 by Nitin Pothen, MD; Shveta Kansal, MD; Theodor Rais, MD; Stacy Doumas, MD; and Ramon Solhkhah, MD
by Nitin Pothen, MD; Shveta Kansal, MD; Theodor Rais, MD; Stacy Doumas, MD; and Ramon Solhkhah, MD
Dr. Pothen and Dr. Kansal are with Ocean Medical Center, Hackensack Meridian Health in Brick, New Jersey. Dr. Rias is with Firelands Regional Health System-Sandusky in Sandusky, Ohio, and Mercy Health-St. Vincent Medical Center in Toledo, Ohio. Dr. Doumas is Psychiatry Residency Program Director at Jersey Shore University Medical Center in Neptune, New Jersey. Dr. Solhkhah is Chairman of Department of Psychiatry at Jersey Shore University Medical Center in Neptune, New Jersey.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Clozapine-induced agranulocytosis, malignant hyperthermia (MH), statin-induced myopathy, and neuroleptic malignant syndrome (NMS) are all serious drug reactions with significant overlap in terms of clinical symptomatology. The use of clozapine can lead to neutropenia, as well as the development of NMS; thus, it seemed logical to explore a possible common genetic background for the development of these two adverse effects. Furthermore, due to the overwhelming clinical resemblance between NMS, MH, and statin-induced myopathy, we decided specifically to search for a common genetic background in the development of these conditions. Methods: We searched the PubMed, OMIM, WikiGenes, Medline, and Google Scholar databases to identify articles pertinent to our subject published over the last 30 years. Articles were reviewed according to our inclusion/exclusion criteria, and irrelevant articles were excluded. Results and Conclusions: In our exploration for a common genetic background between clozapine-induced agranulocytosis, MH, NMS, and statin-induced myopathy, we identified the SLCO1B1 gene, which was common to three of these four conditions (MH, statin-induced myopathy, and clozapine-induced agranulocytosis). Although we did not find a gene common among NMS and the other conditions, the overlap of clinical symptoms between NMS, MH, and statin-induced myopathy did not allow us to rule out the possibility of a common factor, in terms of genetic predisposition, between these conditions. Future studies can aid to fill in the gaps of knowledge in terms of any genetic linkage between these three conditions and the mechanism of their associations.
Keywords: clozapine-induced agranulocytosis, drug reaction, malignant hyperthermia, statin-induced myopathy, neuroleptic malignant syndrome
Innov Clin Neurosci. 2019;16(11–12):28–31
Clozapine is an atypical antipsychotic used in the management of treatment-resistant schizophrenia. After its introduction in the 1960s, it was widely used; however, due to the serious adverse effect of agranulocytosis, it was banned in Europe. Clozapine was reintroduced in the United States (US) and obtained approval from the US Food and Drug Administration in 1989. Although studies have shown that 1 to 2 percent of patients on clozapine developed agranulocytosis, its use has continued in the US due to its effectiveness, with the added precaution of hematologic monitoring of white blood cell count, as well as a required enrollment into a national database for clozaril patients.1
Neuroleptic malignant syndrome (NMS) is a life-threatening reaction that presents with high fever, muscle rigidity, confusion, variable blood pressure, and tachycardia, which is associated with the use of both typical and atypical antipsychotics. Complications of NMS include rhabdomyolysis, kidney failure, hyperkalemia, and seizures.2 There are several reports of the atypical antipsychotic clozapine alone or in addition with lithium therapy causing NMS.3,4
Malignant hyperthermia (MH) is a pharmacogenetic disorder of the skeletal muscle caused by exposure to volatile anesthetic gases; depolarizing muscle relaxants; and, occasionally, by stressors such as vigorous exercise and heat.5 MH presents with high fever, muscle rigidity, and tachycardia and can lead to complications, such as hyperkalemia and rhabdomyolysis. Studies have shown a definite association of the two gene mutations, RYR1 and CACNA1S, with MH.6
Statins are the most important class of medication used in the treatment of hypercholesterolemia. The benefits of statins, as well as their adverse effects in coronary artery disease, have been demonstrated in multiple randomized, controlled trials. One of the common adverse effects of statins is myopathy, which presents as a spectrum of muscle cramps, tenderness, pain, weakness, stiffness, or heaviness and, in severe cases, rhabdomyolysis.7
NMS and MH have significant overlap in terms of clinical symptoms. Patients with NMS or MH can also present similar muscular involvement as those with statin-induced myopathy, and all three conditions can lead to the severe complication of rhabdomyolysis.8,9 Mutations in RYR3 and TaqI A polymorphism of the dopamine D2-receptor gene have been found to correlate with the predisposition for developing NMS.10 In addition to the development of neutropenia, clozapine has also been rarely associated with NMS.13 The objective of this study was to review the existing literature to explore the possibility of a common genetic background between clozapine-induced agranulocytosis, NMS, MH, and statin-induced myopathy. The identification of a common genetic basis could aide with the possible prevention of these serious conditions through extensive monitoring or even directing the withholding of medication in patients with a higher genetic predisposition toward the development of these conditions.
Methods
An initial search was performed in the PubMed gene database and OMIM by two of the authors independently. The first search was carried out using the key phrase “neuroleptic malignant syndrome,” which identified four genes. A second search with the key phrase “malignant hyperthermia” yielded 17 genes. A third search using the key phrase “statin-induced myopathy” yielded 10 genes. Finally, a fourth search using the key phrase “clozapine-induced agranulocytosis” identified 73 genes.
Each of the identified genes were reviewed in depth by the authors and, after cross-matching the results from the four searches, the three genes RYR1, SLCO1B1, and SLCO1B3 were found to have a common association with one another. Upon further investigation of the three genes, the two genes SLCO1B1 and SLCO1B3 were selected for further research and are the primary focus of this study. A systematic search of SLCO1B1 and SLCO1B3 was carried out in the following databases: PubMed, WikiGenes, Google Scholar, and IUHAR/BPS. The following keywords were used, as well as their combinations: “neuroleptic malignant syndrome,” “malignant hyperthermia,” “statin-induced myopathy,” “clozapine-induced agranulocytosis,” “SLCO,” and “OATP1B1.” Our search was limited to studies published from 1983 to 2018.
Hits received from the aforementioned databases were as follows: 421 from PubMed, 25 from WikiGenes, and 121 from Google Scholar. Of the total 567 articles identified, 30 were mutually selected per the inclusion criteria and were included in our study. The full texts of these studies were obtained by the authors, reviewed independently, and subsequently sorted into relevant and irrelevant categories. Studies that were agreed upon as being relevant were included in the present research. Relevant papers covered a variety of topics, including studies in which clozapine was the principle drug prescribed, genetic polymorphisms leading to a variety of drug interactions, common symptomology between the conditions of interest, and various anesthetics or muscle relaxants used in anesthesia. Articles found to be irrelevant were excluded from this study. Our selected articles consisted of English-language scientific studies published over the last 25 years that were both primary and secondary in nature, and the last search was carried out on November 10, 2018.
Results
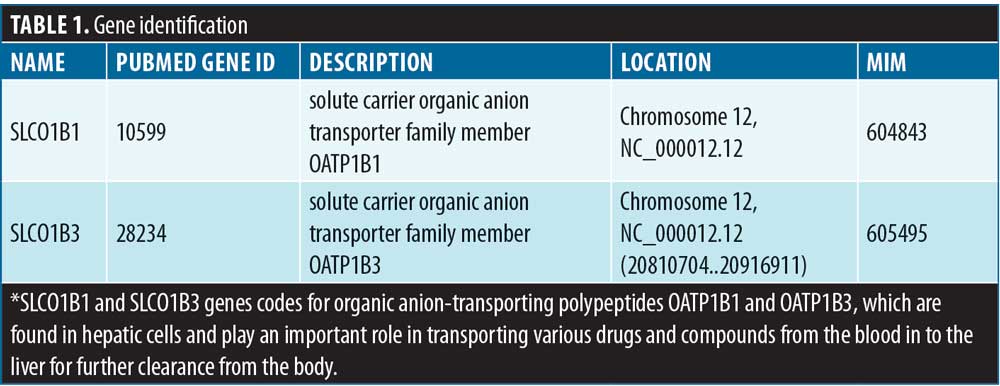
An overview of the SLCO gene family. The SLCO superfamily of genes is divided into six families and 10 subfamilies based on amino acid identity. These six families of SLCO genes code for 11 organic anion transporting polypeptides (OATPs). A system-wide search in PubMed revealed two genes associated with clozapine-induced agranulocytosis, MH, and statin-induced myopathy. These were the SLCO1B1 and SLCO1B3 genes, and Table 1 outlines their gene identification information from PubMed, the protein they code for, their chromosomal location, and their MIM number.
The impact of polymorphisms of the SLCO1B1 gene on statin-induced myopathy. A randomized clinical trial in the United Kingdom (UK) called “the SEARCH trial” was conducted to study the efficacy and safety of intensive statin treatment in patients with high cardiovascular risk. In this trial, 12,064 patients with a prior history of myocardial infarction who met the inclusion criteria for the clinical trial were included, from which 6,301 were randomly assigned to receive 80mg of simvastatin and 6,033 were randomly assigned to receive 20mg of simvastatin daily. Over the course of about six years, 98 cases of myopathy were observed in patients taking 80mg of simvastatin, whereas eight cases were observed among patients taking 20mg of simvastatin. A further analysis showed that a strong association of statin-induced myopathy in patients concomitantly taking amiodarone with an RR of 10ms.11 A genome-wide association study (GWAS), which is an observational study of a genome-wide set of genetic variants in different individuals, was conducted as part of the SEARCH trial to determine the genetic association.12 This GWAS study involved 85 patients with suspected myopathy and 90 controls, who were assigned to receive 80mg of simvastatin daily from the SEARCH trial. A single-nucleotide polymorphism (SNP) analysis revealed a strong link of myopathy with the noncoding rs4363657 SNP located in intron 11 of SLCO1B1 on chromosome 12 (p=4 × 10−9 and p=0.001) (k). The prevalence of the rs4363657 C allele was found to be 0.13 among the controls, with odds ratios (ORs) for myopathy of 4.3 (95% confidence interval [CI]: 2.5–7.2) per copy of the C allele and 17.4 (95% CI: 4.8–62.9) in CC homozygotes versus TT homozygotes.11
In addition, another UK-based study called the Heart Protection Study involved a clinical trial conducted among patients with preexisting occlusive vascular disease or diabetes. In this study, 20,536 patients who met the inclusion criteria were assigned to receive 40mg of simvastatin daily or placebo. Over the course of five years, 24 cases of myopathy were identified in 10,269 patients who were receiving 40mg of simvastatin, as opposed to 12 cases among the 10,267 patients who were receiving the placebo. Deoxyribonucleic acid (DNA) samples were obtained from 19,856 of the total 20,536 patients and, among 16,664 patients, the rs2306283 and rs4149056 SNPs in SLCO1B1 were successfully genotyped. There were no significant differences between the pretreatment levels of low-density lipoprotein (LDL) cholesterol between individuals with variants rs4149056 and rs2306283. Prior to the randomization phase of the study, all of these patients took 40mg of simvastatin daily for up to 4 to 6 weeks with a mean reduction in LDL levels of 40.57±0.12 percent. When both variants were compared together, the LDL levels were reduced by 1.28±0.25 percent per copy of the rs4149056 C allele with p<0.001, compared to the 0.62±0.18 percent increase per copy of the rs2306283 G allele with p<0.001. Hence, it was concluded that the individuals with rs4149056 SNP did not have expected reduction in LDL level and had higher risk of statin-induced myopathy.11
The aforementioned trial, along with the Heart Protection Study, revealed an association existed between the rs4149056 and rs4363657 variants of the SLCOB1 gene in both trials, supporting the cholesterol-lowering effects of simvastatin, as well as statin-induced myopathy. No other SNPs were found to be associated with statin-induced myopathy.
The involvement of the SLCO1B1 and SLCO1B3 genes in clozapine-induced agranulocytosis. Clozapine is an effective medication for treatment-resistant schizophrenia, which is defined as a failure to respond to at least two antipsychotic medication trials with adequate dose and duration. Even though it is the only treatment with proven efficacy in this severely impaired group of patients, it is not often prescribed due to the risk of hematological side effects, including agranulocytosis and neutropenia.1 Although the exact etiology of clozapine-induced blood disorders is currently unknown, it is hypothesized that genetic factors play a role in the development of clozapine-induced agranulocytosis. A meta-analysis study from the GWAS study, conducted by the Clozapine-associated Agranulocytosis Consortium (CIAC), found an association between neutropenia and the rs149104283 SNP. As per the GWAS meta-analysis, 10 SNPs that were most strongly associated with clozapine-induced agranulocytosis were outlined. One of these 10 SNPs, rs149104283 on chromosome 12, surpassed the genome-wide significance threshold for association with clozapine-associated neutropenia (OR: 4.32, p=1.79 × 10−8).13 The variant rs149104283 is intronic to transcripts of SLCO1B3 and SLCO1B7, the associated region of which also contained a third member of this organic anion transporter family, SLCO1B3, which was present in 7.37 percent of cases versus 1.52 percent of controls and 4.20 percent of cases versus 1.67 percent of controls in the CIAC sample. SLCO1B7, which encodes a putative protein, OAT1B7, is poorly characterized based on coding sequence prediction, and its functionality is unknown. Both the SLCO1B3 and SLCO1B1 genes share significant sequence homology and encode for the liver-specific organic anion transporter polypeptides OATP1B3 and OATP1B1. These are multipass transmembrane proteins found in the basolateral membrane of hepatocytes that are involved in the uptake of various exogenous substances, including drugs from portal veins to hepatic cells, which are then either modified by cyp450 enzymes or excreted.13
The involvement of the SLCO1B1 gene in MH. MH is a severe reaction that can be caused by anesthetic and other perioperative medications, such as fentanyl, suxamethonium, rocuronium, and ondansetron. MH is inherited in an autosomal dominant pattern and has an increased risk of occurrence due to inherited abnormalities. With the help of pharmacogenetic studies, variations in anesthetic drugs that affect enzymes, transport proteins, and various drug receptors in an individual’s genetic sequences can be elucidated. This could lead to improved knowledge and a better understanding of the variations seen in the areas of pharmacokinetics, drug metabolism, and efficacy in patients.9
In 2015, Mei et al14 explored the role of polymorphisms in the SLCO1B1, ABCB1, and CHRNA genes on the efficacy and duration of action of rocuronium in Chinese patients. The study consisted of 207 patients scheduled for elective surgery, with 200 completing the study. It was found that both the clinical duration and recovery time of rocuronium was prolonged in patients with the rs1128503 TT SNP in the ABCB1 gene and the rs2306283 AG and GG genotypes of the SLCO1B1 gene. Mei et al14 thus demonstrated that these particular gene variants of the SLCO1B1 and ABCB1 genes could affect the pharmacodynamics of rocuronium.
Since the clinical duration and recovery time of rocuronium were prolonged in the presence of SLCO1B1 variant mutations, we can hypothesize that muscle fibers are exposed to rocuronium for a prolonged period of time, causing the adverse event of MH.
Discussion
The SLCO family of genes, including particularly the SLCO1B1 gene, codes for the OATP1B1 protein. This protein is found in liver cells and transports certain hormones, drugs, and toxins to the liver to be excreted from the body. Drugs transported by the OATP1B1 protein include statins, chemotherapeutic drugs, antibiotics, and neuroleptics, as well as exogenous compounds. So, this leads us to hypothesize that this transport, involving several exogenous substances in the hepatocyte, can facilitate the uptake of medications in the liver that interact with neuroleptics, which could explain the overlap in clinical manifestations of the three conditions MH, statin-induced myopathy, and NMS.
The aforementioned GWAS study from the SEARCH trial identified the involvement of common genetic variants of the SLCO1B1 gene, which are associated with alterations that increase the risk of simvastatin-induced myopathy. These findings are likely to apply to other statin medications since myopathy is a class effect and SLCO1B1 polymorphism affects the blood levels of several other statins.
A meta-analysis from the GWAS study identified a SNP, rs149104283, which is a missense variant of SLCO1B3. This variant shares a sequence homology with SLCO1B1, which is known to be associated with clozapine-induced agranulocytosis. Both SLCO1B1 and SLCO1B3 share sequence homology and encode for OATP1B1 and OAT1B3, liver-specific organic anion transporter polypeptides involved in adverse reactions of several other drugs. This leads us to hypothesize that individuals with particular genetic variants of the SLCO1B1 gene have an increased risk of clozapine-associated neutropenia through a pharmacokinetic mechanism.
The study by Mei et al suggested that there was a decreased clearance of rocuronium in individuals with SLCO1B1 genetic polymorphism, which led to an increased risk of MH. The duration of muscle fiber exposure to the anesthetic was decreased due to reduced liver uptake. We can hypothesize that a similar defect in transport leading to decreased clearance and subsequently increased exposure time to the drug is also the mechanism involved in statin-induced muscular adverse effects. In addition, in individuals with SLCO1B1 mutations, the increased exposure of bone marrow to neuroleptics could increase the risk of development of neutropenia. While this study revealed an association between an increased risk of MH with the use of rocuronium in individuals with particular gene variants of the SLCO1B1 gene, this study was limited in that it involved only a Chinese population. Increased monitoring might be required in populations with an increased frequency and intensity of adverse effects. Future research with diverse samples can be useful to further investigate the role of the SLCO1B1 gene and MH across a variety of populations.
Although the initial intent of our review aimed to include NMS, we were unable to find direct support of involvement of the SLCO family of genes in the development of NMS. However, the significant clinical overlap between NMS, statin-induced myopathy, and MH warrants further research in investigating the role of the SLCO family of genes’ role in the pathophysiology of NMS. Our review highlighted that mutations in the SLCO1B1 gene can lead to an increased risk of neuroleptic-induced adverse effects, such as clozapine-induced agranulocytosis, as well as statin-induced myopathy in individuals with particular genetic polymorphisms. This leads to the consideration of pursuing increased monitoring in patients who are concurrently taking statins and neuroleptics.
In the future, the identification of SLCO1B1 polymorphisms via genotyping can be useful in identifying individuals at increased risk of developing adverse effects and harboring an increased susceptibility to drug interactions. This can be useful in tailoring the statin dose and outlining the need for increased monitoring in individuals taking statins in combination with neuroleptics, especially during the first year of treatment when findings of muscle-related complications are relatively frequent.
References
- Wiciński M, Węclewicz MM. Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr Opin Hematol. 2018;25(1):22–28.
- Becker BN, Ismail N. The neuroleptic malignant syndrome and acute renal failure. J Am Soc Nephrol. 1994;4(7):1406–1412.
- Leonardo QF, Juliana GR, Fernando CJ. Atypical neuroleptic malignant syndrome associated with use of clozapine. Case Rep Emerg Med. 2017;2017:2174379.
- Pope HG Jr, Cole JO, Choras PT, Fulwiler CE. Apparent neuroleptic malignant syndrome with clozapine and lithium. J Nerv Ment Dis. 1986;174(8):493–495.
- Kim DC. Maliganant hyperthermia. Korean J Anesthesiol. 2012;63(5):391–401.
- Riazi S, Kraeva N, Hopkins PM. Malignant hyperthermia in the post-genomics era: new perspectives on an old concept. Anesthesiology. 2018;128(1):168–180.
- Moßhammer D, Schaeffeler E, Schwab M, Mörike K. Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol. 2014;78(3):454–466.
- Tomaszewski M, Stępień KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859–866.
- Merck. Malignant Hyperthermia. https://www.merckmanuals.com/professional/injuries-poisoning/heat-illness/malignant-hyperthermia. Accessed January 3, 2019.
- Jahan MS, Rodnitzky RL, Kawanishi C, et al. Neuroleptic Malignant Syndrome. https://www.wikigenes.org/e/mesh/e/9488.html. Accessed January 3, 2019.
- SEARCH Collaborative Group, Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359(8):789–799.
- Tam V, Patel N, Turcotte M, et al. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–484.
- Legge SE, Hamshere ML, Ripke S, et al. Genome-wide common and rare variant analysis provides novel insights into clozapine-associated neutropenia. Mol Psychiatry. 2017;22(10):1502–1508
- Mei Y, Wang SY, Li Y, et al. Effect of ABCB1 rs12720464 and rs1055302 polymorphisms in Chinese patients on the time course of action of rocuronium administered as a single dose. Int J Clin Pharmacol Ther. 2016;54(6):462–470.





