 by Waguih William IsHak, MD, FAPA; Gabriel Edwards, MD, MPH; Nathalie Herrera, MD; Tiffany Lin, MD; Kathryn Hren, LCSW; Michael Peterson, LCSW; Ashley Ngor; Angela Liu; Asher Kimchi, MD; Brennan Spiegel, MD; Rebecca Hedrick, MD; Robert Chernoff, PhD; Marcio Diniz, PhD; James Mirocha, MS; Vicki Manoukian, MS; Michael Ong, MD; John Harold, MD; Itai Danovitch, MD, MBA; and Michele Hamilton, MD
by Waguih William IsHak, MD, FAPA; Gabriel Edwards, MD, MPH; Nathalie Herrera, MD; Tiffany Lin, MD; Kathryn Hren, LCSW; Michael Peterson, LCSW; Ashley Ngor; Angela Liu; Asher Kimchi, MD; Brennan Spiegel, MD; Rebecca Hedrick, MD; Robert Chernoff, PhD; Marcio Diniz, PhD; James Mirocha, MS; Vicki Manoukian, MS; Michael Ong, MD; John Harold, MD; Itai Danovitch, MD, MBA; and Michele Hamilton, MD
Drs. IsHak, Edwards, Herrera, Lin, Spiegel, Hedrick, Chernoff, Diniz, Danovitch; Mr. Mirocha and Mr. Peterson; and Ms. Hren, Ms. Nigor, Ms. Liu, and Ms. Manoukian and are with the Department of Psychiatry and Behavioral Neurosciences, Cedars-Sinai Medical Center in Los Angeles, California. Drs. Ishak, Spiegel, and Ong are with the David Geffen School of Medicine at UCLA in Los Angeles, California. Drs. Kimchi, Harold, and Hamilton are with the Smidt Heart Institute, Cedars-Sinai Medical Center in Los Angeles, California.
FUNDING: Research reported in this article was partially funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (2017C2-7716) given to Dr. IsHak.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. This paper sought to identify the instruments used to measure depression in heart failure (HF) and elucidate the impact of treatment interventions on depression in HF.
Methods. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. Studies published from 1988 to 2018 covering depression and HF were identified through the review of the PubMed and PsycINFO databases using the keywords: “depres*” AND “heart failure.” Two authors independently conducted a focused analysis, identifying 27 studies that met the specific selection criteria and passed the study quality checks.
Results. Patient-reported questionnaires were more commonly adopted than clinician-rated questionnaires, including the Beck Depression Inventory, the Patient Health Questionnaire (PHQ-9), and the Hospital Anxiety and Depression Scale. Six common interventions were observed: antidepressant medications, collaborative care, psychotherapy, exercise, education, and other nonpharmacological interventions. Except for paroxetine, selective serotonin reuptake inhibitors failed to show a significant difference from placebo. However, the collaborative care model including the use of antidepressants showed a significant decrease in PHQ-9 score after one year. All of the psychotherapy studies included a variation of cognitive behavioral therapy and patients showed significant improvements. The evidence was mixed for exercise, education, and other nonpharmacological interventions.
Conclusion. This study suggests which types of interventions are more effective in addressing depression in heart failure patients.
Keywords: Adult attention-deficit/hyperactivity disorder, comorbid disorders, externalizing disorder, internalizing disorder, nosology
Innov Clin Neurosci. 2020;17(4–6):27–38
Heart failure (HF) and depression, considered separately, are both highly prevalent illnesses. HF is a chronic syndrome affecting more than 5.7 million adults in the United States and 26 million adults worldwide.1,2 Depression is a leading cause of disability and premature mortality, affecting roughly 350 million people worldwide.3
Additionally, the two diagnoses often go hand in hand. Depressive symptoms in patients with HF are common; a Cochrane review detected depressive symptoms in up to 85 percent of patients with HF.4 Other systematic reviews place the prevalence of depressive symptoms in patients with HF in the range of 10 to 60 percent.5 Due to the heightened prevalence of depression in patients with cardiovascular disease (CVD), the American Heart Association has recommended screening for depression among patients with CVD with the Patient Health Questionnaire-2 (PHQ-2) and the Patient Health Questionnaire-9 (PHQ-9).6
Beyond commonly coexisting, the two diagnoses magnify one another. Depressive symptoms are associated with negative outcomes in HF.7 Depression and HF have bidirectional effects through both biological and psychosocial mechanisms.8–11 In general, functioning impairments are closely correlated with depression severity.12–14 Also, HF symptoms greatly restrict patients in their ability to partake in daily physical activities.15–17 Among cardiac patients, those with HF have reported more depressive symptoms and significant mood disruption in comparison with patients with other cardiac illnesses.18 In the context of chronic illnesses, patients with HF have reported the lowest physical and social functioning.19 Health-related quality of life (HRQoL) is significantly lower among patients with HF compared to the general population.20,21
Surprisingly, a weak predictor of poor HRQoL is HF severity, while one of the largest predictors of poor HRQoL is the severity of depression.22 Patients with depression and HF experience a worsening in both cardiac functioning and performance on physical exams, such as the six-minute walk test.23 Studies have reported that patients with HF and more severe physical symptoms experience greater depression severity.19,24 After adjusting for relevant variables, patients with depression and HF have reported lower mental and physical health scores.25
Other studies have concluded a negative impact on the psychosocial and physical health of caregivers, due to their feelings of being unprepared for their caregiving responsibilities in addition to being inadequately supported by their given healthcare team.26 Spouses of patients with HF have reported feeling a significant reduction in well-being and feeling burdened in the caregiving role. In 2004, Dracup et al27 found that the spouses of patients with HF were at greater risk for low levels of emotional well-being and tended to feel a lack of control over the health outcomes of their loved ones. Caregiver burden is also correlated with caregiver depression. Hooley et al28 found that depressed caregivers [Beck Depression Inventory (BDI) II score ≥10 points] have greater burden scores.
Studies have consistently shown that emergency department visits are increased in patients with HF and depression.29,30 Moreover, patients with depression and HF are 4.1 times more likely to be at risk of hospitalization than patients without depression on antidepressants (95% confidence interval: 1.2–13.9; p=0.022.31 A history of depression in patients with HF might also be a predictor of key quality outcome measures, such as prolonged hospital stays. A study performed in the United Kingdom found that more than 986,000 bed-days were distributed among 54,000 male patients diagnosed with HF and more than 1.37 million bed-days were distributed among 59,000 women diagnosed with HF. The increased severity of depressive symptoms was found to increase the risk for functional decline or death at six months among patients with HF.32 After adjusting for confounders, a study of 1,017 outpatient patients with HF concluded that depression was an independent risk factor for mortality.33
The present systematic review of published literature relating to depression in HF was performed to identify areas where future studies could elaborate further by addressing the following questions: 1) what are the instruments used to measure depression in HF, and 2) what is the impact of treatment interventions on depression in HF?
Methods
Search strategy. We performed this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.34 A systematic literature search was conducted on articles in the PubMed and PsycINFO databases published within the past 30 years, from January 1988 to July 2018, after setting exclusion and inclusion criteria. The keywords used for the search were “depres*” AND “heart failure.” We also conducted a manual search of reference lists for identified papers and previous reviews of HF and depression.
Study selection criteria and methodology. The following inclusion criteria were used: 1) articles published in English or that had a published English translation; 2) articles published in a peer-reviewed journal (with all articles in PubMed being published); 3) original studies in human adults (no reviews, no animal studies, age ≥18 years); 4) original studies of any design that focused on describing or treating depression in HF; and 5) studies that used at least one depression assessment measure. Exclusion criteria included editorials, opinion pieces, and case reports. Two authors independently conducted a focused analysis, then together reached a consensus on 27 studies that were able to meet the specific selection criteria. An independent additional reviewer examined the quality of each study by identifying its strengths and limitations using criteria adapted from Lohr and Carey by the Agency for Healthcare Research and Quality.35,36 The reviewer assessed sample size, patient selection methods, bias, study group comparisons, blinding, intervention details, outcome measures, and statistical analysis plans. The findings from this study quality check method eventually led to the exclusion of studies that had significant limitations. The search method is displayed in a flow diagram in Figure 1.
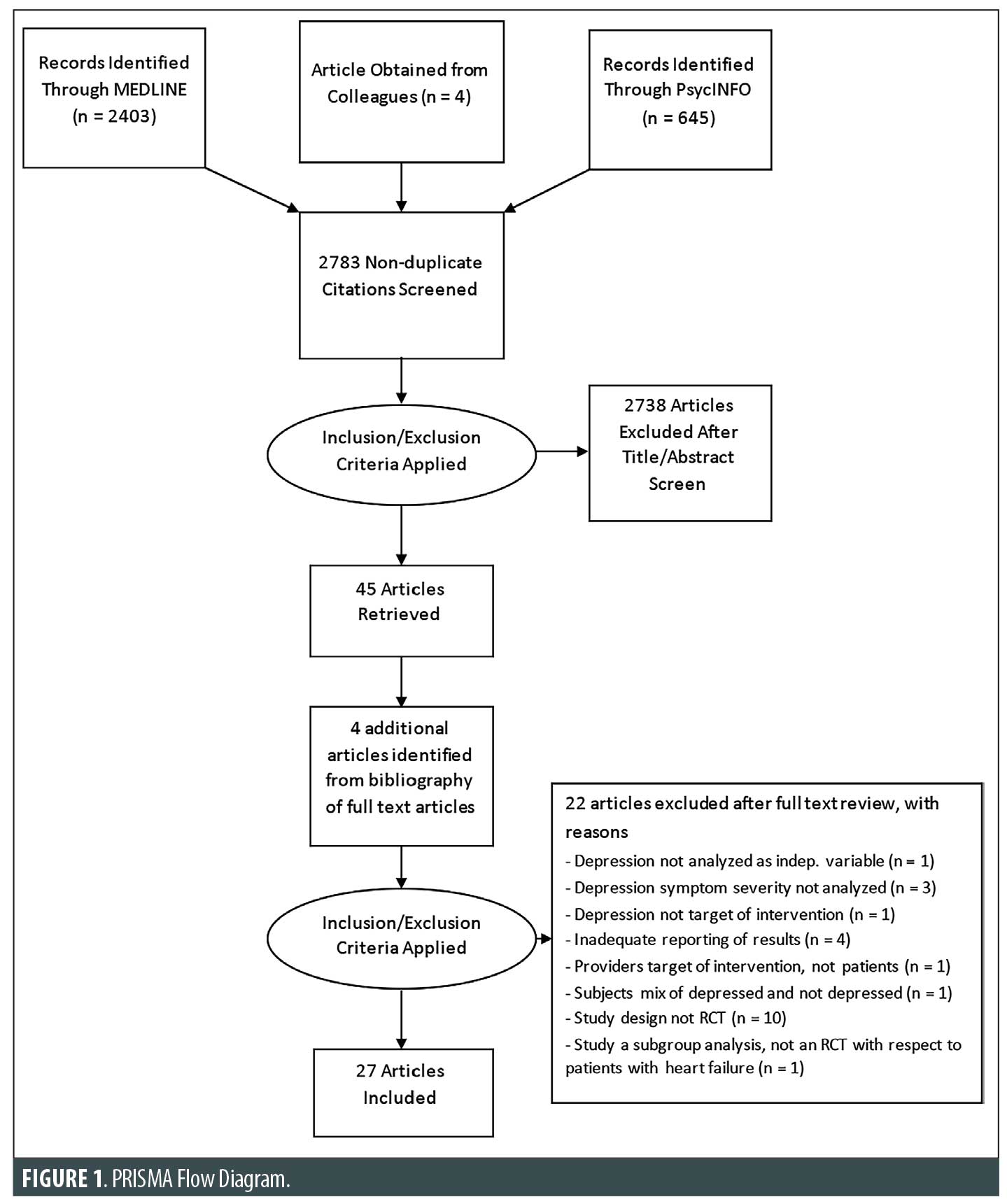
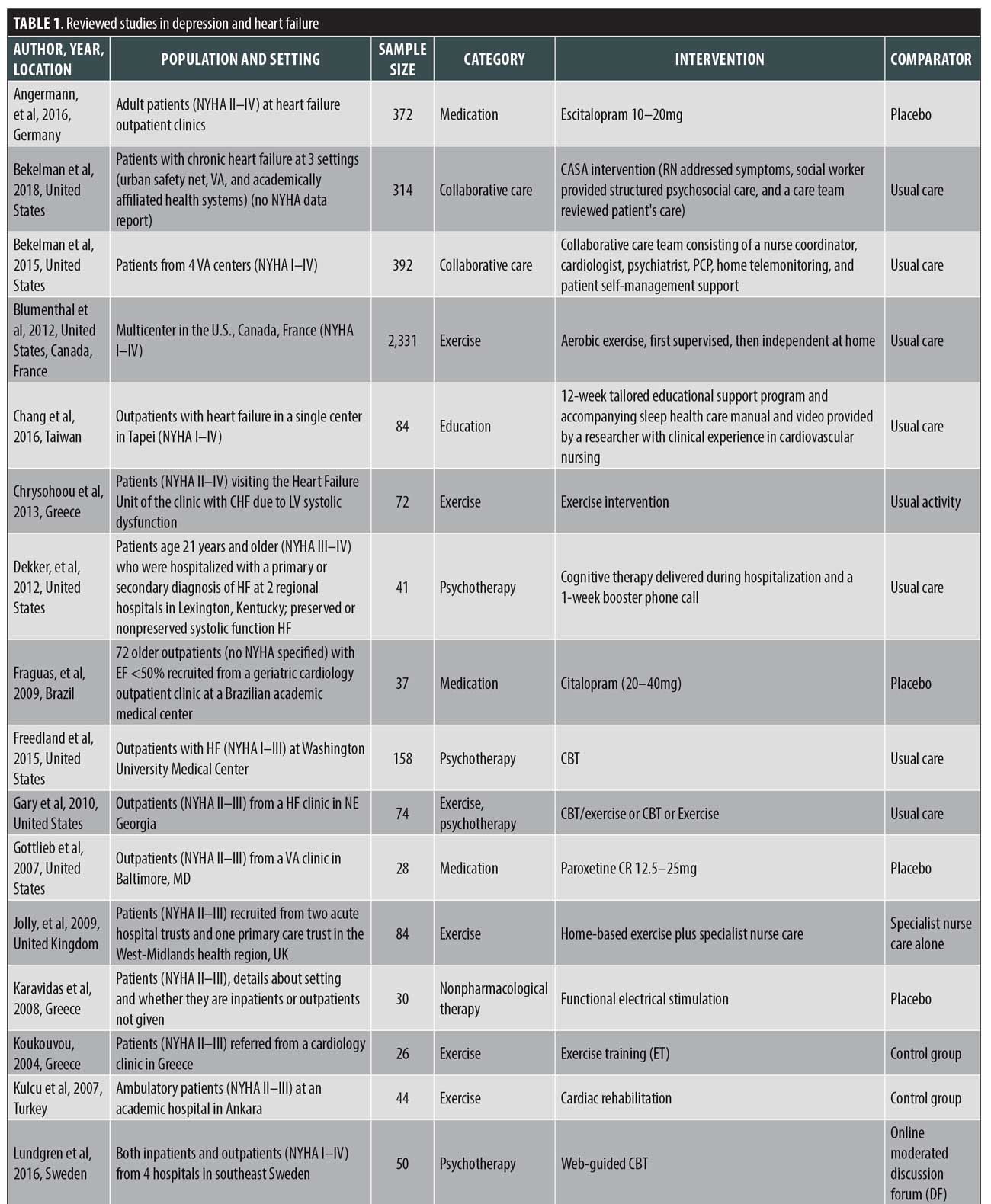
Data extraction and yield. Key findings were derived from the full text and tables of the selected 27 studies. The study designs and findings were analyzed for quality and are detailed in Table 1.


Results
Our search strategy identified 3,052 articles. After the elimination of the duplicates, the abstracts of 2,783 studies were reviewed. The studies that did not meet the selection criteria were excluded, leaving 51 studies. Two authors independently conducted a focused analysis using the gathered 51 full-text articles. The two authors then reached a consensus about what studies to include in this review, which yielded 31 studies. The quality check method led to the exclusion of four studies due to the inadequate reporting of results, where depression scores were not explicitly reported pre- and postintervention, and this resulted in a final 27 studies.
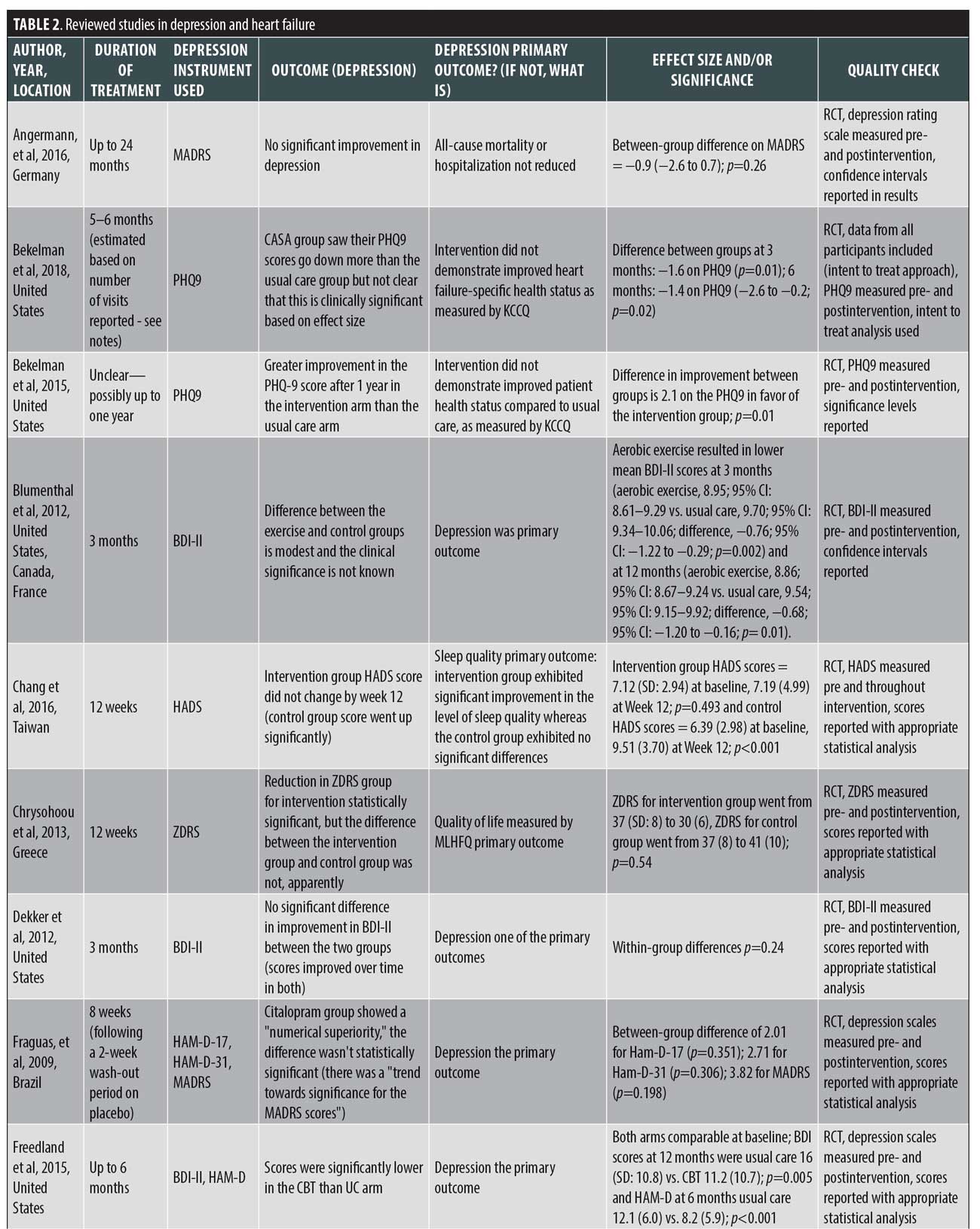
The findings from the reviewed studies are displayed in Table 1.
Instruments used to measure depression in HF. The studies in this review employed both patient-reported and clinician-administered depression symptom inventories. For the studies included in this review, the following instruments were used: nine studies used the BDI and BDI II;37,38 seven studies used the Patient Health Questionnaire (PHQ) exclusively;39 six studies used the Hospital Anxiety and Depression Scale (HADS) exclusively;40 six studies used a version of the BDI;37,38 two studies used the Hamilton Rating Scale for Depression (HAM-D) exclusively,41 and one of those two studies used two versions of the assessment, the HAM–D–17 and HAM–D-21;42 one study each used the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Zung Depression Rating Scale (ZDRS) exclusively;43,44 a single study used the Center for Epidemiologic Studies Depression Scale (CES-D) exclusively.45 The remaining four studies used more than one instrument to measure depression in patients with HF.
Impact of treatment interventions on depression in HF. Treatment for depression in HF encompasses a wide range of psychopharmacological interventions, cognitive behavioral therapy (CBT), physical exercise programs, psychoeducation, case management, telehealth interventions, palliative care, home-based services, mindfulness, and biofeedback. We classified the study findings into the following seven intervention categories: antidepressant medications,42,47–49 collaborative care,50–53 psychotherapy,54–57 exercise,57–64 education,46 65,72 in-home care,66 and other nonpharmacological interventions.67–71 One study included in this review combined the exercise and psychotherapy categories.57 Below, we summarize the results of the included studies broken down by intervention.
Antidepressant medications. The pharmacological agents and the range of doses used include: escitalopram 10 to 20mg,47 citalopram 20 to 40mg,42 paroxetine CR 12.5 to 25mg,49 and sertraline 50 to 200mg.48 All the interventions in this category were compared to placebos, and the duration of treatment in the studies ranged from eight weeks to 24 months.
The study in which paroxetine was used as the intervention showed significantly more remission of depressive symptoms relative to the placebo and lower BDI II scores during intervention (69.2% rate of remission, defined as BDI score <10 points) vs. 23.1 percent for controls (p=0.018).49
In the other three medication studies, however, the antidepressant medication failed to outperform the placebo. In research by O’Connor,48 patients taking sertraline had a reduction in the mean delta HADS score of 0.4 (p=0.89). Citalopram numerically outperformed placebo in terms of observed Montgomery-Asberg Depression Rating Scale (MADRS) scores but not on HAM-D scores, while the overall performance did not rise to the level of statistical significance for any of the assessment scores (between-group p-values on depression screens ranged from 0.198 on MADRS to 0.351 on HAM-D-17).42 For escitalopram, the between-group difference in MADRS score (−0.9 for the intervention group) was not statistically significant (p=0.26).47
Collaborative care. Out of the four studies in this category, two looked at a palliative care intervention delivered alongside standard HF care.52,53 The third study in this category employed and focused upon a specific collaborative care intervention called Collaborative Care to Alleviate Symptoms and Adjust to Illness (CASA),50 while the fourth employed and focused upon a Patient-centered Disease Management (PCDM) intervention involving three components: multidisciplinary collaborative care for managing HF, screening for the treatment of depression, and telemonitoring with patient self-care support.51 The range of the treatment duration ranged from a single consult to one year; however, the duration of one of the palliative care interventions was not explicitly stated.51 Similarly, the duration of the CASA intervention was estimated based on the number of visits reported because its duration was also not explicitly stated in the study.50
All four studies reported statistically significant reductions in depression severity. For Rogers et al,52 the HADS-depression scores for the intervention group decreased by 1.94 points more than in the usual care group (p=0.020). Sidebottom et al53 reported a PHQ-9 score reduction at three months of 2.90 for the intervention group versus 2.18 for the control group (p=0.00). The CASA intervention study saw significant differences in between-group reductions in PHQ-9 score in the intervention group compared to control (reduction in score of 2.2 points for intervention group versus only 0.8 points for the control group; p=0.02)).50 The paper looking at the PCDM intervention found a greater reduction in depressive symptoms for the subset of patients initially screened as positive for depression receiving the intervention compared to controls screening positive for depression as measured by the PHQ-9. The between-group difference in score reduction was 2.1 points (p=0.01).51
Psychotherapy. Three papers exclusively studying psychotherapy were included. Two studies compared psychotherapy to usual care.55,56 One study compared psychotherapy to an online-moderated discussion instead.54 The duration of the treatment for these studies varied between nine weeks and six months.
One study found significantly lower BDI II scores at 12 months with CBT intervention than in the usual care arm (p=0.005) and significantly lower HAM-D scores at six months (p<0.001).55 A second study found no significant difference in improvement in BDI II between the two groups (p=0.24), with scores improving over time in both.56 The third study, which used the online-moderated discussion forum as a comparison group and had the shortest treatment duration (nine weeks), found no significant difference in depressive symptoms between the groups at follow-up according analysis of covariance analysis (p=0.21). However, secondary within-group analysis of depressive symptoms showed that such symptoms decreased significantly in the CBT group from baseline to follow-up relative to in the discussion forum group (p=0.02).54
Exercise. Exercise was the most frequent studied intervention, with eight different studies testing it. The duration of the exercise intervention varied from eight weeks to six months. Only three of the studies found a significant improvement in the intervention group compared to the control.62–64 One of the studies reported p-values of less than 0.05 for the results of both depression screening measures used (BDI, HADS) for the intervention group relative to the control group.64 A second study reported p-values of 0.02 for the within-group changes for the intervention (a treadmill walking program) and 0.245 for the within-group changes for the control group.63 A third study using a home-based walking and resistance exercise program found the between-group difference in HADS score reductions was not statistically significant at six months (p=0.2) but was statistically significant at 12 months (p=0.02).62 An additional study found that aerobic exercise resulted in a lower mean BDI II score at three, six, and 12 months (p=0.02). Though the between-group difference was significant (0.68-point reduction on the BDI II scale; p=0.01), the clinical significance was characterized by the authors as unknown.61
The remaining four studies all reported reductions in depressive symptom severity for the intervention group, but the magnitude did not differ significantly from the control groups.57–60
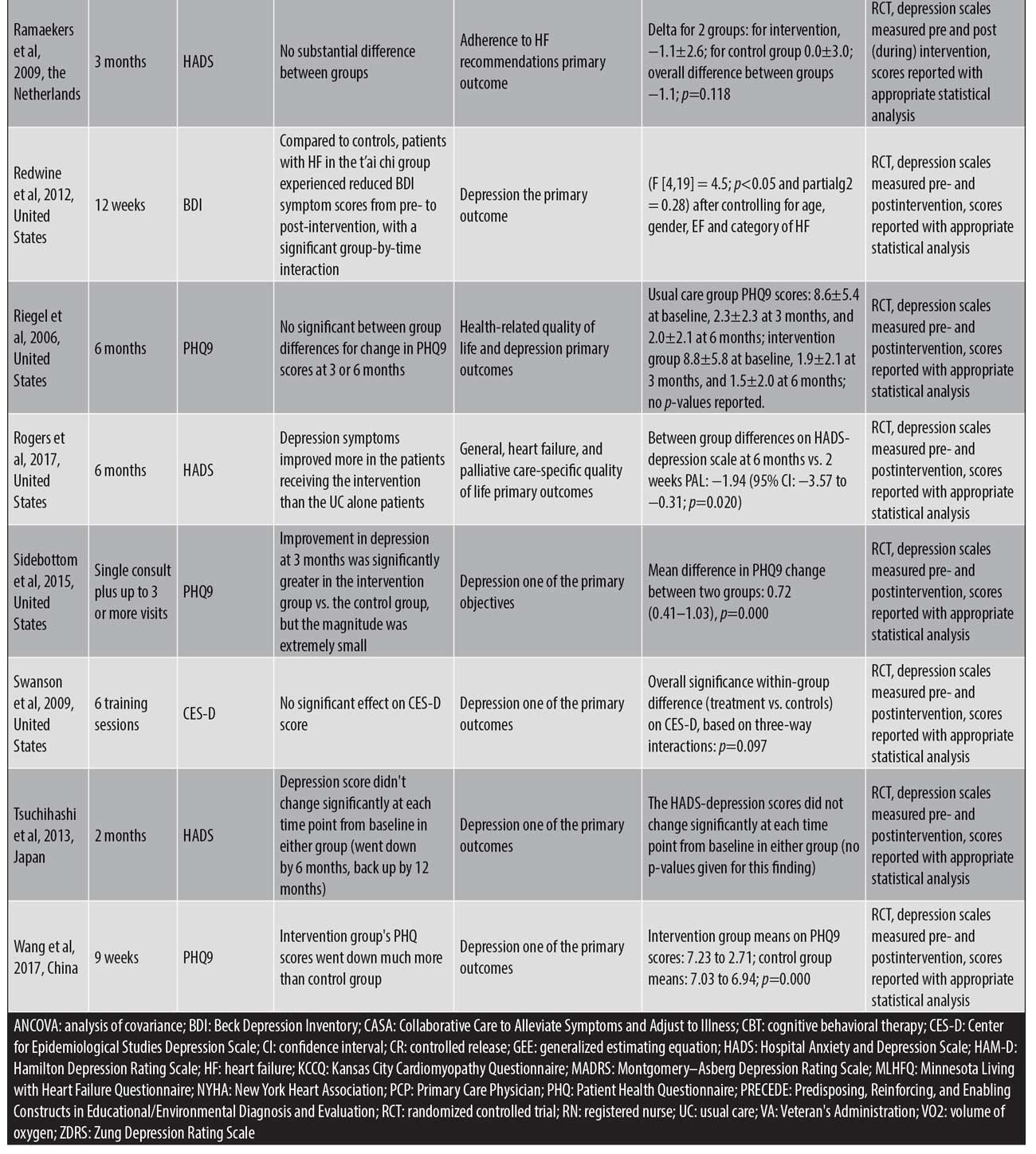
Education. One study using an education intervention showed that anxiety and depression scores significantly increased at 12 weeks in the control group (p<0.001), while individuals in the intervention group did not exhibit significant change at 12 weeks in the supportive nursing care program (p>0.05).46 Another study in this category reported that, after the intervention, the mean PHQ-9 score decreased significantly in the intervention group (p=0.000).72 A randomized controlled trial of a 12-week supportive educational nursing care program showed that, relative to in the control group, patients in the intervention group experienced significantly greater decreases in fatigue and significantly greater improvements in quality of life after 12 weeks of intervention.65
In-home care. A single study looked at a home-based nursing intervention in comparison with usual care.66 Overall, the HADS score for the intervention group actually went up by 12 months after a slight reduction at six months (from eight points at baseline to seven points at six months, then back over eight points by 12 months). These results were expressed in graphical form, without reporting the results numerically.
Other nonpharmacological interventions. Five studies total were included in this category.67–71
T’ai chi. T’ai chi for 12 weeks was compared to usual care. Relative to controls, patients with HF in the T’ai chi group experienced reduced BDI symptom scores from pre- to postintervention (p<0.05) and a significant group-by-time interaction (p<0.05).67
Biofeedback. A study comparing biofeedback to sham was unable to show that breathing retraining and biofeedback intervention had a significant effect on the CES-D score (p=0.097).68
Functional electrical stimulation (FES). Comparing pre and post scores, FES led to the improvement of depressive symptoms as measured by the Zung Self-rating Depression Scale (p<0.001) and the BDI (p<0.001) as well as quality of life measured by the KCCQ (p<0.001).69
Telemonitoring. Compared to usual care, telemonitoring was unable to show substantial differences (p=0.118).70
Telephone case management. Compared to standard care groups, telephone case management did not show a significant difference (p-values not reported).71
Discussion
Summary of the review findings. The studies used in this systematic review included a variety of depression assessment tools, protocol lengths, and sample sizes while also employing different intervention modalities to measure the effects of depression in patients with HF. Patient-reported questionnaires were more commonly used than clinician-rated questionnaires, with the BDI, the PHQ, and the HADS being those most frequently used with regard to measuring depression in HF. The interventions mentioned encompassed a broad range of categories that included antidepressant medications, collaborative care, psychotherapy, exercise, education, and other nonpharmacological interventions. The impact of the six treatment interventions were used to address and analyze how depression varied across the included studies.
All psychopharmacological agents in this review were tested against placebos and included the following medications: escitalopram, citalopram, paroxetine, and sertraline.42,47–49 The duration of the treatment regimens ranged from two to 24 months and were unable to show any significant preference to antidepressant medication over placebos, with the exception of one treatment: the use of paroxetine CR 12.5 to 25mg was able to provoke a significant reduction in depressive symptoms and BDI II score.49 Five studies included in this review tested the effect of collaborative care intervention on depression in patients with HF. The two studies that included palliative care interventions were both able to demonstrate an improvement in depressive symptoms.52,53 Meanwhile, the study that included the CASA group demonstrated improvements in PHQ-9 scores.50 In addition, another study by the same first author (Bekelman) reported a decrease in PHQ-9 scores after one year.51 Three studies in this review employed psychotherapy techniques by comparing the effects of the intervention groups to usual care and an online-moderated discussion forum.54–56 All the psychotherapy studies included a variation of CBT as the intervention. The Freedland study exhibited the best results due to its weekly one-hour CBT sessions, occurring for a duration of up to six months, that resulted in significantly lower BDI II scores reported at 12 months and significantly lower HAM-D scores reported at six months.55
Although exercise was the most frequently used intervention, there appeared to be many limitations in determining the overall effect on depression in patients with HF. One exercise intervention study failed to include statements regarding the clinical significance of its outcomes.61 Another study had varying results at different time points, with no proven statistical significance when considering within-group analysis.62 Although a different study reported clinical significance, it lacked proper calculations between the groups for BDI.58 The remaining exercise intervention studies were unable to show any statistical clinical significance.57,59
Two studies included in the education intervention category reported significant improvements in depression scores within their intervention groups.46,72 One education intervention study showed significant improvements in sleep quality outcomes within the intervention group receiving a 12-week educational support program and additional education on sleep; in contrast, the control group receiving usual care experienced significant increases in depression. However, the intervention group did not show significant results in terms of decreasing depression scores.46 The second study reported that, after receiving nine weeks of education intervention, PHQ-9 scores decreased significantly.72
Three studies included in the other nonpharmacological intervention category compared interventions to usual and/or standard care.67,70,71 One study compared interventions to sham biofeedback.68 A study that included T’ai chi as the intervention showed significant improvements in depressive symptoms but it should be noted that the study had a small sample size.67 All other studies mentioned in this category that involved telemonitoring, telephone case management, breathing retraining, and biofeedback were unable to yield significant results in terms of reducing depressive symptoms.68,70,71
Interpretation of the review findings. Through the analysis of the 27 studies that met the specific selection criteria and passed the study quality checks of this review, it can be interpreted that treatment interventions might greatly impact depression in HF; in particular, psychotherapy intervention had the most significant influence. A study using psychotherapy as the intervention was able to result not only in significantly lower BDI II scores but also significantly lower HAM-D scores; notably, both outcomes correspond to improvements in depressive symptoms.55 Collaborative care also proved to be an impactful form of intervention with its ability to improve depressive symptoms.50–53 Collaborative care follows psychotherapy in terms of the extent of impact on depression in HF due to its results being supported by the use of only one instrument (PHQ-9) and the results of psychotherapy intervention being supported by the use of two instruments (BDI II and HAM-D). Education was the third-most impactful form of intervention: it resulted in significant improvements in depression scores within the intervention groups.46,65,72 However, the intervention group in one study was unable to show significant effects on decreasing depression scores.46
Antidepressant medications serve as the fourth-most impactful form of intervention for depression in HF because paroxetine CR 12.5 to 25mg was the only medication out of the four mentioned that resulted in a significant reduction of depressive symptoms and BDI II scores; escitalopram, citalopram, and sertraline were all unable to demonstrate any significant preference over the placebos.49 While a study using nonpharmacological intervention resulted in significant improvements in depressive symptoms, this fifth form of intervention cannot be interpreted as greatly impacting depression in HF due to the study’s small sample size.67 This could possibly lead to bias in addition to other studies in this category failing to yield significant results in reducing depressive symptoms.68,70,71 The sixth intervention category of exercise was determined to be the least significant in impacting depression in HF because of its lack of clinical significance statistical significance, or proper calculations for BDI within the studies included in this category.58,61,62
Comparing this review’s findings to previous reviews. Prior research has explored the impact of different types of intervention for depression in patients with HF—mainly antidepressant medication, psychotherapy, exercise, and collaborative care—to establish the most impactful form of intervention for this population.7,73–76 However, the academic community has found this task challenging because of the substantial heterogeneity in the type, quality, and/or appropriateness of the interventions for target patient groups.75
Likewise, this paper corroborates that studies describing the instruments used to measure depression in HF and the adopted interventions are heterogeneous; notwithstanding, to date, to our knowledge, this is the only systematic review that has identified and classified six different modalities of intervention, assessing their quality and impact in the target population.
Notably, this study confirms that psychotherapy is the most impactful intervention regarding the improvement of depressive symptoms in patients with HF as underlined by prior reviews and recommended as first-line treatment for patients with CVD.7,74,77
This review positions psychopharmacological therapy as the fourth most impactful modality of treatment, supporting existing literature that questions its superiority over other interventions.48,77,78,79
Similarly, exercise has been highlighted by prior research as an effective therapy with an important impact in the reduction of depressive symptoms.61,77 However, this paper calls in to question these assertions, having found a lack of clinical and statistical significance.
Strengths and limitations. The strengths in this review are that this is a systematic review of articles published in the last 30 years. All analyzed studies are randomized, controlled trials that report measurements pre- and postintervention, with only one study reporting measurements pre- and throughout intervention.46
The limitations of this paper include a possible decrease in the statistical power of some of the evaluated studies due to small sample size. The substantial heterogeneity in the sensitivity and specificity of the instruments used to measure depressive symptoms could also be interpreted as a weakness since the studies analyzed take as a target population for intervention those patients with HF that screen positive using these instruments.74 This method could fail to include patients with subsyndromal depression; higher mortality rates have been observed in patients after acute myocardial infarction who have lower levels of depressive symptoms, which is not generally considered clinically significant.80 In general, it is important to distinguish between statistical significance and clinical significance when looking at the numerical decrease of depression assessment scores, which not every included paper did.
Conclusions and Future Directions
Depression is widely accepted as a major cause of morbidity and poor quality of life among patients with CVD, especially those with HF, and leads to staggering social, economic, and psychological costs worldwide.74,81 This situation has critically influenced the academic dialogue regarding the tools used to measure depressive symptoms in patients with HF and the impact of different modalities of treatment in this population. Despite its prevalence, current studies describing evaluation instruments and interventions among patients with HF and depression are too heterogeneous to permit definitive conclusions.
The review of articles included in this paper show that interventions exist that possess a demonstrated benefit for patients suffering from depression in the setting of HF, while some types of intervention (psychotherapy) tend to yield superior results relative to others (e.g., exercise). Future research is needed to create evidence-based evaluation and treatment algorithms tailored to the specific needs of the target population.
References
- Heart Failure Fact Sheet 2016. Center for Disease Control and Prevention website. https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm. Accessed June 17, 2019.
- Bui A, Horwich T, Fonarow G. Epidemiology and risk profile of heart failure. Nature. 2011;8(1):30–41.
- World Health Organization. Depression and Other Common Mental Disorder: Global Health Estimates. Geneva, Switzerland: World Health Organization; 2017.
- Lane D, Chong A, Lip G. Psychological interventions for depression in heart failure. Cochrane Database Syst Rev. 2005;(1):CD003329.
- Yohannes A, Willgoss T, Baldwin R, Connolly M. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221.
- Lichtman J, Bigger J, Blumenthal J, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–7175.
- Jeyanatham K, Kotecha D, Thanki D. Effects of cognitive behavioural therapy for depression in heart failure patients: a systematic review and meta-analysis. Heart Failure Rev. 2017;22(6):731–741.
- Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161(14):1725–1730.
- Glassman A, Shapiro P. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155(1):4–11.
- Cohen H, Gibson G, Alderman M. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108(1):2–8.
- Sherwood A, Blumenthal JA, Hinderliter AL, et al. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2011;57(4):418–423.
- Turvey CL, Klein DM, Pies CJ, Arndt S. Attitudes about impairment and depression in elders suffering from chronic heart failure. Int J Psychiatry Med. 2003;33(2):117–132.
- Johansson P, Dahlstrom U, Brostrom A. Consequences and predictors of depression in patients with chronic heart failure: implications for nursing care and future research. Prog Cardiovasc Nurs. 2006;21(4):202–211.
- Skotzko CE, Krichten C, Zietowski G, et al. Depression is common and precludes accurate assessment of functional status in elderly patients with congestive heart failure. J Card Fail. 2000;6(4):300–305.
- Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;11(2 Pt 1):273–279.
- Mayou R, Blackwood R, Bryant B, Garnham J. Cardiac failure: symptoms and functional status. J Psychosom Res. 1991;35(4-5):399–407.
- Friedman M, King K. Correlates of fatigue in older women with heart failure. Heart Lung. 1995;24(6):512–518.
- Hawthorne M, Hixon M. Functional status, mood disturbance and quality of life in patients with heart failure. Prog Cardiovasc Nurs. 1994;9(1):22–32.
- Stewart A, Greenfield S, Hays R, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262(7):907–913.
- Blyth F, Lazarus R, Ross D, et al. Burden and outcomes of hospitalisation for congestive heart failure. Med J Aust. 1997;167(2):67–70.
- Gottlieb S, Khatta M, Friedmann E, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43(9):1542–1549.
- Muller-Tasch T, Peters-Klimm F, Schellberg D, et al. Depression is a major determinant of quality of life in patients with chronic systolic heart failure in general practice. J Card Fail. 2007;13(10):818–824.
- Sullivan M, Levy WC, Russo JE, Spertus JA. Depression and health status in patients with advanced heart failure: a prospective study in tertiary care. J Card Fail. 2004;10(5):390–396.
- Friedman M. Older adults’ symptoms and their duration before hospitalization for heart failure. Heart Lung. 1997;26(3):634–640.
- Lesman-Leegte I, Jaarsma T, Sanderman R, et al. Depressive symptoms are prominent among elderly hospitalised heart failure patients. Eur J Heart Fail. 2006;8(6):634–640.
- Evangelista L, Stromberg A, Dionne-Odom J. An integrated review of interventions to improve psychological outcomes in caregivers of patients with heart failure. Curr Opin Support Palliat Care. 2016;10(1):24–31.
- Dracup K, Evangelista L, Doering L, et al. Emotional well-being in spouses of patients with advanced heart failure. Heart Lung. 2004;33(6):354–361.
- Hooley PJ, Butler G, Howlett JG. The relationship of quality of life, depression, and caregiver burden in outpatients with congestive heart failure. Congest Heart Fail. 2005;11(6):303–310.
- Moraska AR, Chamberlain AM, Shah ND, et al. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail. 2013;6(3):387–394.
- Bhatt K, Kaleogeropoulos A, Dunbar S, et al. Depression in heart failure: can PHQ-9 help? Int J Cardiol. 2016;221(15):246–250.
- Dekker R, Tovar E, Doering L, et al. Abstract 13961: A single cognitive behavior therapy session improves short-term depressive symptoms in hospitalized patients with heart failure. Circulation. 2014;130(Suppl_2).
- Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38(1):199–205.
- Diez-Quevedo C, Lupon J, Gonzalez B, et al. Depression, antidepressants, and long-term mortality in heart failure. Int J Cardiol. 2013;167(4):1217–1225.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6.
- Lohr K, Carey T. Assessing “best evidence”: issues in grading the quality of studies for systematic reviews. Jt Comm J Qual Improv. 1999;25(9):470–479.
- West S, King V, Carey T, McKoy K, et al. Systems to Rate the Strength Of Scientific Evidence. Report No.: 02-E016. Rockville, Maryland: Agency for Healthcare Research and Quality; 2002.
- Beck A, Ward C, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961(4):561–571.
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
- Kroenke K, Spitzer R, Williams J. The PHQ-9: Validity of a Brief Depression Severity Measure. J Gen Intern Med. 2001;16(9):606–613.
- Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Hamilton M. A Rating Scale for Depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62.
- Fraguas R, da Silva Telles RM, Alves TC, et al. A double-blind, placebo-controlled treatment trial of citalopram for major depressive disorder in older patients with heart failure: the relevance of the placebo effect and psychological symptoms. Contemp Clin Trials. 2009;30(3):205–211.
- Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389.
- Zung W. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63–70.
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
- Chang YL, Chiou AF, Cheng SM, Lin KC. Tailored educational supportive care programme on sleep quality and psychological distress in patients with heart failure: a randomised controlled trial. Int J Nurs Stud. 2016;61:219–229.
- Angermann CE, Gelbrich G, Stork S, et al. Effect of escitalopram on all-cause mortality and hospitalization in Patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683–2693.
- O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–699.
- Gottlieb SS, Kop WJ, Thomas SA, et al. A double-blind placebo-controlled pilot study of controlled-release paroxetine on depression and quality of life in chronic heart failure. Am Heart J. 2007;153(5):868–873.
- Bekelman DB, Allen LA, McBryde CF, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med. 2018;178(4):511–519.
- Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for Heart Failure study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725–732.
- Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017;70(3):331–341.
- Sidebottom AC, Jorgenson A, Richards H, et al. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134–142.
- Lundgren JG, Dahlstrom O, Andersson G, et al. The effect of guided Web-based cognitive behavioral therapy on patients with depressive symptoms and heart failure: a pilot randomized controlled trial. J Med Internet Res. 2016;18(8):e194.
- Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773–1782.
- Dekker RL, Moser DK, Peden AR, Lennie TA. Cognitive therapy improves three-month outcomes in hospitalized patients with heart failure. J Card Fail. 2012;18(1):10–20.
- Gary RA, Dunbar SB, Higgins MK, et al. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119–131.
- Piotrowicz E, Piotrowski W, Piotrowicz R. Positive effects of the reversion of depression on the sympathovagal balance after telerehabilitation in heart failure patients. Ann Noninvasive Electrocardiol. 2016;21(4):358–368.
- Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol. 2015;22(5):582–593.
- Chrysohoou C, Tsitsinakis G, Vogiatzis I, et al. High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM. 2014;107(1):25–32.
- Blumenthal JA, Babyak MA, O’Connor, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: The HF-ACTION randomized trial. JAMA. 2012;308(5):465–474.
- Jolly K, Taylor RS, Lip GY, et al. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM-CHF) study. Eur J Heart Fail. 2009;11(2):205–213.
- Kulcu DG, Kurtais Y, Tur BS, et al. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trial, short-term results. Eura Medicophys. 2007;43(4):489–497.
- Koukouvou G, Kouidi E, Iacovides A, et al. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med. 2004;36(1):36–41.
- Wang TC, Huang JL, Ho WC, Chiou AF. Effects of a supportive educational nursing care programme on fatigue and quality of life in patients with heart failure: a randomised controlled trial. Eur J Cardiovasc Nurs. 2016;15(2):157–167.
- Tsuchihashi-Makaya M, Matsuo H, Kakinoki S, et al. Home-based disease management program to improve psychological status in patients with heart failure in Japan. Circ J. 2013;77(4):926–933.
- Redwine LS, Tsuang M, Rusiewicz A, et al. A pilot study exploring the effects of a 12-week t’ai chi intervention on somatic symptoms of depression in patients with heart failure. J Altern Complement Med. 2012;18(8):744–748.
- Swanson KS, Gevirtz RN, Brown M, et al. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback. 2009;34(2):71–91.
- Karavidas A, Parissis J, Arapi S, et al. Effects of functional electrical stimulation on quality of life and emotional stress in patients with chronic heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy: a randomised, placebo-controlled trial. Eur J Heart Fail. 2008;10(7):709–713.
- Ramaekers BL, Janssen-Boyne JJ, Gorgels AP, Vrijhoef HJ. Adherence among telemonitored patients with heart failure to pharmacological and nonpharmacological recommendations. Telemed J E Health. 2009;15(6):517–524.
- Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12(3):211–219.
- Wang Q, Dong L, Jian Z, Tang X. Effectiveness of a PRECEDE-based education intervention on quality of life in elderly patients with chronic heart failure. BMC Cardiovasc Disord. 2017;17(1):262.
- Rajeswaran T, Plymen C, Doherty A. The effect of antidepressant medications in the management of heart failure on outcomes: mortality, cardiovascular function and depression—a systematic review. Int J Psychiatry Clin Pract. 2018;22(8):164–169.
- Rustad J, Stern T, Herbert K, Musselman D. Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Companion CNS Disord. 2013;15(4).
- Rutledge T, Reis V, Linke S, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537.
- Celano C, Villegas A, Albanese A, et al. Depression and anxiety in heart failure: A Review. Harv Rev Psychiatry. 2018;26(4):175–184.
- Jha M, Qamar A, Vaduganathan M, et al. Screening and management of depression in patients with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(14):1827–1845.
- Angermann C, Gelbrich G, Stork S, et al. Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re-uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD-HF). Eur J Heart Fail. 2007;9(12):1212–1222.
- Freedland KE, Hesseler MJ, Carney RM, et al. Major depression and long-term survival of patients with heart failure. Psychosom Med. 2016;78(8):896–903.
- Bush D, Ziegelstein R, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88(4):337–341.
- Correll C, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180.





