were located for each identified outcome assessment, if available. Where possible, the reliability and validity of each outcome assessment, as well as a brief interpretation of each scale or scoring system, were extracted. The performances of outcome assessments were evaluated according to the Consensus‐Based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines.17 Internal consistency (reliability) was evaluated by the Cronbach’s α method. Reliability was determined to be good if α was 0.8 or greater (+++), acceptable if α ranged from 0.6 to 0.79 (++), and poor or unacceptable if α was less than 0.6 (+/-). From each identified study, the reported intraclass correlation coefficients (ICCs) were assessed for each outcome assessment to determine the external validity. External validity was considered to be strong if ICC was 0.5 or greater (+++), moderate if ICC was 0.25 to 0.49 (++), and weak if ICC was less than 0.25 (+). Furthermore, information on MCIDs was extracted when possible, making note of the treatment modalities.
Results
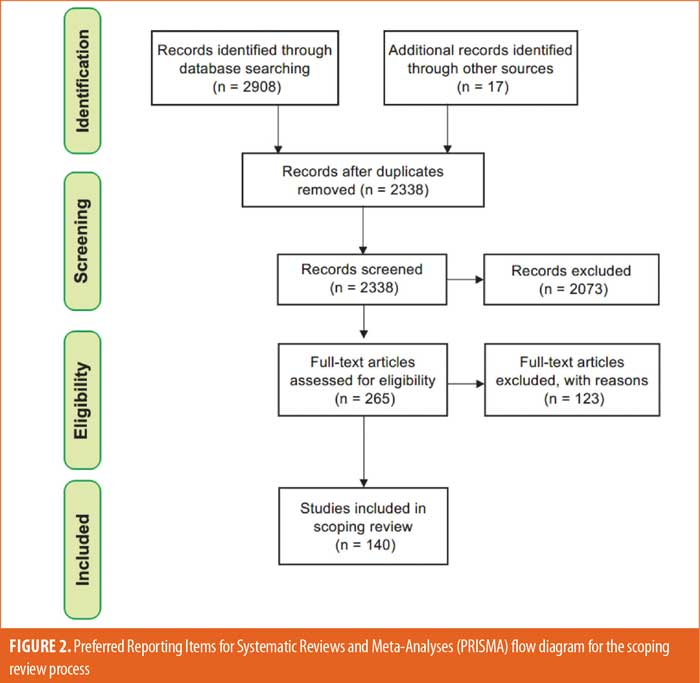
Literature search. Overall, 2,908 studies were identified by searching the key databases, and 17 studies of interest were identified through known sources prior to the primary literature search (Figure 2). After removing duplicate studies, the titles and abstracts of the remaining 2,329 studies were screened against the inclusion and exclusion criteria. The initial screening yielded 256 studies, which then underwent a full-text review for eligibility. Of those 256 studies, 140 studies were included in this scoping review.11,18–156 Our search of ClinicalTrials.gov identified 294 ongoing or completed randomized controlled trials (RCTs) on schizophrenia with relevant information concerning clinical outcome assessments. A total of 15 EMA-/FDA-approved drugs and the related clinical outcome assessments were extracted from the PROLABELS™ database (Supplementary Table 2). Notably, none of the antipsychotic agents approved between 2010 and 2020 included a mention of PRO endpoints in their respective labels, and all mentioned were ClinROs/ObsROs. Additionally, two outcome assessments (Virtual Reality Functional Capacity Assessment Tool [VRFCAT] and Epidemiological Study of Cognitive Impairment in Schizophrenia [EPICOG]) were identified from schizophrenia-related submissions to the COA Qualification Submissions on the FDA’s website.
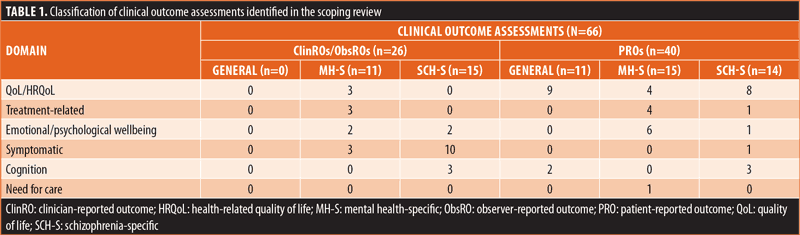
Summary of clinical outcome assessments. A total of 66 clinical outcome assessments were identified from the included studies and secondary sources (Table 1). Of these outcome assessments, 26 were ClinROs/ObsROs and 40 were PROs. Among the 26 ClinROs/ObsROs, there were 11 mental health-specific and 15 schizophrenia-specific outcome assessments; no generic ClinROs/ObsROs were reported in the included literature. Among the 40 PROs, there were 11 generic, 15 mental health-specific, and 14 schizophrenia-specific outcome assessments. Of these generic and schizophrenia-specific outcome assessments, select measures specific to cognition (n=5) were added to supplement and strengthen the cognition domain representation.
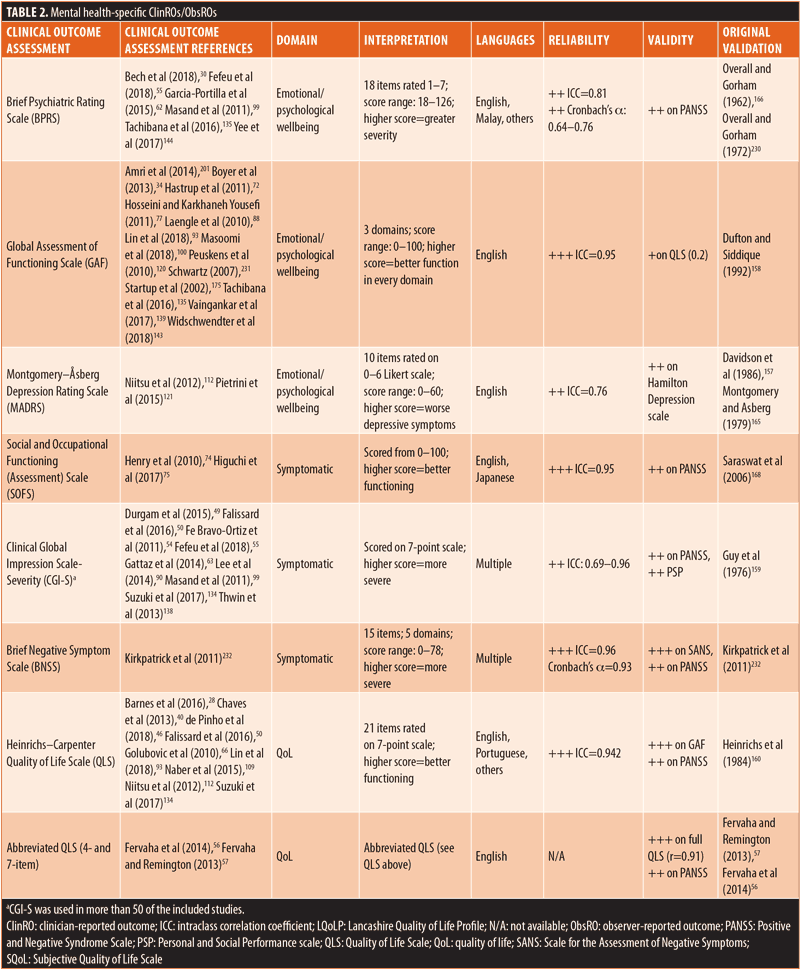
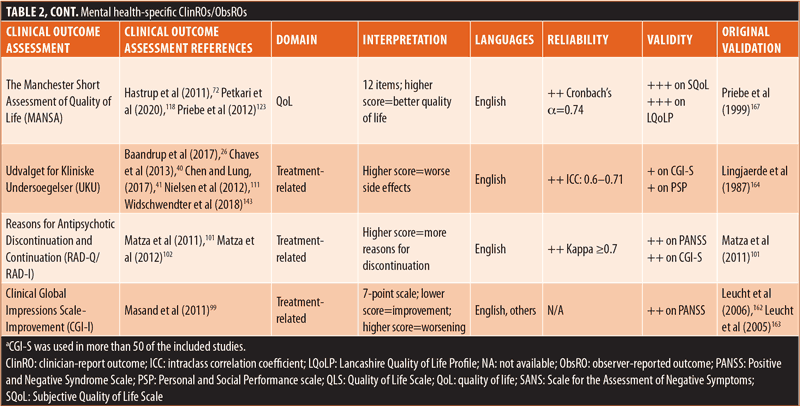
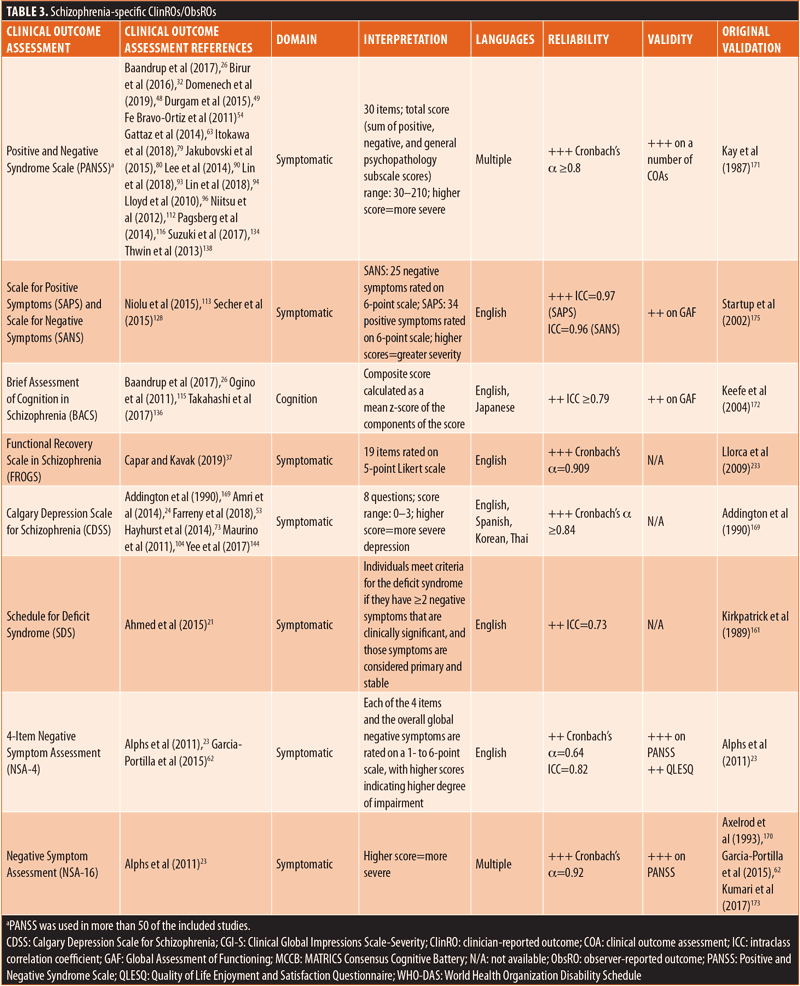
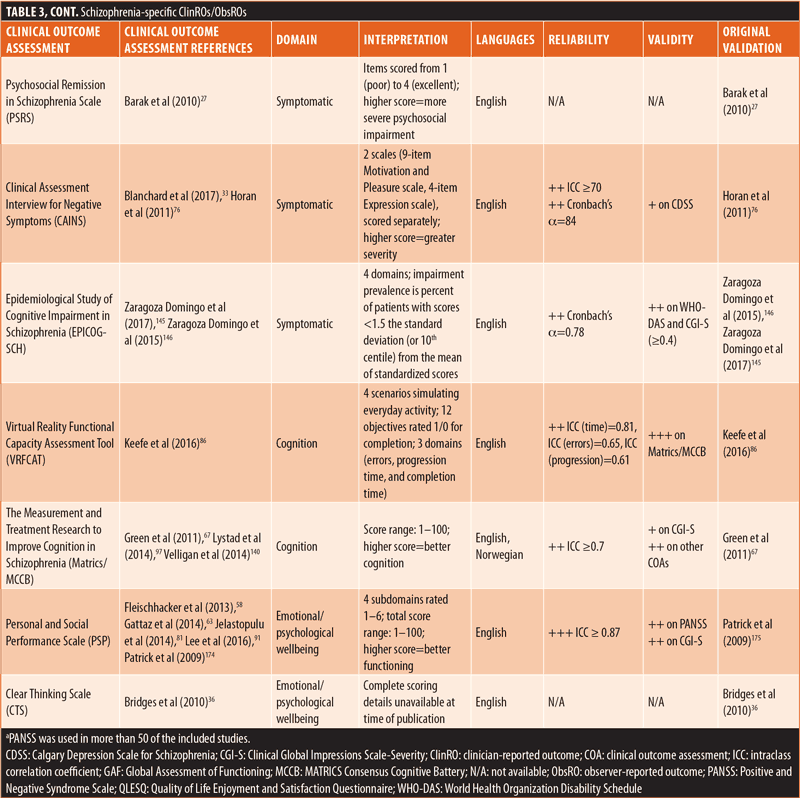
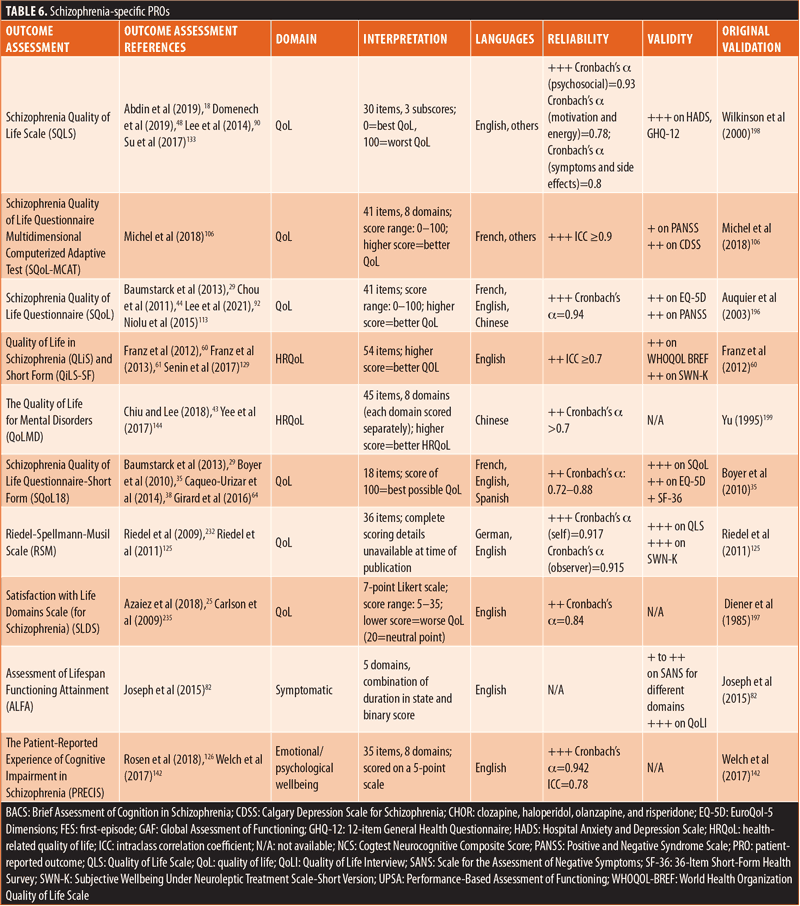
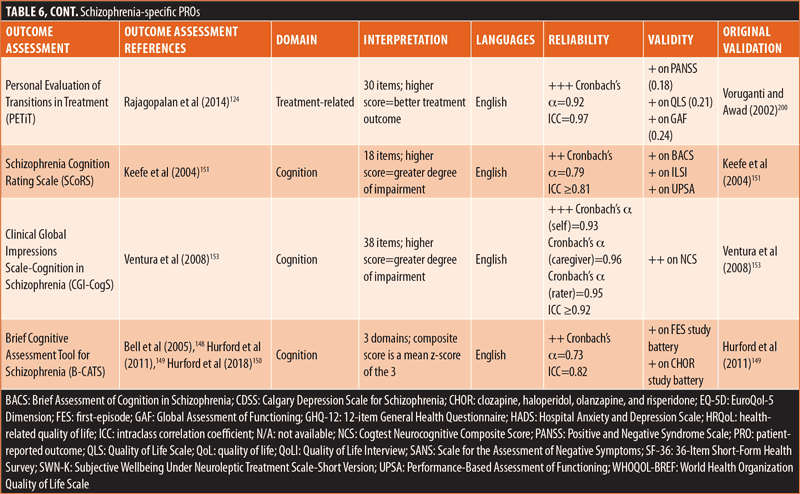
ClinROs/ObsROs. Nearly half (n=11) of the ClinROs/ObsROs were mental health-specific (Table 2). These 11 outcome assessments were distributed between the QoL/HRQoL (n=3), treatment-related (n=3), symptomatic (n=3), and emotional/psychological wellbeing (n=2) domains. Overall, the mental health-specific outcome assessments identified here had relatively high reliability and strong validity, compared to other outcome assessments.56,57,102,157–168 The remaining 15 ClinROs/ObsROs were schizophrenia-specific (Table 3). Of these 15 outcome assessments, 10 focused on symptoms, while three focused on cognition, and two focused on emotional/psychological wellbeing. The reliability and external validity of the identified schizophrenia-specific assessments varied considerably.23,62,67,76,86,145,146,161,169–175 Certain outcome assessments, such as the Positive and Negative Syndrome Scale (PANSS), had good reliability and strong validity, while newly developed outcome assessments generally demonstrated lower reliability and validity. Reliability and external validity were not available for two outcome assessments.27,36
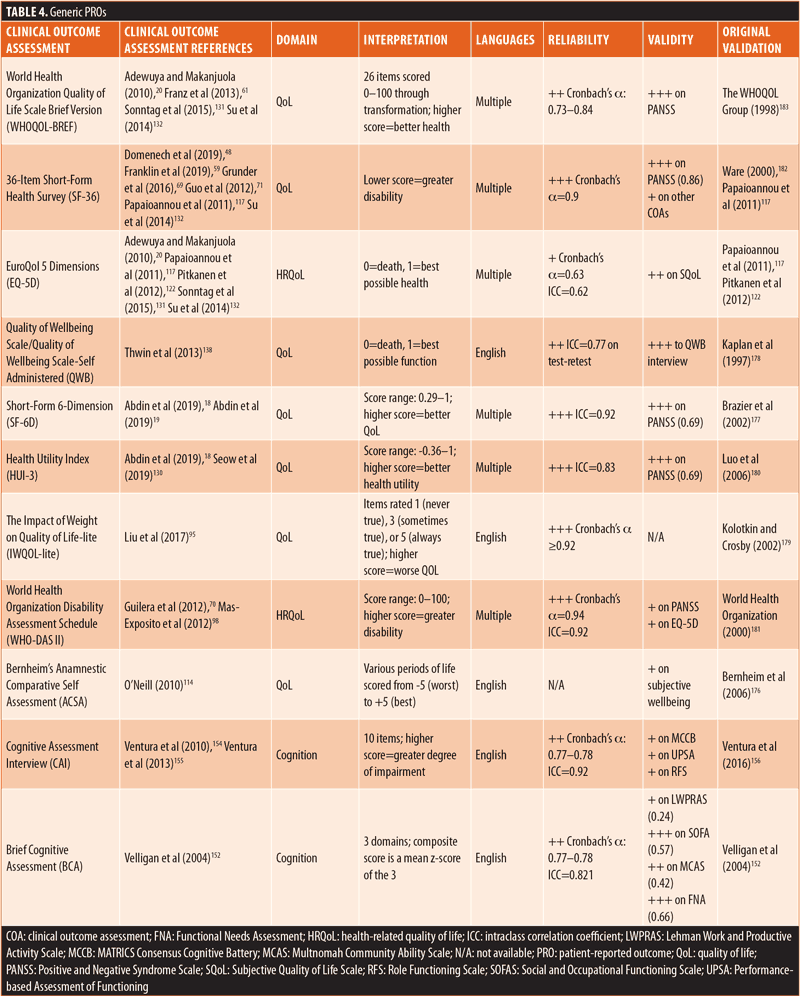
PROs. A summary of the nine generic PROs is presented in Table 4. All generic PROs were focused on QoL/HRQoL or cognition. Most of the identified generic PROs displayed acceptable reliability, with Cronbach’s α of 0.8 or greater.117,122,176–183 External validity, however, was not particularly strong, whereas variation among the generic outcome assessments was quite high.117,122,176–178,180–183
Most (n=15) of the identified PROs were mental health-specific (Table 5). Overall, six assessments focused on emotional/psychological wellbeing, four centered on QoL/HRQoL, four assessed treatment-related factors, and one evaluated the need for care. Similar to the generic PROs, the mental health-specific PROs had good reliability, whereas external validity varied.68,107,109,184–193 However, external validity was not available for several mental health-specific PROs.68,107,188,190 Reliability or external validity were not available for two of the treatment-related PROs and the need for care PRO.45,194,195
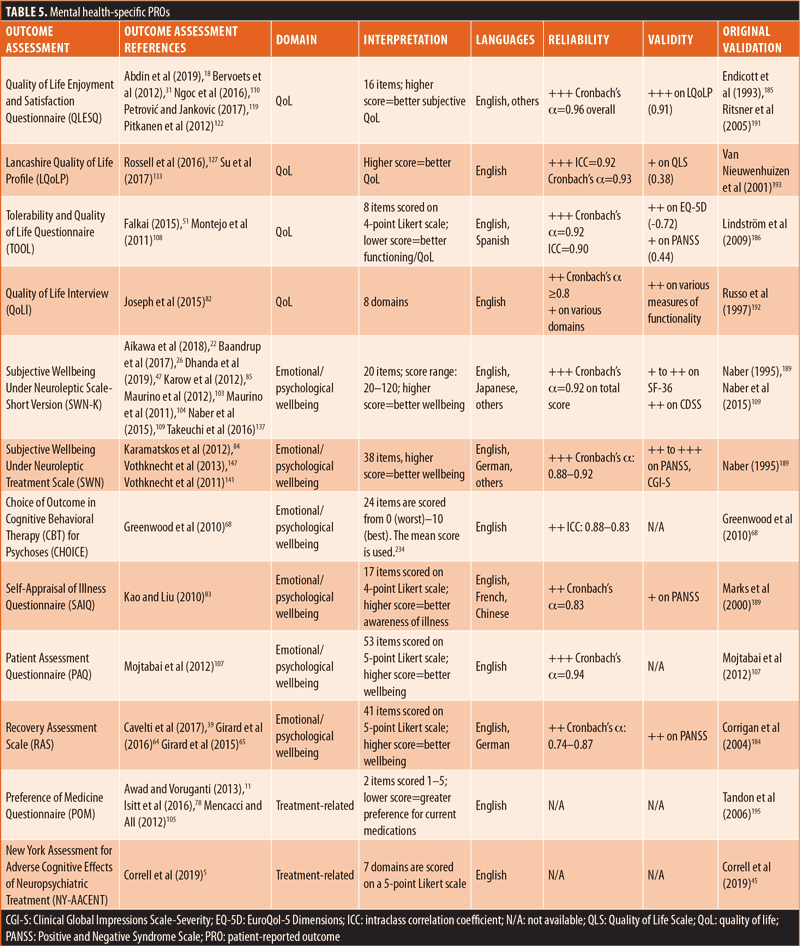
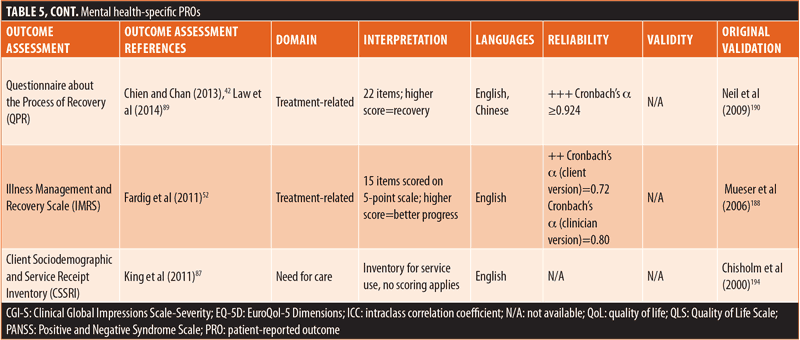
The remaining PROs were comprised of 14 schizophrenia-specific outcome assessments (Table 6). Among these 14 schizophrenia-specific PROs, more than half (n=8) evaluated QoL/HRQoL and three evaluated cognition. The remaining three schizophrenia-specific PROs were evenly distributed between the treatment-related, emotional/psychological wellbeing, and symptomatic domains. Generally, the QoL/HRQoL PROs were reliable (Cronbach’s α ≥0.8) and had strong validity.35,60,106,125,196–199 The treatment-related schizophrenia-specific PRO had good reliability and acceptable external validity.200 The emotional/psychological wellbeing schizophrenia-specific PRO had good reliability but lacked data on external validity.142 The symptomatic schizophrenia-specific PRO had variable external validity but lacked data on reliability.82
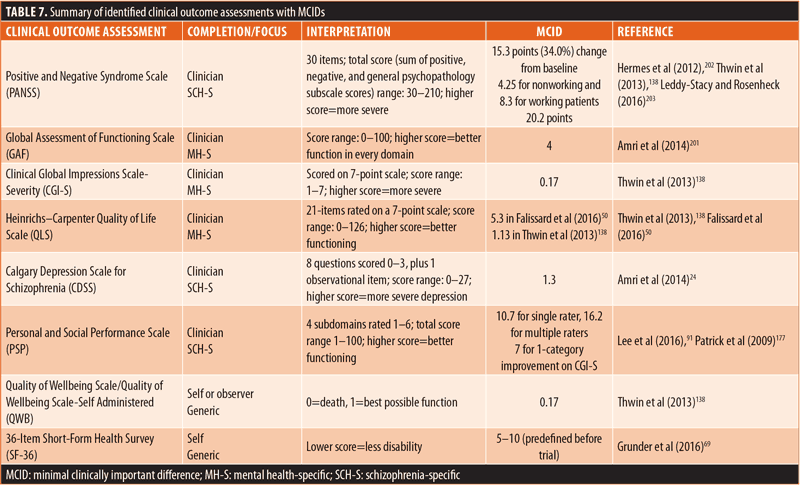
MCIDs. MCIDs were reported for eight of the 66 identified outcome assessments (Table 7).24,50,69,91,138,201–203 Of these eight outcome assessments, six were ClinROs/ObsROs (3 mental health-specific and 3 schizophrenia-specific), while only two were PROs (both generic). One of the outcome assessments, the 36-item Short Form Health Survey (SF-36), reported predetermined MCIDs rather than derived values.69
Discussion
This analysis of schizophrenia-related literature identified 66 individual clinical outcome assessments, which represented patient- and nonpatient-reported outcome assessments, as well as various domains and focuses of outcome assessments for patients with schizophrenia. An advantage of a scoping review over a systematic review, in this context, was the ability to include a broader range of studies and review a larger share of the published literature. This benefit was reflected in the greater number of studies and outcome assessments that we evaluated in this review, as compared to previous efforts over the past decade.25,204,205
The findings of this scoping review highlighted the heterogeneity of existing assessments in terms of scope, focus, and performance. While the reliability of most of the identified ClinROs/ObsROs was good, external validity varied considerably. Weak external validity was noted among newly developed schizophrenia-specific outcome assessments, suggesting a need for further validation through additional research. Overall, mental health- and schizophrenia-specific PROs demonstrated strong psychometric properties (reliability and external validity); these findings were consistent with previous studies.130
The presence of published MCID values for only eight of the 66 identified outcome assessments indicates a substantial gap in knowledge. In particular, none of the schizophrenia-specific PROs had reported MCID values. Additionally, searches performed using the PROLABELS™ database confirmed that PRO endpoints were not mentioned in the labels of any antipsychotic agents approved over the last decade (2010–2020), further highlighting this gap.
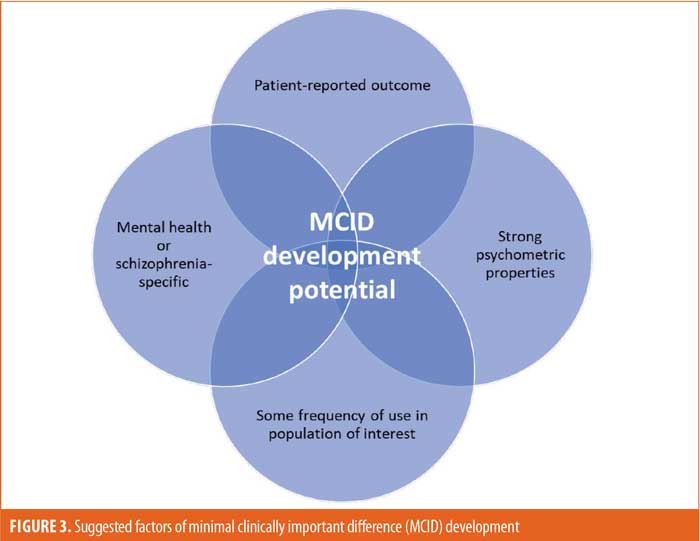
While MCIDs are needed to help guide clinical practice and assess treatment effectiveness in research, the development of MCIDs requires substantial investments in research and trial resources. There is no consensus on a universal method for calculating MCIDs for PROs; anchor-based and distributive methods have been the most commonly used for the calculation of MCIDs, with distributive methods being the preferred method in schizophrenia.206,207 By definition, PROs rely on self-report by patients, and the issue of reliability of PROs for MCID calculation has been raised in the literature.207 The main criticism has been that MCIDs based on PROs are unreliable in a population of patients with schizophrenia because of discrepancies between the perceptions of patients and those of other raters (i.e., clinician, observer, or proxy [e.g., family, caregiver]).208 Therefore, the selection of outcome measures should be considered carefully. In this review, criteria for the selection of an outcome measure for MCID evaluation that prioritized multiple aspects of an outcome measure (PROs, mental health- or schizophrenia-specific measures with strong psychometric properties, evidence of use in patients with schizophrenia, and established psychometric properties) were proposed. The application of this criteria to identified outcome measures resulted in a list of recommended outcome assessments for MCID research development. However, the list of suggested outcome measures for MCID evaluation is not exhaustive and only considers the outcome measures that met our recommendation criteria in the strictest sense.
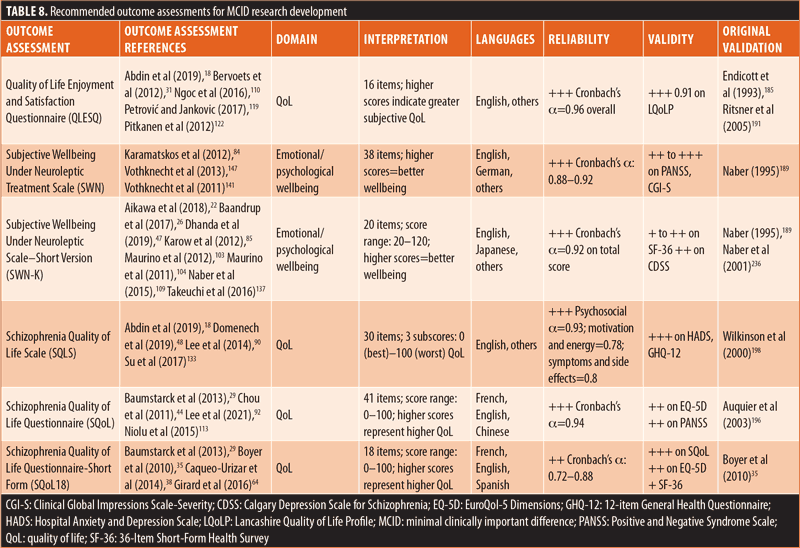
Recommendations for MCID research. Based on the findings of this review, it is recommended that researchers consider the following priorities when selecting an outcome measure for MCID evaluation (Figure 3): type (prioritize PROs because of the lack of reported MCIDs among existing outcome measures), focus (prioritize mental health- or schizophrenia-specific measures based on the strong psychometric properties observed in this scoping review), evidence of use in patients with schizophrenia, and established and acceptable psychometric properties. By applying these criteria, three mental health-specific PROs were identified that should be evaluated for MCIDs: Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ), Subjective Wellbeing Under Neuroleptic Treatment Scale (SWN), and SWN–Short Version (SWN-K) (Table 8). Furthermore, three schizophrenia-specific PROs with potential for MCID evaluation were identified: Schizophrenia Quality of Life Scale (SQLS), Schizophrenia Quality of Life Questionnaire (SQoL), and SQoL–Short Form (SQoL18; Table 8). As of May 2020, the QLESQ, SWN, and SQLS were being utilized in ongoing Phase III trials indexed on ClinicalTrials.gov.209–229
Limitations. The literature search was limited to studies published during or after 2010 and written in English. The review may have missed relevant outcome assessments used in earlier studies or published in other languages. However, if these missed outcomes were not used in the last decade or were not published in the de facto language of most regulatory agencies and medical research, the impact of their exclusion from our review can be considered minimal. In addition, some outcome assessments lacked reliability and/or validity, which makes comparisons with other assessments difficult. Please note that the categories of mental health- and schizophrenia-specific can appear arbitrary for those measures that can be employed transdiagnostically. Lastly, the strength of the reliability and validity of outcome assessments were only determined with Cronbach’s α method and ICC, respectively, which may have limited the breadth of this review.
Conclusion
This scoping review provided a comprehensive overview of the clinical outcome assessments utilized during the past ten years in the field of schizophrenia and related disorders. The 66 identified outcome assessments were found to be of varying focus, scope, and validity. A large portion of these outcome assessments were patient-reported, indicating the importance of a growing implementation of PROs in the field of schizophrenia. However, none of the schizophrenia-specific PROs had estimated MCIDs, suggesting significant gaps in knowledge and opportunities for future research. Mental health- and schizophrenia-specific PROs overall demonstrated strong psychometric properties (reliability and external validity), and mental health-specific ClinROs/ObsROs generally had good reliability. Additional research is warranted to interpret individual and group level changes in the most sensitive disease-specific PROs.
Acknowledgments
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Adel Chowdhury, PharmD; Mark Skopin, PhD, CMPP; Jennifer C. Jaworski, MS, BCMAS, CMPP; and Frederique H. Evans, MBS, all of whom are with Ashfield MedComms, an Inizio company. Medical writing and editorial support were funded by Teva Branded Pharmaceutical Products R&D, Inc.
Author Contributions
All authors contributed to the conceptual design and writing of the manuscript. All authors have approved the final manuscript.
References
- Kane J, Correll CU, Tohami O, et al. TV-46000 provided continued symptom improvement in patients with schizophrenia during the phase 3, multicenter, randomized, double-blind, placebo-controlled relapse prevention RISE study. Presented at the 34th Annual Psych Congress; Oct 29–Nov 1 2021: San Antonio, Texas.
- Thornicroft G, Slade M. New trends in assessing the outcomes of mental health interventions. World Psychiatry. 2014;13(2):118–124.
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77(2):201–210.
- Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014;39(9):638–645.
- Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci. 2011;13(2):155–172.
- Leucht S, Heres S, Hamann J, Kane JM. Methodological issues in current antipsychotic drug trials. Schizophr Bull. 2008;34(2):275–285.
- Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144.
- Mercieca-Bebber R, King MT, Calvert MJ, et al. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367.
- United States Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Devices and Radiological Health (CDRH). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Dec 2009. https://www.fda.gov/media/77832/download. Accessed 5 Apr 2023.
- Durand D, Strassnig M, Sabbag S, et al. Factors influencing self-assessment of cognition and functioning in schizophrenia: implications for treatment studies. Eur Neuropsychopharmacol. 2015;25(2):185–191.
- Awad AG, Voruganti LN. The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia. CNS Drugs. 2013;27(8):625–636.
- Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494.
- Kristensen S, Mainz J, Baandrup L, et al. Conceptualizing patient-reported outcome measures for use within two Danish psychiatric clinical registries: description of an iterative co-creation process between patients and healthcare professionals. Nord J Psychiatry. 2018;72(6):409–419.
- Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther. 2012;20(3):160–166.
- Peters MD, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–146.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473.
- Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157.
- Abdin E, Chong SA, Seow E, et al. A comparison of the reliability and validity of SF-6D, EQ-5D and HUI3 utility measures in patients with schizophrenia and patients with depression in Singapore. Psychiatry Res. 2019;274:400–408.
- Abdin E, Chong SA, Seow E, et al. Mapping the Positive and Negative Syndrome Scale scores to EQ-5D-5L and SF-6D utility scores in patients with schizophrenia. Qual Life Res. 2019;28(1):177–186.
- Adewuya AO, Makanjuola RO. Subjective life satisfaction and objective living conditions of patients with schizophrenia in Nigeria. Psychiatr Serv. 2010;61(3):314–316.
- Ahmed AO, Strauss GP, Buchanan RW, et al. Are negative symptoms dimensional or categorical? Detection and validation of deficit schizophrenia with taxometric and latent variable mixture models. Schizophr Bull. 2015;41(4):879–891.
- Aikawa S, Kobayashi H, Nemoto T, et al. Social anxiety and risk factors in patients with schizophrenia: relationship with duration of untreated psychosis. Psychiatry Res. 2018;263:94–100.
- Alphs L, Morlock R, Coon C, et al. Validation of a 4-item Negative Symptom Assessment (NSA-4): a short, practical clinical tool for the assessment of negative symptoms in schizophrenia. Int J Methods Psychiatr Res. 2011;20(2):e31–e37.
- Amri I, Millier A, Toumi M. Minimum clinically important difference in the Calgary Depression Scale for Schizophrenia. Value Health. 2014;17(7):A766.
- Azaiez C, Millier A, Lancon C, et al. Health related quality of life in patients having schizophrenia negative symptoms – a systematic review. J Mark Access Health Policy. 2018;6(1):1517573.
- Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well-being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add-on melatonin versus placebo. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):163–171.
- Barak Y, Bleich A, Aizenberg D. Psychosocial remission in schizophrenia: developing a clinician-rated scale. Compr Psychiatry. 2010;51(1):94–98.
- Barnes TR, Leeson VC, Paton C, et al. Antidepressant Controlled Trial for Negative Symptoms In Schizophrenia (ACTIONS): a double-blind, placebo-controlled, randomised clinical trial. Health Technol Assess. 2016;20(29):1–46.
- Baumstarck K, Boyer L, Boucekine M, et al. Self-reported quality of life measure is reliable and valid in adult patients suffering from schizophrenia with executive impairment. Schizophr Res. 2013;147(1):58–67.
- Bech P, Austin SF, Timmerby N, et al. A clinimetric analysis of a BPRS-6 scale for schizophrenia severity. Acta Neuropsychiatr. 2018;30(4):187–191.
- Bervoets C, Morrens M, Vansteelandt K, et al. Effect of aripiprazole on verbal memory and fluency in schizophrenic patients: results from the ESCAPE study. CNS Drugs. 2012;26(11):975–982.
- Birur B, Thirthalli J, Janakiramaiah N, et al. Dimensions of schizophrenia and their time course of response to a second generation antipsychotic olanzapine-a clinical study. Asian J Psychiatr. 2016;24:17–22.
- Blanchard JJ, Bradshaw KR, Garcia CP, et al. Examining the reliability and validity of the Clinical Assessment Interview for Negative Symptoms within the Management of Schizophrenia in Clinical Practice (MOSAIC) multisite national study. Schizophr Res. 2017;185:137–143.
- Boyer L, Richieri R, Guedj E, et al. Validation of a functional remission threshold for the Functional Remission of General Schizophrenia (FROGS) scale. Compr Psychiatry. 2013;54(7):1016–1022.
- Boyer L, Simeoni MC, Loundou A, et al. The development of the S-QoL 18: a shortened quality of life questionnaire for patients with schizophrenia. Schizophr Res. 2010;121(1–3):241–250.
- Bridges IF, Chan KS, Stuart EA. The reliability and validity of the Clear Thinking Scale (CTS) among patients diagnosed with schizophrenia. Value Health. 2010;13(3):A119.
- Capar M, Kavak F. Effect of internalized stigma on functional recovery in patients with schizophrenia. Perspect Psychiatr Care. 2019;55(1):103–111.
- Caqueo-Urizar A, Boyer L, Boucekine M, Auquier P. Spanish cross-cultural adaptation and psychometric properties of the Schizophrenia Quality of Life short-version questionnaire (SQoL18) in 3 middle-income countries: Bolivia, Chile and Peru. Schizophr Res. 2014;159(1):136–143.
- Cavelti M, Wirtz M, Corrigan P, Vauth R. Recovery assessment scale: examining the factor structure of the German version (RAS-G) in people with schizophrenia spectrum disorders. Eur Psychiatry. 2017;41:60–67.
- Chaves KM, Serrano-Blanco A, Ribeiro SB, et al. Quality of life and adverse effects of olanzapine versus risperidone therapy in patients with schizophrenia. Psychiatr Q. 2013;84(1):125–135.
- Chen KP, Lung FW. Reliability and validity of the short version of Udvalg for Kliniske Undersogelser in antipsychotic treatment. Psychiatr Q. 2017;88(4):787–796.
- Chien WT, Chan ZC. Chinese translation and validation of the questionnaire on the process of recovery in schizophrenia and other psychotic disorders. Res Nurs Health. 2013;36(4):400–411.
- Chiu EC, Lee SC. Factor structure of the quality of life scale for mental disorders in patients with schizophrenia. J Nurs Res. 2018;26(3):185–190.
- Chou CY, Ma MC, Yang TT, Chen-Sea MJ. Psychometric validation of the S-QoL Chinese (Taiwan) version for patients with schizophrenia. Qual Life Res. 2011;20(5):763–767.
- Correll C, Kohegyi E, Baker R, et al. T45. The New York assesment of adverse cognitive effects of neuropsyhiatric treatment (NY-AACENT): intitial validation findings. Schizophr Bull. 2019;45(Suppl 2):S221.
- de Pinho LMG, Pereira AMS, Chaves CMCB, Batista P. Quality of Life Scale and symptomatology of schizophrenic patients–a systematic review. Eur J Psychiatry. 2018;32(1):1–10.
- Dhanda R, Varghese D, Nadipelli VR, et al. Patient-reported outcomes in schizophrenia patients treated with once-monthly extended-release risperidone in a long-term clinical study. Patient Prefer Adherence. 2019;13:1037–1050.
- Domenech C, Pastore A, Altamura AC, et al. Correlation of health-related quality of life in clinically stable outpatients with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:3475–486.
- Durgam S, Cutler AJ, Lu K, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574–e1582.
- Falissard B, Sapin C, Loze JY, et al. Defining the minimal clinically important difference (MCID) of the Heinrichs-Carpenter quality of life scale (QLS). Int J Methods Psychiatr Res. 2016;25(2):101–111.
- Falkai P. C.05.02 Assessment of functioning and quality of life in schizophrenia and the relevance for clinical practice. Eur Neuropsychopharmacol. 2015;25(Suppl 2):S665–S666.
- Fardig R, Lewander T, Fredriksson A, Melin L. Evaluation of the Illness Management and Recovery Scale in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;132(2–3):157–164.
- Farreny A, Savill M, Priebe S. Correspondence between negative symptoms and potential sources of secondary negative symptoms over time. Eur Arch Psychiatry Clin Neurosci. 2018;268(6):603–609.
- Fe Bravo-Ortiz M, Gutiérrez-Casares JR, Rodríguez-Morales A, et al. Influence of type of treatment on the well-being of Spanish patients with schizophrenia and their caregivers. Int J Psychiatry Clin Pract. 2011;15(4):286–295.
- Fefeu M, De Maricourt P, Cachia A, et al. One-year mirror-image study of the impact of olanzapine long-acting injection on healthcare resource utilization and costs in severe schizophrenia. Psychiatry Res. 2018;270:205–210.
- Fervaha G, Foussias G, Siddiqui I, et al. Abbreviated quality of life scales for schizophrenia: comparison and utility of two brief community functioning measures. Schizophr Res. 2014;154(1–3):89–92.
- Fervaha G, Remington G. Validation of an abbreviated quality of life scale for schizophrenia. Eur Neuropsychopharmacol. 2013;23(9):1072–1077.
- Fleischhacker WW, Perry P, Sanchez R, et al. Functional outcomes with aripiprazole once-monthly in two double-blind, placebo- and active-controlled studies (Aspire US 246 and Aspire EU 247) for the treatment of schizophrenia. Value Health. 2013;16(7):A550.
- Franklin M, Mukuria C, Mulhern B, et al. Measuring the burden of schizophrenia using clinician and patient-reported measures: an exploratory analysis of construct validity. Patient. 2019;12(4):405–417.
- Franz M, Fritz M, Gallhofer B, Meyer T. QLiS—development of a schizophrenia-specific quality-of-life scale. Health Qual Life Outcomes. 2012;10:61.
- Franz M, Fritz M, Meyer T. Discriminant and convergent validity of a subjective quality-of-life instrument aimed at high content validity for schizophrenic persons. Qual Life Res. 2013;22(5):1113–1122.
- Garcia-Portilla MP, Garcia-Alvarez L, Saiz PA, et al. Psychometric evaluation of the negative syndrome of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265(7):559–566.
- Gattaz WF, Campos JA, Lacerda AL, et al. Switching from oral risperidone to flexibly dosed oral paliperidone extended-release: core symptoms, satisfaction, and quality of life in patients with stable but symptomatic schizophrenia: the RISPALI study. Curr Med Res Opin. 2014;30(4):695–709.
- Girard V, Tinland A, Boucekine M, et al. Validity of a common quality of life measurement in homeless individuals with bipolar disorder and schizophrenia. J Affect Disord. 2016;204:131–137.
- Girard V, Tinland A, Mohamed EH, et al. Psychometric properties of the recovery measurement in homeless people with severe mental illness. Schizophr Res. 2015;169(1–3):292–297.
- Golubovic B, Misic-Pavkov G, Gajic Z, Ivanovic-Kovacevic S. Evaluation of quality of life for patients with schizophrenic and schizoaffective disorders related to the use of antipsychotic therapy. Med Arh. 2010;64(1):37–40.
- Green MF, Schooler NR, Kern RS, et al. Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry. 2011;168(4):400–407.
- Greenwood KE, Sweeney A, Williams S, et al. CHoice of Outcome In Cbt for psychosEs (CHOICE): the development of a new service user-led outcome measure of CBT for psychosis. Schizophr Bull. 2010;36(1):126–135.
- Grunder G, Heinze M, Cordes J, et al. Effects of first-generation antipsychotics versus second-generation antipsychotics on quality of life in schizophrenia: a double-blind, randomised study. Lancet Psychiatry. 2016;3(8):717–729.
- Guilera G, Gomez-Benito J, Pino O, et al. Utility of the World Health Organization Disability Assessment Schedule II in schizophrenia. Schizophr Res. 2012;138(2-3):240–247.
- Guo X, Zhang Z, Zhai J, et al. Effects of antipsychotic medications on quality of life and psychosocial functioning in patients with early-stage schizophrenia: 1-year follow-up naturalistic study. Compr Psychiatry. 2012;53(7):1006–1012.
- Hastrup L, Nordentoft M, Hjorthoj C, Gyrd-Hansen D. Does the EQ-5D measure quality of life in schizophrenia? J Ment Health Policy Econ. 2011;14(4):187–196.
- Hayhurst KP, Massie JA, Dunn G, et al. Validity of subjective versus objective quality of life assessment in people with schizophrenia. BMC Psychiatry. 2014;14:365.
- Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716–728.
- Higuchi Y, Sumiyoshi T, Seo T, et al. Associations between daily living skills, cognition, and real-world functioning across stages of schizophrenia; a study with the Schizophrenia Cognition Rating Scale Japanese version. Schizophr Res Cogn. 2017;7:13–18.
- Horan WP, Kring AM, Gur RE, et al. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132(2–3):140–145.
- Hosseini SH, Karkhaneh Yousefi M. Quality of life and GAF in schizophrenia correlation between quality of life and global functioning in schizophrenia. Iran J Psychiatry Behav Sci. 2011;5(2):120–125.
- Isitt JJ, Nadipelli VR, Kouassi A, et al. Health-related quality of life in acute schizophrenia patients treated with RBP-7000 once monthly risperidone: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. Schizophr Res. 2016;174(1–3):126–131.
- Itokawa M, Miyashita M, Arai M, et al. Pyridoxamine: a novel treatment for schizophrenia with enhanced carbonyl stress. Psychiatry Clin Neurosci. 2018;72(1):35–44.
- Jakubovski E, Carlson JP, Bloch MH. Prognostic subgroups for remission, response, and treatment continuation in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial. J Clin Psychiatry. 2015;76(11):1535–1545.
- Jelastopulu E, Giourou E, Merekoulias G, et al. Correlation between the Personal and Social Performance scale (PSP) and the Positive and Negative Syndrome Scale (PANSS) in a Greek sample of patients with schizophrenia. BMC Psychiatry. 2014;14:197.
- Joseph J, Kremen WS, Glatt SJ, et al. Assessment of Lifespan Functioning Attainment (ALFA) scale: a quantitative interview for self-reported current and functional decline in schizophrenia. J Psychiatr Res. 2015;65:102–107.
- Kao YC, Liu YP. The clinical applicability of the Self-Appraisal of Illness Questionnaire (SAIQ) to chronic schizophrenic patients in Taiwan. Psychiatr Q. 2010;81(3):215–225.
- Karamatskos E, Mulert C, Lambert M, Naber D. Subjective well-being of patients with schizophrenia as a target of drug treatment. Curr Pharm Biotechnol. 2012;13(8):1490–1499.
- Karow A, Naber D, Lambert M, et al. Remission as perceived by people with schizophrenia, family members and psychiatrists. Eur Psychiatry. 2012;27(6):426–431.
- Keefe RSE, Davis VG, Atkins AS, et al. Validation of a computerized test of functional capacity. Schizophr Res. 2016;175(1-3):90–96.
- King D, Knapp M, Thomas P, et al. Cost-effectiveness analysis of aripiprazole vs standard-of-care in the management of community-treated patients with schizophrenia: STAR study. Curr Med Res Opin. 2011;27(2):365–374.
- Laengle G, Bayer W, Eschweiler G, et al. PW01-186 – effects of longterm treatment with atypical neuroleptics for patients with schizophrenia (ELAN): medication use, adherence, functional impairment, quality of life. Eur Psychiatry. 2010;25(S1):1-1.
- Law H, Neil ST, Dunn G, Morrison AP. Psychometric properties of the questionnaire about the process of recovery (QPR). Schizophr Res. 2014;156(2–3):184–189.
- Lee NY, Kim SH, Cho SJ, et al. A prospective, open-label study to evaluate symptomatic remission in schizophrenia with risperidone long-acting injectable in Korea. Int Clin Psychopharmacol. 2014;29(5):279–287.
- Lee SC, Tang SF, Lu WS, et al. Minimal detectable change of the Personal and Social Performance scale in individuals with schizophrenia. Psychiatry Res. 2016;246:725–729.
- Lee SJ, Lawrence R, Bryce S, et al. Emotional discomfort mediates the relationship between self-efficacy and subjective quality of life in people with schizophrenia. J Ment Health. 2021;30(1):20–26.
- Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2018;84(6):422–432.
- Lin CH, Lin HS, Lin SC, et al. Early improvement in PANSS-30, PANSS-8, and PANSS-6 scores predicts ultimate response and remission during acute treatment of schizophrenia. Acta Psychiatr Scand. 2018;137(2):98–108.
- Liu Y, Crosby R, Sun X, et al. SU128. Psychometric properties of the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire in a clinical trial sample in schizophrenia. Schizophr Bull. 2017;43(Suppl 1):S208.
- Lloyd K, Latif MA, Simpson S, Shrestha KL. Switching stable patients with schizophrenia from depot and oral antipsychotics to long-acting injectable risperidone: efficacy, quality of life and functional outcome. Hum Psychopharmacol. 2010;25(3):243–252.
- Lystad JU, Falkum E, Mohn C, et al. The MATRICS Consensus Cognitive Battery (MCCB): performance and functional correlates. Psychiatry Res. 2014;220(3):1094–1101.
- Mas-Exposito L, Amador-Campos JA, Gomez-Benito J, Lalucat-Jo L. The World Health Organization Short Disability Assessment Schedule: a validation study in patients with schizophrenia. Compr Psychiatry. 2012;53(2):208–216.
- Masand P, O’Gorman C, Mandel FS. Clinical Global Impression of Improvement (CGI-I) as a valid proxy measure for remission in schizophrenia: analyses of ziprasidone clinical study data. Schizophr Res. 2011;126(1–3):174–183.
- Masoomi M, Shadloo B, Nedjat S, et al. Validity and reliability of the Persian version of the “Quality of Life Scale” in schizophrenia. Iran J Psychiatry Behav Sci. 2018;12(3):e67632.
- Matza LS, Phillips GA, Revicki DA, et al. Development of a clinician questionnaire and patient interview to assess reasons for antipsychotic discontinuation. Psychiatry Res. 2011;189(3):463–468.
- Matza LS, Phillips GA, Revicki DA, et al. Validation of a clinician questionnaire to assess reasons for antipsychotic discontinuation and continuation among patients with schizophrenia. Psychiatry Res. 2012;200(2–3):835–842.
- Maurino J, Cordero L, Ballesteros J. The subjective well-being under neuroleptic scale – short version (SWN-K) and the SF-36 health survey as quality of life measures in patients with schizophrenia. Patient Prefer Adherence. 2012;6:83–85.
- Maurino J, Sanjuan J, Haro JM, et al. Impact of depressive symptoms on subjective well-being: the importance of patient-reported outcomes in schizophrenia. Patient Prefer Adherence. 2011;5:471–474.
- Mencacci C, All I. Efficacy and tolerability of switching to ziprasidone in Italian patients with acute exacerbation of schizophrenia: an open-label trial. Pharmacopsychiatry. 2012;45(6):236–240.
- Michel P, Baumstarck K, Lancon C, et al. Modernizing quality of life assessment: development of a multidimensional computerized adaptive questionnaire for patients with schizophrenia. Qual Life Res. 2018;27(4):1041–1054.
- Mojtabai R, Corey-Lisle PK, Ip EH, et al. The Patient Assessment Questionnaire: initial validation of a measure of treatment effectiveness for patients with schizophrenia and schizoaffective disorder. Psychiatry Res. 2012;200(2–3):857–866.
- Montejo AL, Lauffer JC, Cuervo J, et al. Validation of a specific measure to assess health-related quality of life in patients with schizophrenia and bipolar disorder: the ’tolerability and quality of life’ (TOOL) questionnaire. Ann Gen Psychiatry. 2011;10:6.
- Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1–2):498–504.
- Ngoc TN, Weiss B, Trung LT. Effects of the family schizophrenia psychoeducation program for individuals with recent onset schizophrenia in Viet Nam. Asian J Psychiatr. 2016;22:162–166.
- Nielsen J, Emborg C, Gydesen S, et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2012;32(2):173–178.
- Niitsu T, Fujisaki M, Shiina A, et al. A randomized, double-blind, placebo-controlled trial of fluvoxamine in patients with schizophrenia: a preliminary study. J Clin Psychopharmacol. 2012;32(5):593–601.
- Niolu C, Bianciardi E, Di Lorenzo G, et al. Enhancing adherence, subjective well-being and quality of life in patients with schizophrenia: which role for long-acting risperidone? Ther Adv Psychopharmacol. 2015;5(5):278–288.
- O’Neill P. Quality of life in schizophrenia: preliminary validity investigation of Bernheim’s ACSA. Doctoral dissertation. Alliant International University, California School of Professional Psychology; 2010.
- Ogino S, Miyamoto S, Tenjin T, et al. Effects of discontinuation of long-term biperiden use on cognitive function and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):78–83.
- Pagsberg AK, Jeppesen P, Klauber DG, et al. Quetiapine versus aripiprazole in children and adolescents with psychosis—protocol for the randomised, blinded clinical Tolerability and Efficacy of Antipsychotics (TEA) trial. BMC Psychiatry. 2014;14:199.
- Papaioannou D, Brazier J, Parry G. How valid and responsive are generic health status measures, such as EQ-5D and SF-36, in schizophrenia? A systematic review. Value Health. 2011;14(6):907–920.
- Petkari E, Giacco D, Priebe S. Factorial structure of the Manchester Short Assessment of Quality of Life in patients with schizophrenia-spectrum disorders. Qual Life Res. 2020;29(3):833–841.
- Petrović A, Jankovic S. Translation, cultural adjustment and evaluation of reliability and validity of “Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form” for patients with schizophrenia. Acta Facultatis Medicae Naissensis. 2017;34(1):35–42.
- Peuskens J, Taeter C, Duquenne V. P.3.c.035 QUALITY: a non-interventional study evaluating QOL in schizophrenic patients treated with atypical antipsychotics. Eur Neuropsychopharmacology. 2010;20(Suppl 3):S478.
- Pietrini F, Spadafora M, Talamba GA, et al. The effects of switching from oral to LAI antipsychotic treatment on subjective experience of schizophrenic and schizoaffective patients: preliminary results. Int J Psychiatry Clin Pract. 2015;19(2):106–113.
- Pitkanen A, Valimaki M, Endicott J, et al. Assessing quality of life in patients with schizophrenia in an acute psychiatric setting: reliability, validity and feasibility of the EQ-5D and the Q-LES-Q. Nord J Psychiatry. 2012;66(1):19–25.
- Priebe S, Golden E, McCabe R, Reininghaus U. Patient-reported outcome data generated in a clinical intervention in community mental health care—psychometric properties. BMC Psychiatry. 2012;12:113.
- Rajagopalan K, Hassan M, Rajagopalan K, et al. Poster #S145 Health-related quality of life outcomes among patients with schizophrenia: results from a long-term naturalistic trial of patients switching to lurasidone from other antipsychotics. Schizophrenia Res. 2014;153(1):S141.
- Riedel M, Spellmann I, Schennach-Wolff R, et al. The RSM-scale: a pilot study on a new specific scale for self- and observer-rated quality of life in patients with schizophrenia. Qual Life Res. 2011;20(2):263–272.
- Rosen R, Trudeau J, Silverstein S, et al. T66. Psychometric validation of a novel patient-reported outcome measure for assessing patients’ subjective experience of cognitive impairment of schizophrenia (PRECIS). Schizophr Bull. 2018;44(Suppl 1):S139–S140.
- Rossell SL, Francis PS, Galletly C, et al. N-acetylcysteine (NAC) in schizophrenia resistant to clozapine: a double blind randomised placebo controlled trial targeting negative symptoms. BMC Psychiatry. 2016;16(1):320.
- Secher RG, Hjorthoj CR, Austin SF, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2015;41(3):617–626.
- Senin T, Franz M, Deuschle M, et al. QLiS-SF: Development of a short form of the quality of life in schizophrenia questionnaire. BMC Psychiatry. 2017;17(1):149.
- Seow LSE, Tan THG, Abdin E, et al. Comparing disease-specific and generic quality of life measures in patients with schizophrenia. Psychiatry Res. 2019;273:387–393.
- Sonntag M, Konig HH, Konnopka A. The responsiveness of the EQ-5D and time trade-off scores in schizophrenia, affective disorders, and alcohol addiction. Health Qual Life Outcomes. 2015;13:114.
- Su CT, Ng HS, Yang AL, Lin CY. Psychometric evaluation of the Short Form 36 Health Survey (SF-36) and the World Health Organization Quality of Life Scale Brief Version (WHOQOL-BREF) for patients with schizophrenia. Psychol Assess. 2014;26(3):980–989.
- Su CT, Yang AL, Lin CY. Comparing two schizophrenia-specific quality of life instruments in institutionalized people with schizophrenia. Psychiatry Res. 2017;258:274–282.
- Suzuki H, Hibino H, Inoue Y, et al. The effect of switching from oral low-dose aripiprazole to aripiprazole once-monthly 300 mg on the quality of life in three patients with schizophrenia. SAGE Open Med Case Rep. 2017;5:2050313X17710594.
- Tachibana M, Niitsu T, Watanabe M, et al. Effectiveness of blonanserin for patients with drug treatment-resistant schizophrenia and dopamine supersensitivity: a retrospective analysis. Asian J Psychiatr. 2016;24:28–32.
- Takahashi T, Higuchi Y, Komori Y, et al. Quality of life in individuals with attenuated psychotic symptoms: possible role of anxiety, depressive symptoms, and socio-cognitive impairments. Psychiatry Res. 2017;257:431–437.
- Takeuchi H, Fervaha G, Remington G. Reliability of a patient-reported outcome measure in schizophrenia: results from back-to-back self-ratings. Psychiatry Res. 2016;244:415–419.
- Thwin SS, Hermes E, Lew R, et al. Assessment of the minimum clinically important difference in quality of life in schizophrenia measured by the Quality of Well-Being Scale and disease-specific measures. Psychiatry Res. 2013;209(3):291–296.
- Vaingankar JA, Abdin E, Chong SA, et al. Psychometric properties of the short Warwick Edinburgh mental well-being scale (SWEMWBS) in service users with schizophrenia, depression and anxiety spectrum disorders. Health Qual Life Outcomes. 2017;15(1):153.
- Velligan DI, Fredrick M, Mintz J, et al. The reliability and validity of the MATRICS functional assessment battery. Schizophr Bull. 2014;40(5):1047–1052.
- Vothknecht S, Schoevers RA, de Haan L. Subjective well-being in schizophrenia as measured with the Subjective Well-Being under Neuroleptic Treatment scale: a review. Aust N Z J Psychiatry. 2011;45(3):182–192.
- Welch LC, Trudeau JJ, Silverstein SM, et al. Initial development of a patient-reported outcome measure of experience with cognitive impairment associated with schizophrenia. Patient Relat Outcome Meas. 2017;8:71–81.
- Widschwendter CG, Kemmler G, Rettenbacher MA, et al. Subjective well-being, drug attitude, and changes in symptomatology in chronic schizophrenia patients starting treatment with new-generation antipsychotic medication. BMC Psychiatry. 2018;18(1):212.
- Yee A, Ng BS, Hashim HMH, et al. Cultural adaptation and validity of the Malay version of the brief psychiatric rating scale (BPRS-M) among patients with schizophrenia in a psychiatric clinic. BMC Psychiatry. 2017;17(1):384.
- Zaragoza Domingo S, Bobes J, Garcia-Portilla MP, Morralla C. EPICOG-SCH: a brief battery to screen cognitive impact of schizophrenia in stable outpatients. Schizophr Res Cogn. 2017;8:7–20.
- Zaragoza Domingo S, Bobes J, Garcia-Portilla MP, et al. Cognitive performance associated to functional outcomes in stable outpatients with schizophrenia. Schizophr Res Cogn. 2015;2(3):146–158.
- Vothknecht S, Meijer C, Zwinderman A, et al. Psychometric evaluation of the Subjective Well-being Under Neuroleptic Treatment Scale (SWN) in patients with schizophrenia, their relatives and controls. Psychiatry Res. 2013;206(1):62–67.
- Bell MD, Bryson GJ, Greig TC, et al. Neurocognitive enhancement therapy with work therapy: Productivity outcomes at 6- and 12-month follow-ups. J Rehabil Res Dev. 2005;42(6):829–838.
- Hurford IM, Marder SR, Keefe RS, et al. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37(3):538–545.
- Hurford IM, Ventura J, Marder SR, et al. A 10-minute measure of global cognition: validation of the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS). Schizophr Res. 2018;195:327–333.
- Keefe RS, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426–432.
- Velligan DI, DiCocco M, Bow-Thomas CC, et al. A brief cognitive assessment for use with schizophrenia patients in community clinics. Schizophr Res. 2004;71(2–3):273–283.
- Ventura J, Cienfuegos A, Boxer O, Bilder R. Clinical global impression of cognition in schizophrenia (CGI-CogS): reliability and validity of a co-primary measure of cognition. Schizophr Res. 2008;106(1):59–69.
- Ventura J, Reise SP, Keefe RS, et al. The Cognitive Assessment Interview (CAI): development and validation of an empirically derived, brief interview-based measure of cognition. Schizophr Res. 2010;121(1–3):24–31.
- Ventura J, Reise SP, Keefe RS, et al. The Cognitive Assessment Interview (CAI): reliability and validity of a brief interview-based measure of cognition. Schizophr Bull. 2013;39(3):583–591.
- Ventura J, Subotnik KL, Ered A, et al. Cognitive Assessment Interview (CAI): validity as a co-primary measure of cognition across phases of schizophrenia. Schizophr Res. 2016;172(1–3):137–142.
- Davidson J, Turnbull CD, Strickland R, et al. The Montgomery-Asberg Depression Scale: reliability and validity. Acta Psychiatr Scand. 1986;73(5):544–548.
- Dufton BD, Siddique CM. Measures in the day hospital. I. The global assessment of functioning scale. Int J Partial Hosp. 1992;8(1):41–49.
- Guy W, National Institute of Mental Health, Early Clinical Drug Evaluation Program. ECDEU Assessment Manual for Psychopharmacology. United States Department of Health, Education, and Welfare; 1976.
- Heinrichs DW, Hanlon TE, Carpenter WT, Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–398.
- Kirkpatrick B, Buchanan RW, McKenney PD, et al. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119–123.
- Leucht S, Kane JM, Etschel E, et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacol. 2006;31(10):2318–2325.
- Leucht S, Kane JM, Kissling W, et al. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005;187:366–371.
- Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389.
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812.
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry. 1999;45(1):7–12.
- Saraswat N, Rao K, Subbakrishna DK, Gangadhar BN. The Social Occupational Functioning Scale (SOFS): a brief measure of functional status in persons with schizophrenia. Schizophr Res. 2006;81(2–3):301–309.
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247–251.
- Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptom Assessment. J Psychiatr Res. 1993;27(3):253–258.
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276.
- Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297.
- Kumari S, Malik M, Florival C, et al. An assessment of five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used symptoms rating scales in schizophrenia and comparison to newer scales (CAINS, BNSS). J Addict Res Ther. 2017;8(3):324.
- Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25(2):325–338.
- Startup M, Jackson MC, Bendix S. The concurrent validity of the Global Assessment of Functioning (GAF). Br J Clin Psychol. 2002;41(Pt 4):417–422.
- Bernheim JL, Theuns P, Mazaheri M, et al. The potential of Anamnestic Comparative Self-Assessment (ACSA) to reduce bias in the measurement of subjective well-being. J Happiness Stud. 2006;7(2):227–250.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292.
- Kaplan RM, Sieber WJ, Ganiats TG. The quality of well-being scale: comparison of the interviewer-administered version with a self-administered questionnaire. Psychol Health. 1997;12(6):783–791.
- Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11(2):157–171.
- Luo N, Seng BK, Thumboo J, et al. A study of the construct validity of the Health Utilities Index Mark 3 (HUI3) in patients with schizophrenia. Qual Life Res. 2006;15(5):889–898.
- World Health Organization. World Health Organization Disabilty Assessment Schedule: WHODAS II. Phase 2 field trials. Health services research. World Health Organization; 2000. https://apps.who.int/iris/handle/10665/68350. Accessed 5 Apr 2023.
- Ware JE, Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–3139.
- The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551–558.
- Corrigan PW, Salzer M, Ralph RO, et al. Examining the factor structure of the recovery assessment scale. Schizophr Bull. 2004;30(4):1035–1041.
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326.
- Lindström E, Jönsson L, Berntsson A. PMH56 a patient perspective on side effects of antipsychotic therapy: the Tool instrument. Value Health. 2009;12(7):A361.
- Marks KA, Fastenau PS, Lysaker PH, Bond GR. Self-Appraisal of Illness Questionnaire (SAIQ): relationship to researcher-rated insight and neuropsychological function in schizophrenia. Schizophr Res. 2000;45(3):203–211.
- Mueser KT, Meyer PS, Penn DL, et al. The Illness Management and Recovery Program: rationale, development, and preliminary findings. Schizophr Bull. 2006;32(Suppl 1):S32–S43.
- Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. Int Clin Psychopharmacol. 1995;10(Suppl 3):133–138.
- Neil ST, Kilbride M, Pitt L, et al. The questionnaire about the process of recovery (QPR): a measurement tool developed in collaboration with service users. Psychosis. 2009;1(2):145–155.
- Ritsner M, Kurs R, Gibel A, et al. Validity of an abbreviated quality of life enjoyment and satisfaction questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. Qual Life Res. 2005;14(7):1693–1703.
- Russo J, Roy-Byrne P, Reeder D, et al. Longitudinal assessment of quality of life in acute psychiatric inpatients: reliability and validity. J Nerv Ment Dis. 1997;185(3):166–175.
- van Nieuwenhuizen C, Schene AH, Koeter MW, Huxley PJ. The Lancashire Quality of Life Profile: modification and psychometric evaluation. Soc Psychiatry Psychiatr Epidemiol. 2001;36(1):36–44.
- Chisholm D, Knapp MR, Knudsen HC, et al. Client Socio-Demographic and Service Receipt Inventory – European version: development of an instrument for international research: EPSILON Study 5. Br J Psychiatry. 2000;177(S39):S28–S33.
- Tandon R, Marcus RN, Stock EG, et al. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA). Schizophr Res. 2006;84(1):77–89.
- Auquier P, Simeoni MC, Sapin C, et al. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophr Res. 2003;63(1–2):137–149.
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75.
- Wilkinson G, Hesdon B, Wild D, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–46.
- Yu Y, Cheng HL, Long FW, et al. The development of quality of life scale for patients with mental disorders. Public Health Quarterly. 1995;2(1):29–40.
- Voruganti LN, Awad AG. Personal evaluation of transitions in treatment (PETiT): a scale to measure subjective aspects of antipsychotic drug therapy in schizophrenia. Schizophr Res. 2002;56(1–2):37–46.
- Amri I, Millier A, Toumi M. Minimum clinically important difference in the global assessment functioning in patients with schizophrenia. Value Health. 2014;17(7):A765–A766.
- Hermes ED, Sokoloff D, Stroup TS, Rosenheck RA. Minimum clinically important difference in the Positive and Negative Syndrome Scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry. 2012;73(4):526–532.
- Leddy-Stacy MA, Rosenheck R. Obtaining employment as an anchor for estimating the minimum clinically important difference on the Positive and Negative Syndrome Scale (PANSS) in schizophrenia. Psychiatry Res. 2016;238:304–309.
- Germain N, Kymes S, Lof E, et al. A systematic literature review identifying associations between outcomes and quality of life (QoL) or healthcare resource utilization (HCRU) in schizophrenia. J Med Econ. 2019;22(5):403–413.
- Millier A, Clay E, Charaf I, et al. Patient reported outcomes instruments in schizophrenia: a review of psychometric properties. Open J Med Psychol. 2014;3(2):141–156.
- Pouchot J, Kherani RB, Brant R, et al. Determination of the minimal clinically important difference for seven fatigue measures in rheumatoid arthritis. J Clin Epidemiol. 2008;61(7):705–713.
- Sedaghat AR. Understanding the minimal clinically important difference (MCID) of patient-reported outcome measures. Otolaryngol Head Neck Surg. 2019;161(4):551–560.
- Becchi A, Rucci P, Placentino A, et al. Quality of life in patients with schizophrenia—comparison of self-report and proxy assessments. Soc Psychiatry Psychiatr Epidemiol. 2004;39(5):397–401.
- Dose finding of quetiapine fumarate 200mg vs 400mg in first episode psychosis. ClinicalTrials.gov Identifier: NCT00449397. Updated 27 Jan 2011. https://clinicaltrials.gov/ct2/show/NCT00449397. Accessed 5 Apr 2023.
- Comparison of the subjective well-being and tolerability of quetiapine XR to risperidone (RECOVER). ClinicalTrials.gov Identifier: NCT00600756. Updated 5 Oct 2012. https://clinicaltrials.gov/ct2/show/NCT00600756. Accessed 5 Apr 2023.
- Open-label study of intramuscular olanzapine depot in patients with schizophrenia or schizoaffective disorder. ClinicalTrials.gov Identifier: NCT00088465. Updated 11 Jan 2012. https://clinicaltrials.gov/ct2/show/NCT00088465. Accessed 5 Apr 2023.
- Aripiprazole once-monthly in patients with schizophrenia. ClinicalTrials.gov Identifier: NCT01959035. Updated 17 Mar 2017. https://clinicaltrials.gov/ct2/show/NCT01959035. Accessed 5 Apr 2023.
- Aripiprazole once-monthly versus paliperidone palmitate in adult patients with schizophrenia. ClinicalTrials.gov Identifier: NCT01795547. Updated 17 Mar 2017. https://clinicaltrials.gov/ct2/show/NCT01795547. Accessed 5 Apr 2023.
- Paliperidone palmitate flexible dosing in schizophrenia (PALMFlexS). ClinicalTrials.gov Identifier: NCT01281527. Updated 10 Mar 2014. https://clinicaltrials.gov/ct2/show/NCT01281527. Accessed 5 Apr 2023.
- Prevention of relapse with injectable paliperidone palmitate versus oral antipsychotics (PROSIPAL). Updated 18 Feb 2015. https://clinicaltrials.gov/ct2/show/NCT01081769. Accessed 5 Apr 2023.
- Trial evaluating the efficacy and safety of 2 fixed doses of paliperidone extended-release (ER) tablets in the treatment of adult patients with schizophrenia. ClinicalTrials.gov Identifier: NCT00077714. Updated 8 Jun 2011. https://clinicaltrials.gov/ct2/show/NCT00077714. Accessed 5 Apr 2023.
- Safety study with paliperidone ER extended-release (ER) tablets in geriatric patients with schizophrenia. ClinicalTrials.gov Identifier: NCT00085748. Updated 8 Jun 2011. https://www.clinicaltrials.gov/ct2/show/NCT00085748. Accessed 5 Apr 2023.
- A study of the long-term safety and tolerability and the long-term effectiveness of extended-release oral paliperidone in patients diagnosed with schizophrenia. ClinicalTrials.gov Identifier: NCT00210769. Updated 18 May 2011. https://clinicaltrials.gov/ct2/show/NCT00210769. Accessed 5 Apr 2023.
- Trial evaluating three fixed doses of paliperidone extended-release (ER) tablets and olanzapine in the treatment of patients with schizophrenia. ClinicalTrials.gov Identifier: NCT00083668. Updated 8 Jun 2011. https://clinicaltrials.gov/ct2/show/NCT00083668. Accessed 5 Apr 2023.
- A 52 week open-label extension trial following the double-blind efficacy and safety study R076477-SCH-305. ClinicalTrials.gov Identifier: NCT00668837. Updated 8 Jun 2011. https://clinicaltrials.gov/ct2/show/NCT00668837. Accessed 5 Apr 2023.
- Long-term efficacy and safety of asenapine using olanzapine as a positive control (41512)(COMPLETED)(P05784). ClinicalTrials.gov Identifier: NCT00156091. Updated 16 Feb 2022. https://clinicaltrials.gov/ct2/show/NCT00156091. Accessed 5 Apr 2023.
- Efficacy and safety of asenapine with placebo and haloperidol (41023)(P05926)(COMPLETED). ClinicalTrials.gov Identifier: NCT00156104. Updated 16 Feb 2022. https://www.clinicaltrials.gov/ct2/show/NCT00156104. Accessed 5 Apr 2023.
- Efficacy and safety of asenapine using an active control in subjects with schizophrenia or schizoaffective disorder (25517)(P05935). ClinicalTrials.gov Identifier: NCT00212784. Updated 16 Feb 2023. https://clinicaltrials.gov/ct2/show/NCT00212784. Accessed 5 Apr 2023.
- Fixed dose efficacy and safety study of asenapine for the treatment of schizophrenia in adolescents (P05896). ClinicalTrials.gov Identifier: NCT01190254. Updated 9 Feb 2022. https://clinicaltrials.gov/ct2/show/NCT01190254. Accessed 5 Apr 2023.
- Study to evaluate the efficacy and safety of Risperidone ISM® in patients with acute schizophrenia: open label extension (PRISMA-3_OLE). ClinicalTrials.gov Identifier: NCT03870880. Updated 25 Mar 2022. https://clinicaltrials.gov/ct2/show/NCT03870880. Accessed 5 Apr 2023.
- Pediatric schizophrenia efficacy and safety study. ClinicalTrials.gov Identifier: NCT01911429. Updated 18 Apr 2017. https://clinicaltrials.gov/ct2/show/NCT01911429. Accessed 5 Apr 2023.
- Pediatric open-label extension study. ClinicalTrials.gov Identifier: NCT01914393. Updated 19 Dec 2019. https://clinicaltrials.gov/ct2/show/NCT01914393. Accessed 5 Apr 2023.
- An adaptive phase II/III, two-part, double-blind, randomized, placebo-controlled, dose-finding, multi-center study of the safety and efficacy of NaBen®, as an add-on therapy with clozapine, for residual symptoms of refractory schizophrenia in adults. ClinicalTrials.gov Identifier: NCT03094429. Updated 13 Sep 2021. https://www.clinicaltrials.gov/ct2/show/NCT03094429. Accessed 5 Apr 2023.
- Efficacy and safety of asenapine using an active control in subjects with schizophrenia or schizoaffective disorder (25520)(P05846) (ACTAMESA). ClinicalTrials.gov Identifier: NCT00212771. Updated 16 Feb 2022. https://clinicaltrials.gov/ct2/show/NCT00212771. Accessed 5 Apr 2023.
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. In: Guy W, National Institute of Mental Health, Early Clinical Drug Evaluation Program. ECDEU Assessment Manual for Psychopharmacology. United States Department of Health, Education, and Welfare; 1976:157.
- Schwartz RC. Concurrent validity of the Global Assessment of Functioning Scale for clients with schizophrenia. Psychol Rep. 2007;100(2):571–574.
- Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305.
- Llorca PM, Lançon C, Lancrenon S, et al. The “Functional Remission of General Schizophrenia” (FROGS) scale: development and validation of a new questionnaire. Schizophr Res. 2009;113(2–3):218–225.
- Webb R, Bartl G, James B, et al. Exploring the development, validity, and utility of the Short-Form version of the CHoice of Outcome In Cbt for PsychosEs: a patient-reported outcome measure of psychological recovery. Schizophr Bull. 2021;47(3):653–661.
- Riedel M, Spellmann I, Schennach-Wolff R, et al. Validation of a new scale to assess quality of life in schizophrenic patients treated with antipsychotic drugs, the RSM-scale. Pharmacopsychiatry. 2009;42(5):A134.
- Carlson J, Ochoa S, Haro JM, et al. Adaptation and validation of the quality-of-life scale: Satisfaction with Life Domains Scale by Baker and Intagliata. Compr Psychiatry. 2009;50(1):76–80.
- Naber D, Moritz S, Lambert M, et al. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50(1–2):79–88.



