
by Michelle D. Po, PhD; Roberto Gomeni, PhD, HDR, FCP; and Bev Incledon, PhD
Dr. Po is with Highland Therapeutics Inc. in Toronto, Ontario, Canada. Dr. Gomeni is with PharmacoMetrica France in La Fouillade, France and is an Adjunct Professor with the Division of Pharmacotherapy and Experimental Therapeutics in the UNC Eshelman School of Pharmacy at The University of North Carolina at Chapel Hill in Chapel Hill, North Carolina. Dr. Incledon is with Ironshore Pharmaceuticals & Development, Inc. in Grand Cayman, Cayman Islands.
Funding: This research was funded by Ironshore Pharmaceuticals & Development, Inc.
Disclosures: Dr. Po is an employee of Highland Therapeutics Inc., the parent company of Ironshore Pharmaceuticals & Development, Inc. Dr. Gomeni is a paid consultant for Ironshore Pharmaceuticals & Development, Inc.; Sunovion Pharmaceuticals, Inc.; Supernus Pharmaceuticals, Inc.; Teva Branded Pharmaceutical Products R&D, Inc.; Tris Pharma; Biomedical Science Institutes, Singapore; Nanomi BV, the Netherlands; Laboratorios Liconsa SA, Spain; Recordati Rare Diseases, Italy; 4SC AG, Germany; General Hospital Corporation, Boston, Massachusetts; and UCB Biopharma SPRL. Dr. Incledon is an employee of Ironshore Pharmaceuticals & Development, Inc.
Innov Clin Neurosci. 2022;19(7–9):32–37.
Abstract
Objective: Extended-release methylphenidate (ER-MPH) formulations used to treat attention deficit hyperactivity disorder (ADHD) have complex pharmacokinetic (PK) profiles, resulting from differing ratios of immediate-release and extended-release components and/or their site of absorption. This study aimed to evaluate the smoothness of PK curves of ER-MPHs.
Design: The integral of the second derivative squared was evaluated for modeled PK curves, with smaller values indicating a smoother curve. The calculated smoothness of each PK curve was normalized by dividing by Cmax 2 to derive a normalized smoothness parameter appropriate across the dose range of each formulation. Calculations used modeled PK curves from 100mg delayed-release and ER-MPH (DR/ER-MPH), 54mg osmotic release oral system MPH (OROS MPH), 60mg MPH controlled-release delivery (MPH CD), 60mg ER-MPH oral suspension (MEROS), 20mg ER dexmethylphenidate (d-MPH ER), and 60mg multilayer-release MPH (MLR-MPH).
Results: The Cmax2-normalized smoothness value was consistent across DR/ER-MPH doses, allowing for relevant comparisons across formulations. Normalized smoothness values differed widely; the lowest normalized smoothness was 0.05 with DR/ER-MPH and ranged up to 9.56 with d-MPH ER.
Conclusion: DR/ER-MPH demonstrated a smoother PK profile compared to the highest dose of other ER-MPH formulations. While the benefits of a smooth PK profile remain to be tested clinically, having fewer peaks and troughs has been hypothesized to reduce waxing and waning of therapeutic effects throughout the day, and more gradual changes in MPH plasma levels have been hypothesized to lower the risk of likeability and potentially abate afternoon symptom rebound.
Keywords: Pharmacokinetics, modeling, methylphenidate, ADHD, smoothness
Stimulant medications, including methylphenidate (MPH), have been used to treat attention deficit hyperactivity disorder (ADHD) for over 60 years and are recommended as first-line pharmacotherapy for children and adolescents with ADHD.1,2 MPH inhibits the reuptake of dopamine and norepinephrine into presynaptic neurons by blocking their respective reuptake transporters, and the resulting increase in concentration of these neurotransmitters within the synaptic cleft is considered the basis for its clinical effect.3,4
Immediate-release MPH (IR-MPH) is rapidly absorbed from the stomach and upper gastrointestinal (GI) tract, reaching peak serum concentration and maximum clinical effect within 1 to 2 hours of oral administration.5–7 Because of its short duration of efficacy (2–3 hours), IR-MPH requires twice- or thrice-daily dosing to achieve symptom control over the day, and these regimens are characterized by fluctuating peak and trough drug plasma concentrations that might increase the risk of adverse events or lead to inconsistent efficacy.3,6,7 To address these challenges and others, such as convenience, adherence, stigma, and potential for diversion, extended-release MPH (ER-MPH) formulations have been developed. All morning-dosed oral ER-MPH formulations include an IR component to shorten the time gap between treatment administration and the onset of therapeutic effect, followed by an ER component that provides a prolonged phase of MPH delivery.8
Because the pharmacokinetic (PK) profiles of stimulant formulations are predictive of their pharmacodynamic (PD) profiles (i.e., efficacy and safety) with little-to-no time delay,9–11 United States (US) Food and Drug Administration (FDA) draft guidance12 asserts that stimulant formulations can be developed with the objective of creating specific PK profiles, specifically, altering the shape of the PK curve, onset of effect, or duration of effect. The development of novel formulations with unique PK profiles has attempted to address remaining unmet needs in the treatment of ADHD with previous formulations. One of these newer formulations designed to achieve a specific PK profile is delayed-release (DR)/ER-MPH (formerly HLD200; trade name: JORNAY PM® [Ironshore Pharmaceuticals & Development, Inc.; Grand Cayman, Cayman Islands]), which was approved by the FDA in 2018. DR/ER-MPH was developed with a drug delivery technology such that an IR component is not necessary.13,14 Dosed in the evening, with a predictable delay to target drug release in the colon approximately 8 to 10 hours after dosing, therapeutic MPH levels are achieved in the early morning. Compared to the rapid absorption that occurs in the small intestine, more gradual absorption in the colon underlies the dose-dependent duration of effect of DR/ER-MPH that was predicted from PK/PD modeling, where increases in the duration of clinical benefit into the evening are predicted with increases in dose.15 As a result of colonic absorption and lack of an IR component, the monophasic PK profile of DR/ER-MPH is characterized by a gradual ascending curve coinciding with the early morning peaking at about 14 hours postadministration, followed by a protracted elimination phase.13,14
Peak-to-trough ratio (typically calculated as [Cmax-Cmin]/Cavg) has been used to determine fluctuations in drug plasma concentration,16–18 as well as fluctuations in PD effect across the day.19 However, this definition fails to differentiate multiple peaks over the day (i.e., a biphasic PK profile would have a similar fluctuation index as a multiphasic or monophasic profile as long as the Cmax, Cmin, and Cavg were similar). Nor does the fluctuation index take into account PK profiles with “shoulders” (i.e., regions where the rate of increase or decrease slows or plateaus).
Compared to an IR-MPH with a similar Cmax value, an ER-MPH formulation with longer time to Cmax was associated with less likeability,20 indicating that rapid change in MPH plasma concentrations at onset was associated with reinforcing effects (i.e., detection and likeability).20,21 It has also been hypothesized that a faster decline in MPH levels is related to withdrawal and/or rebound symptoms.20,21 Therefore, not only is low peak-to-trough fluctuation of plasma concentrations important, but so is the smoothness of a PK curve. This study aimed to apply a mathematical definition of curvature smoothness to modeled PK profiles of ER-MPH formulations using the integral of the second derivative squared, a definition that has been applied to many fields, including finance and biomechanics.22,23
Methods
Data sources. Data from model-based PK curves for ER-MPH formulations, previously determined using a population PK modeling approach using data from healthy adults, were obtained.10,15 A similar convolution-based approach was used for all formulations. However, the time-varying absorption rate of DR/ER-MPH was best described by a single Weibull function,15 consistent with its single release process, rather than a double Weibull function that best describes the in vivo release of MPH from the other ER-MPH formulations,10 which is consistent with their dual release properties. Comparisons were conducted assuming that DR/ER-MPH was administered in the evening 10 hours before the ER-MPH formulations were administered in the early morning; therefore, the modeled PK curve for DR/ER-MPH is 34 hours versus 24 hours for the morning-administered formulations. Each of the modeled doses represents the highest dose strength for the formulation: 60mg multilayer-release MPH (MLR-MPH; Aptensio XR®, Rhodes Pharmaceuticals LP; Coventry, RI, US); 54mg osmotic release oral system MPH (OROS MPH; Concerta®, Janssen Pharmaceuticals, Inc.; Titusville, NJ, US); 20mg dexmethylphenidate extended-release (d-MPH ER; Focalin XR®, Novartis Pharmaceuticals Corporation; East Hanover, NJ, US); 60mg MPH controlled-release delivery (MPH CD, Metadate CD®, UCB Inc.; Smyrna, GA, US); 60mg MPH extended-release oral suspension (MEROS, Quillivant XR®; Tris Pharma Inc.; Monmouth Junction, NJ, US); and 100mg DR/ER-MPH.
All of the PK profiles were based on dl-MPH plasma levels, except for d-MPH ER and MEROS, which were based on d-MPH plasma levels.10,15 Because d-MPH contributes about 90 percent of bioavailable MPH,24,25 no corrections were performed between PK profiles.
Smoothness calculation. The smoothness of a curve can be quantified by measuring the rate of change of a time-varying profile in a wide range of fields, such as finance, computer science, and biomechanics,22,23,26 and is commonly defined as the integral of the squared second-order derivative of the curve function. When applied to a PK curve, the first derivative describes the rate of increases and decreases in drug plasma concentration and the second derivative describes changes in the rate of drug concentration increases and decreases. Squaring the second derivative is required so that sharp bends in opposite directions would not be canceled out, because squaring will give a positive number whether second derivative values are positive or negative. Furthermore, as squaring numbers less than one makes them smaller and squaring numbers greater than one makes them bigger, squaring the second derivative penalizes sharp bends and is more forgiving of small bends. To calculate the total smoothness over an interval from t1 to t2, the second derivative squared is integrated from t1 to t2, with lower values indicating a smoother curve. The integral of the second derivative squared from 0 to t hours has dimensions of concentration2/time3. Therefore, smoothness of a PK curve without dependency on amplitude (i.e., dimensionless for amplitude) can be calculated by dividing this integral by concentration2 (represented by Cmax2), resulting in the following equation:
This approach is similar to those used to measure the amplitude-adjusted smoothness of movement; other representatives of concentration2, such as
would also be acceptable to define an amplitude-adjusted smoothness measure.27 Although data are not shown for the latter approach, both give similar results. Because each included formulation has been shown to be dose proportional,8 and peak exposure is proportional to dose, peak exposure-normalized smoothness should be similar for a given formulation, regardless of dose.
Sensitivity analyses. The PK profile of DR/ER-MPH is characterized by an 8-to-10-hour delay in MPH release, following evening administration.13,14 In addition to the main analysis, where peak exposure-normalized smoothness of the DR/ER-MPH PK profile was calculated from evening administration at –10 hours to 24 hours, the same calculation was performed on the DR/ER-MPH PK profile from –5 hours to 24 hours to investigate the effect of the delayed release of DR/ER-MPH on the smoothness calculation (i.e., whether the part of the curve before MPH release, where MPH concentration is approximately zero, affects the smoothness calculation). Additionally, the smoothness of the 20mg DR/ER-MPH PK curve15 was calculated to confirm the appropriateness of normalization by Cmax2.
Data analysis. Smoothness calculations were performed using R version 4.0.2, included in Appendix 1, which can be accessed at https://innovationscns.com/wp-content/uploads/Incledon_Appendix-1.docx.
Results
The modeled PK curves (A), first derivative curves (B), second derivative curves (C), and second derivative squared curves (D) are shown in Figure 1 for single doses of d-MPH ER (20mg), DR/ER-MPH (100mg), MEROS (60mg), MPH CD (60mg), MPH-MLR (60mg), and OROS MPH (54mg).
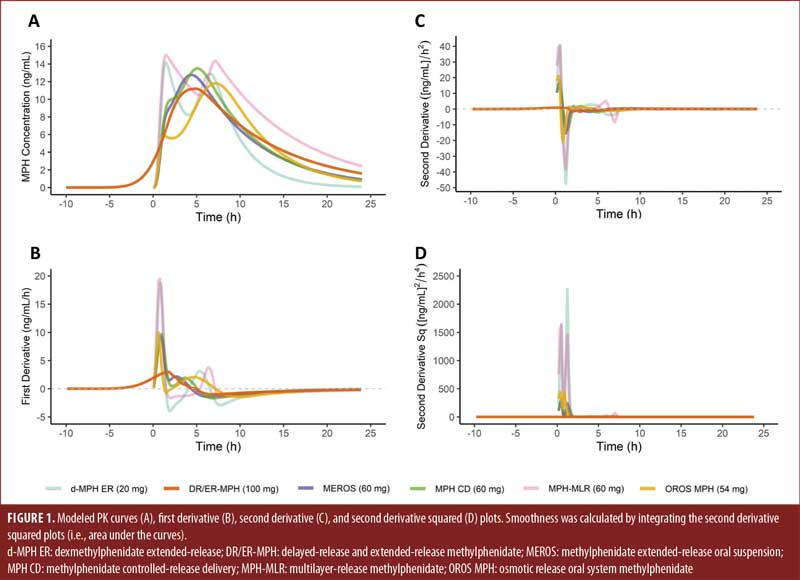
The calculated smoothness values for each ER-MPH formulation are summarized in Table 1. Normalized smoothness values ranged from 0.05 for DR/ER-MPH to 6.65 for d-MPH ER. When compared between PK profiles of 20mg and 100mg DR/ER-MPH, normalized smoothness values were identical, indicating that normalization by Cmax2 appropriately evaluates smoothness across the dose range for a given formulation. Because normalized smoothness is consistent across doses of a formulation, normalized smoothness can be appropriately compared between ER-MPH formulations, despite using PK models developed from doses that are not equivalent for MPH exposure.

When the smoothness of the 100mg DR/ER-MPH PK profile was evaluated from –5 hours to 24 hours, the normalized smoothness remained 0.05, identical to the DR/ER-MPH PK profile evaluated from evening administration at –10 hours to 24 hours. Therefore, the delay between administration of DR/ER-MPH and initial release did not affect the calculated smoothness value.
Discussion
These analyses demonstrate that PK curves of ER-MPH formulations differ in smoothness, and this variation is related to their formulation properties (i.e., composition and ratio of IR and ER components) and rate of MPH absorption, which is influenced by site of absorption in the GI tract (i.e., upper GI tract versus the colon) (Incledon et al. 2022, in press).28 Among the oral ER-MPH formulations evaluated here, DR/ER-MPH demonstrated the smoothest PK profile, while d-MPH ER demonstrated the least smooth PK profile.
Although some PK profiles (Figure 1A) are intuitively less smooth due to having multiple and/or sharper peaks, the smoothness calculations performed here also highlight subtleties that are not immediately apparent from a visual inspection of the PK curves, especially among the formulations with monophasic PK profiles. From the first derivative analysis (Figure 1B), the rate of change in MPH concentration within the first 0.5 hours following morning administration is near-identical in all the evaluated morning-dosed ER-MPH formulations because of the absorption of their IR components in the stomach/upper GI tract, despite some formulations having distinct early morning peaks in a clearly biphasic PK profile and some having only a shoulder in a monophasic PK profile. Although the shoulders seen in the MPH CD and MEROS PK curves are not local maxima (Supplemental Figure 1A), they are followed by an accelerating MPH concentration and correspond to distinct IR and ER release processes. These changes in the rate of MPH increases and decreases, the definition of the second derivative, are reflected in the peaks of the second derivative and second derivative squared plots (Supplemental Figures 1C and 1D) and thus contribute to the higher smoothness value (Table 1), indicating a less smooth curve. Compared to the other formulations with monophasic PK profiles, the smoothness of the DR/ER-MPH profile is driven by two factors: more gradual morning absorption and the absence of a separate second release process, both results of targeting colonic absorption following evening administration (Supplemental Figure 1C). Although DR/ER-MPH also exhibits a protracted elimination phase, compared to many of the other formulations,8 this contributes less to the smoothness differences compared to the two factors above.

Limitations. The study is limited by calculating smoothness using modeled PK curves based on data obtained from healthy adults and not individuals with ADHD. Moreover, because substantial interpatient PK variability exists, the smoothness of modeled MPH profiles might not be fully predictive of the shape of MPH PK curves in any individual. Finally, the clinical significance of the PK profile smoothness of ER-MPH formulations remains to be fully explored. Safety and tolerability data are typically not collected across the day; however, certain adverse events, such as appetite suppression during lunchtime, would be expected to temporally co-occur with peak MPH plasma concentrations.29
Despite these limitations, the shape of a PK curve is acknowledged by the FDA as critical,12,30 especially in modified-release formulations that have different phases of drug release corresponding to different clinical effects. This recognition is based on studies showing that PK profiles of MPH formulations can predict the time course of clinical benefit.9,10 Furthermore, an additional study showed a direct relationship, with no apparent delay, between MPH blood levels and changes in blood pressure and heart rate in healthy adults, with IR formulations yielding larger effects than ER formulations of the same dose.11 The latter finding suggests that the shape and smoothness of an MPH formulation profile does matter when it comes to cardiovascular effects.
Conclusion
Based on the established concentration-response relationship for efficacy and safety in MPHs and other stimulants, the smoothness of a PK profile might contribute to reducing adverse events associated with peak concentrations6 when compared to formulations that have high peak-to-trough fluctuations, improving consistency of effect over the course of the day7,9 and potentially lowering the risk of likeability and afternoon symptom rebound.20,21 Whether these hypothesized benefits of a smooth PK profile are seen in clinical settings warrants further investigation.
Acknowledgments
The authors would like to thank Cassandra Uchida, PhD, and Marina Komolova, PhD, from Highland Therapeutics Inc., and Victor Otcheretko, MD, and Randy Sallee, MD, from Ironshore Pharmaceuticals Inc., who reviewed and edited the article for scientific accuracy.
References
- Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921.
- Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528.
- Swanson J, Gupta S, Guinta D, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66(3):295–305.
- Volkow ND, Fowler JS, Wang G, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(Suppl 1):S31–S43.
- Patrick KS, Straughn AB. Absorption differences between immediate-release dexmethylphenidate and dl-methylphenidate. Drug Metab Dispos. 2016;44(3):418–421.
- Greenhill LL, Pliszka SR, Dulcan M, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 Suppl):26S–49S.
- Buitelaar J, Medori R. Treating attention-deficit/hyperactivity disorder beyond symptom control alone in children and adolescents: a review of the potential benefits of long-acting stimulants. Eur Child Adolesc Psychiatry. 2010;19(4):325–340.
- Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937–974.
- Kimko H, Gibiansky E, Gibiansky L, et al. Population pharmacodynamic modeling of various extended-release formulations of methylphenidate in children with attention deficit hyperactivity disorder via meta-analysis. J Pharmacokinet Pharmacodyn. 2012;39(2):161–176.
- Gomeni R, Bressolle-Gomeni FMM, Spencer TJ, et al. Model-based approach for optimizing study design and clinical drug performances of extended-release formulations of methylphenidate for the treatment of ADHD. Clin Pharmacol Ther. 2017;102(6):951–960.
- Li L, Wang Y, Uppoor RS, et al. Exposure-response analyses of blood pressure and heart rate changes for methylphenidate in healthy adults. J Pharmacokinet Pharmacodyn. 2017;44(3):245–262.
- US Food and Drug Administration. Attention deficit hyperactivity disorder: developing stimulant drugs for treatment guidance for industry draft guidance. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/attention-deficit-hyperactivity-disorder-developing-stimulant-drugs-treatment-guidance-industry. Accessed 12 Dec 2021.
- Childress A, Mehrotra S, Gobburu J, et al. Single-dose pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate formulation, in healthy adults and in adolescents and children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2018;28(1):10–18.
- Liu T, Gobburu JVS, Po MD, et al. Pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate: evaluation of dose proportionality, food effect, multiple-dose modeling, and comparative bioavailability with immediate-release methylphenidate in healthy adults. J Child Adolesc Psychopharmacol. 2019;29(3):181–191.
- Gomeni R, Komolova M, Incledon B, Faraone SV. Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020;40(4):350–358.
- Wakamatsu A, Aoki K, Sakiyama Y, et al. Predicting pharmacokinetic stability by multiple oral administration of atypical antipsychotics. Innov Clin Neurosci. 2013;10(3):23–30.
- Hagen NA, Thirlwell M, Eisenhoffer J, et al. Efficacy, safety, and steady-state pharmacokinetics of once-a-day controlled-release morphine (MS CONTIN XL) in cancer pain. J Pain Symptom Manage. 2005;29(1):80–90.
- Tremblay S, Nigro V, Weinberg J, et al. A steady-state head-to-head pharmacokinetic comparison of all FK506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432–442.
- Gomeni R, Faraone SV, Spencer TJ, Incledon B. Establishing clinical benefit of HLD200, a novel delayed-release and extended-release formulation of methylphenidate, using a model-based approach. Presented at: APSARD; 15 Jan 2017; Washington, DC.
- Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163(3):387–395.
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160(11):1909–1918.
- Ulrich M, Walther S. Option-implied information: what’s the vol surface got to do with it? Rev Deriv Res. 2020;23:323–355.
- Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. 1985;5(7):1688–1703.
- Hubbard JW, Srinivas NR, Quinn D, Midha KK. Enantioselective aspects of the disposition of dl-threo-methylphenidate after the administration of a sustained-release formulation to children with attention deficit-hyperactivity disorder. J Pharm Sci. 1989;78(11):944–947.
- Srinivas NR, Hubbard JW, Quinn D, Midha KK. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561–568.
- Lim KG, Xiao Q. Computing maximum smoothness forward rate curves. Stat Comput. 2002;12(3):275–279.
- Hogan N, Sternad D. Sensitivity of smoothness measures to movement duration, amplitude, and arrests. J Mot Behav. 2009;41(6):529–534.
- Incledon B, Incledon C, Gomeni R, et al. Effect of colonic absorption on the pharmacokinetic properties of delayed-release and extended-release methylphenidate: in vivo, in vitro, and modeling evaluations. Clin Pharmacol Drug Dev. 2022;11(8):966–975.
- Coghill D, Banaschewski T, Zuddas A, et al. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237.
- Fourie Zirkelbach J, Jackson AJ, Wang Y, Schuirmann DJ. Use of partial AUC (PAUC) to evaluate bioequivalence—a case study with complex absorption: methylphenidate. Pharm Res. 2013;30(1):191–202.


