 by Eirini Beneki, MD, MSc; Xenofon Papazarkadas, MD, MSc; Achillefs Karras, MD,MSc; Konstantinos Kalantzis, MD, MSc; and Konstantinos Tsatiris, MD, MSc
by Eirini Beneki, MD, MSc; Xenofon Papazarkadas, MD, MSc; Achillefs Karras, MD,MSc; Konstantinos Kalantzis, MD, MSc; and Konstantinos Tsatiris, MD, MSc
Drs. Beneki and Tsatiris are with the Department of Cardiology at Karditsa General Hospital in Karditsa, Greece. Dr. Papazarkadas is with the Department of General Surgery at the Hospital of Valais (CHVR) in Sion, Switzerland. Drs. Karras and Kalantzis are with the Department of Internal Mediine at Karditsa General Hospital in Karditsa, Greece.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Fahr’s syndrome, also known as basal ganglia calcification, is a rare neurodegenerative disorder characterized by radiological findings of symmetrical and bilateral idiopathic abnormal deposits of calcium in areas of the brain that control motor activity, including the basal ganglia and the cerebral cortex. There is neither a specific cure, nor a standard treatment for Fahr’s syndrome and treatment is primarily symptomatic. Brain imaging has gained widespread use in order to support clinicians in diagnosing intracranial calcifications.
Case presentation. We present a case of an 83-year-old female patient who presented with symptoms of confusion, fever, nausea, and vomiting. Clinical diagnosis of Fahr’s syndrome secondary to hypopathyroidism was based on the neuropsychiatric signs and symptoms, laboratory evidence of hypoparathyroidism, and radiological signs of calcifications in the basal ganglia. The patient improved following replacement therapy with calcium gluconate, followed by oral supplemental calcitriol.
Conclusion. This case highlights the importance of considering organic causes when patients present with neuropsychiatric disturbances, especially following thyroidectomy.
Keywords: Fahr’s syndrome, basal ganglia calcification, bilateral intracranial calcification; hypoparathyroidism
Innov Clin Neurosci. 2020;18(4–6):xx–xx
Fahr’s syndrome, also known as idiopathic basal ganglia calcification, is a rare neurological disorder. Its prevalence is lower than 0.5 percent, and it is characterized by encephalopathy and progressive and symmetric calcifications in the basal ganglia.1,2,3 Karl Theodor Fahr, a German scientist, first described it in 1930 in the case of a man with hypoparathyroidism, dementia, and symmetrical calcifications of the basal ganglia.4 Although most affected people are asymptomatic, the main clinical features of idiopathic basal ganglia calcification include neuropsychiatric symptoms and motor disorders.2,3,5,6 Fahr’s disease, on the other hand, is often inherited in an autosomal dominant pattern, although endocrine disorders, infectious diseases, and other conditions such as developmental, traumatic, toxic, metabolic, and sporadic may also cause Fahr’s syndrome.7,8
We present a case of an 83-year-old female patient who presented with low calcium and parathyroid hormone (PTH) levels, confusion, and fever secondary to Fahr’s syndrome.
Case Report
An 83-year-old female patient presented to the emergency department of our hospital with confusion, fever, nausea, and vomiting. She had a history of polymyalgia rheumatica (PMR) treated with azathioprine plus prednisone, hypoparathyroidism after total thyreoidectomy for nodular goiter, and secondary osteoporosis. Other related comorbidities included arterial hypertension, mixed anxiety–depressive disorder, and regression of gastroesophageal reflux disease. She had no family history of mental illness or neurological disease and no personal history of smoking or alcoholism.
The initial physical examination revealed hemodynamic stability with a heart rate of 80 beats per minute and a blood pressure of 145/90mmHg, oxygen saturation 78 percent on room air accompanied with tachypnea of 27 breaths per minute, and a body temperature of 38.9 degrees Celsius. On lung auscultation, harsh vesicular breathing accompanied with rhonchus was detected. Arterial pulse was palpable at the peripheries. Examination of other systems, including cardiovascular and digestive, were unremarkable. On neurological examination, the patient presented with cognitive impairment and confusion regarding orientation to time, place, and person. Reduced muscle strength of the right arm and leg and presence of Babinski sign were observed. The patient had spontaneous horizontal right-beating nystagmus, and the doll’s eyes reflex (or oculocephalic reflex) was absent on the right side. No neck stiffness was observed.
Blood tests were normal for glucose level, as well as renal and liver functions. Hypocalcaemia (7.1 mg/dL) was observed, but other electrolyte levels were normal. Parathormone serum levels (PTH) were low (14.24pg/mL), as well as hemotocrit (anemia of chronic disease, Ht= 29,1%), as a result of the existing polymyalgia. Electrocardiography demonstrated sinus rhythm with three supraventricular ectopic contractions. Chest radiograph was normal.
Infectious and non-infectious central nervous system (CNS) disease were suspected. Following stabilization of the patient’s ventilation with supplemental oxygen via a Venturi mask, a brain computed tomography (CT) scan and a lumbarspinal puncture were performed. Brain CT scan showed bilateral symmetric and extensive calcifications in the basal ganglia, periventricular white matter, and cerebellar hemispheres (Figures 1, 2).
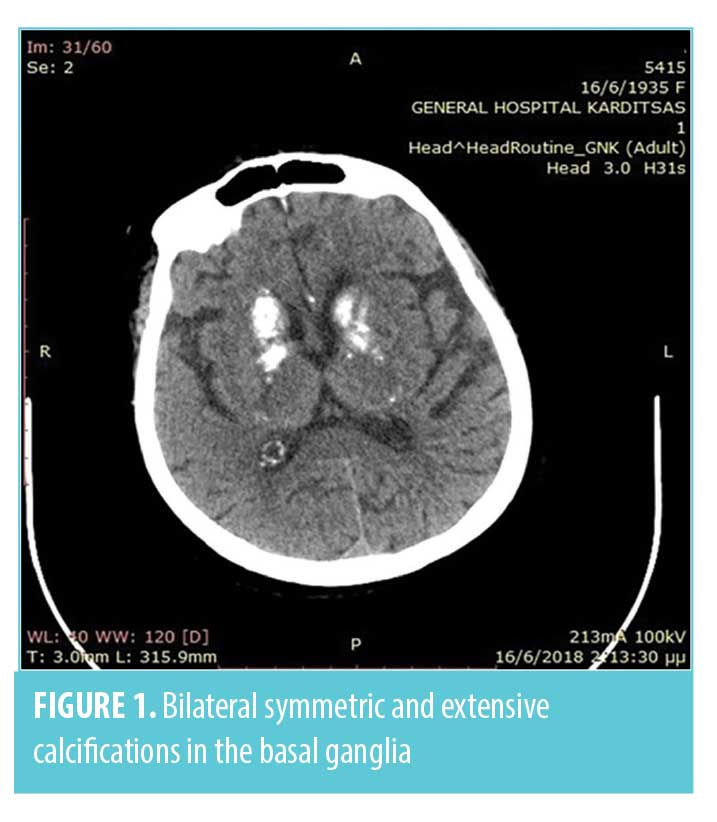
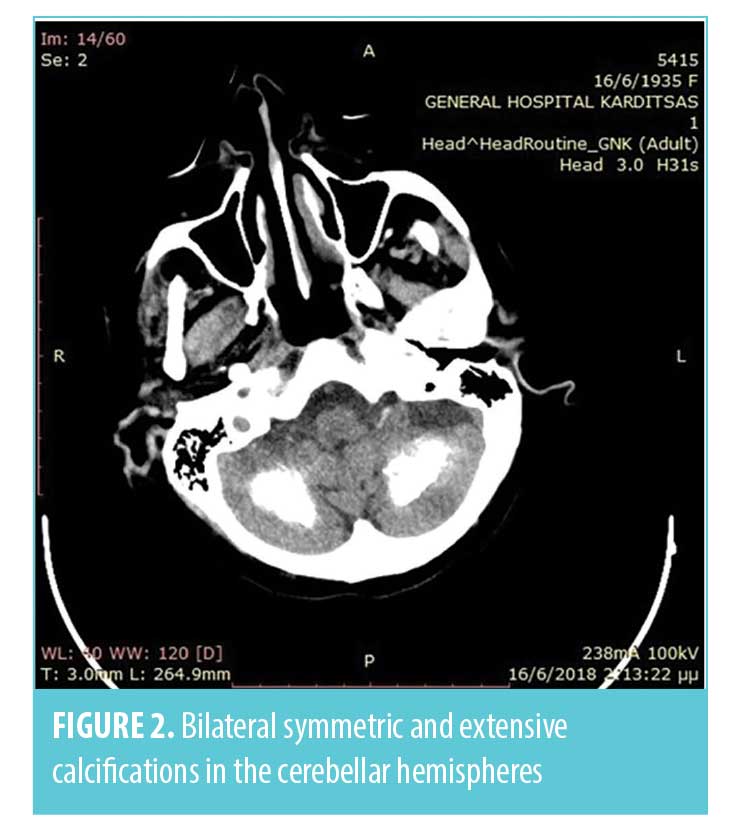
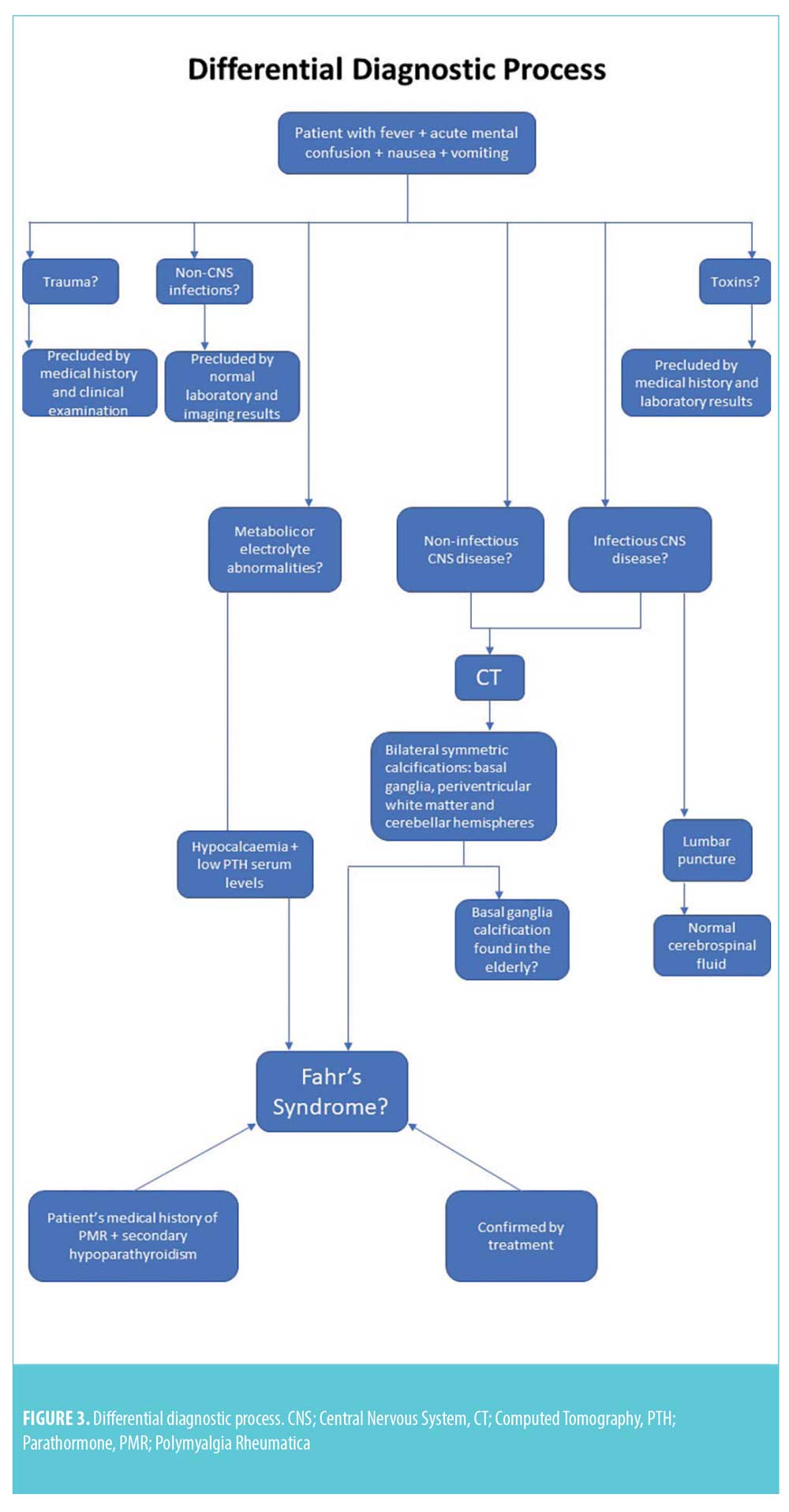
The cerebrospinal fluid analysis test results were normal and excluded the diagnosis of CNS infection. After excluding any metabolic causes, electrolyte abnormalities, infections, and toxic or traumatic etiology, a diagnosis of Fahr’s syndrome was made. This diagnosis was supported by the clinical features, CT findings, and biochemical test results. The coexistence of rheumatic polymyalgia and secondary hypoparathyroidism also supported the diagnosis of Fahr’s syndrome. Differential diagnoses of Fahr’s syndrome are presented in Figure 3.
There is no well-known treatment for Fahr’s syndrome, however, replacement therapy with calcium gluconate was administrated in combination with a broad-spectrum antibiotic for the respiratory infection treatment. The patient did not require anticonvulsant or antipsychotic treatment, and her clinical symptoms improved gradually throughout her hospital stay. On the fourth day of treatment, symptoms had resolved, including neuropsychiatric symptoms and abnormal serum calcium and PTH levels. In addition, brain CT imaging showed no changes. On the fifth day, the patient was discharged from the hospital on oral supplemental calcium and calcitriol preparations for maintenance of serum calcium in the low to normal range. At the six-month follow-up, the patient was still responding to treatment.
Discussion
Fahr’s syndrome, known also as idiopathic basal ganglia calcification, is a rare, progressive neurodegenerative disorder characterized by symmetric bilateral extensive intracranial calcifications. Although an early onset type may occur, the typical age of presentation is the fourth to sixth decade of life, with men being twice as likely to be diagnosed than women.2,3,8-10
These calcifications are separated in two main forms: primary and secondary. The primary form refers to the term Fahr’s disease and characterizes the cases of idiopathic calcifications in the brain that are not secondary to other conditions and are presented with neuropsychiatric and extrapyramidal symptoms. Although Fahr’s disease is generally considered to be idiopathic, it has a strong genetic link, with an autosomal dominant inheritance being most common. There have been several genetic mutations identified, including SLC20A2 on chromosome 8p11.2 and PDGFRB on chromosome 5q32, PDGFB on chromosome 22q13.1, and XPR1 on chromosome 1q25. In addition, other genes lead to similar phenotypes.6,11,12
In contrast to this primary form, a secondary form known as Fahr’s syndrome of bilateral and symmetric calcifications of basal ganglia and other regions of the brain can occur as the consequence of various metabolic, infectious, or degenerative diseases, including endocrinopathies, vasculitis, mitochondrial disorders, infections, radiotherapy, chemotherapy, or carbon monoxide intoxication. The most common reported metabolic disorders that cause Fahr’s syndrome are hypoparathyroidism and pseudoparathyroidism. Although the specific mechanism of brain calcification and hypocalcaemia association has not been identified, it is suggested that increased calcium-phosphorus complex formation, local disruption of the blood-brain barrier or a disorder of neuronal calcium metabolism appear to be involved in the subsidence of calcium in the brain parenchyma.10-16 Fahr’s disease should be distinguished not only from Fahr’s syndrome but from basal ganglia calcification found incidentally in the elderly population. A variety of progressive neuropsychiatric disorders are associated with Fahr’s syndrome and Fahr’s disease, although most cases are asymptomatic. Neurological symptoms may include extrapyramidal symptoms, cerebellar dysfunction, speech difficulty, dementia, vertigo, epilepsy, and syncope. Psychiatric symptoms can vary from difficulty with concentration and memory to more severe alterations in personality and behavior, psychosis, or dementia.5,6,12,17]
What makes our case of Fahr’s syndrome so unusual is that all the neurological symptoms occurred suddenly and were provoked by a respiratory infection, in contrast to the typical slowly progressive neuropsychiatric manifestations. The patient also presented with a disorder in calcium metabolism and with altered parathyroid hormone levels due to secondary medical hypoparathyroidism (following total thyroidectomy). CT scans showed classical bilateral symmetric and extensive calcifications in the basal ganglia, periventricular white matter, and cerebellar hemispheres. As previously mentioned, vasculitis may be associated with intracerebral calcification, so the existence of rheumatic polymyalgia in our patient’s past medical history supported the diagnosis of Fahr’s syndrome.
Neuroimaging with CT remains the most effective screening tool for Fahr’s syndrome and Fahr’s disease, so the radiologist’s role is paramount in not only early detection of the condition but also in correlating it with the clinical findings. Typical findings are abnormal deposition of calcium in areas of the brain that control movements, including basal ganglia, thalamus, dentate nucleus, cerebral cortex, cerebellum, subcortical white matter, and hippocampus.
There is neither a specific cure for Fahr’s syndrome nor a standard course of treatment. Case reports have suggested that correcting underlying abnormalities may improve the presenting symptoms in cases where metabolic disturbances are found, who improved with normalization of plasma calcium and PTH levels.12,14,15 Diet may play a role in the development of Fahr’s syndrome, since hypoparathyroidism is a common cause. This necessitates the intake of calcium-rich food. The recommended daily intake of calcium is 1,200mg for post-menopausal women and 1,000mg for other adults. Consumption of phosphorus-rich food, on the other hand, can lead to calcium transfer from the bones to the blood, hence increasing the likelihood of calcium deposition in the basal ganglia. Generally, fish, poultry, red meat, and whole grains should be consumed in moderation so as not to exceed the suggested daily value of 1,250mg of phosphorus. Nutritional guidance is required to maintain a balanced diet and prevent the progression of Fahr’s syndrome or Fahr’s disease.18 Most authors recommend symptomatic treatment of the neurological and psychiatric symptoms with antipsychotic, antidepressant, and/or antiepileptic medications with appropriate rehydration and electrolyte and hemodynamic maintenance. 12,13,19,20
Conclusion
Fahr’s syndrome is a poorly understood condition due to its rare occurence; however, it should be kept in mind in all cases of patients with classical clinical manifestations and neuroimaging findings. Any suspected hypoparathyroidism should be treated immediately, especially in patients who have undergone thyroidectomy, to prevent the formation and progression of brain calcifications. In Fahr’s disease, no prenatal or genetic test is available for genetic counseling. More research is required to better understand the role of genetics in Fahr’s disease. Identifying these genes could assist researchers in developing effective prevention and treatment modalities. Timely recognition of Fahr’s syndrome of Fahr’s disease is important to address clinical manifestations quickly and prevent progression.
References
- Ellie E, Julien J, Ferrer X. Familial idiopathic striopallidodentate calcifications. Neurology. 1989;39(3):381–385.
- El Hechmi S, Bouhlel S, Melki W, et al. Psychotic disorder induced by Fahr’s syndrome: a case report. Encephale. 2014;40(3):271–275.
- Verulashvili IV, Glonti LSh, Miminoshvili DK,et al. Basal ganglia calcification: clinical manifestations and diagnostic evaluation. Georgian Med News. 2006;140:39–43.
- Fahr T. Idiopathischeverkalkung der hirngefässe. ZentrablAllgPathol. 1930;50:129–133.
- Chiu HF, Lam LC, Shum PP, et al. Idiopathic calcification of the basal ganglia. Postgrad Med J. 1993;69(807):68–70.
- König P. Psychopathological alterations in cases of symmetrical basal ganglia sclerosis. Biol Psychiatry. 1989;15;25(4):459–468.
- Ramos EM, Oliveira J, Sobrido MJ, Coppola G. Primary familial brain calcification. GeneReviews® [Internet]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1421/. Published April 18, 2004. Updated August 24, 2017.
- Saleem S, Aslam HF, Anwar M, et al. Fahr’s syndrome: literature review of current evidence. Orphanet J Rare Dis. 2013;8:156.
- Jiménez-Ruiz A, Cárdenas-Sáenz O, Ruiz-Sandoval JL. Symmetrical and bilateral basal ganglia calcification. Case series and literature review. Gac Med Mex. 2018;154(2):258–262.
- Jaworski K, Styczyńska M, Mandecka M, et al. Fahr syndrome– an important piece of a puzzle in the differential diagnosis of many diseases. Pol J Radiol. 2017;82:490–493.
- Kavirayani DK, Jammulapati S. Fahr’s syndrome with psychotic features: Review and Case report. NMJ. 2014;3(2):71−74.
- Marinković DM, Dragović T, Kiković S,et al. Fahr’s syndrome and idiopathic hypoparathyroidism: a case report. vojnosanitetskipregled. VojnosanitPregl. 2017;74(2):184–188.
- Kumar S, Sher K, Ahmed S, et al. Fahr’s disease: a rare neurological disease frequently misdiagnosed as acute psychosis, or mood disorder. J Neurol Disord. 2013;1:130.
- Soares FB, Amorima FF, Santana AR, et al. Fahr’s Syndrome due to hypoparathyroidism following thyroidectomy. J Med Cases. 2013;4(6):380−384.
- Goswami R, Sharma R, Sreenivas V,et al. Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf). 2012;77(2):200−206.
- Guedes C, Macedo B. Fahr syndrome. Acta Med Port. 2013;26(2):180. Epub 2013 May 31.
- Calili DK, Mutlu NM, MutluTitiz AP, et al. Unexplained neuropsychiatric symptoms in intensive care: a fahr syndrome case. J Pak Med Assoc. 2016;66(8):1029–1031.
- Colditz GA. Healthy diet in adults. UpToDate. https://www.uptodate.com/contents/healthy-diet-in-adults. Online access on December 14, 2018.
- Caliskan T, Gurbuz S. Fahr’s Disease. J Contemp Med. 2013;3(2):133−135.
- Rizvi I, Ansari NA, Beg M, et al. Widespread intracranial calcification, seizures and extrapyramidal manifestations in a case of hypoparathyroidism. N Am J MedSci. 2012;4(8):369−372.





