
by Mark G. A. Opler, PhD, MPH; Amy Claxton, PhD*; James McGrory, PhD; Sabina Gasper, MPH*; Meihua Wang, PhD*; and Sergey Yagoda, MD, PhD
Dr. Opler is with WCG, Inc. in New York, New York. Drs. Claxton, McGrory, Wang, and Yagoda and Ms. Gasper are with Alkermes, Inc. in Waltham, Massachusetts.
*Drs. Claxton and Wang and Ms. Gasper were employees of Alkermes, Inc. at the time of the study.
Funding: This study was sponsored by Alkermes, Inc. (Waltham, MA, USA). Medical writing and editorial support were provided by Kathleen Dorries, PhD, and John H. Simmons, MD, of Peloton Advantage, LLC (Parsippany, NJ, USA), an OPEN Health company, and funded by Alkermes, Inc.
Disclosures: MGAO is an employee and shareholder of WCG, Inc. AC, SG, and MW were employees of Alkermes, Inc., at the time of the study and may own stock/options in the company. JM and SY are employees of Alkermes, Inc., and may own stock/options in the company.
Innov Clin Neurosci. 2024;21(1–3):43–51.
Abstract
Background: Clinical practice guidelines support efforts to improve functioning in patients with schizophrenia. Discrepancies in the perception of cognitive status between clinicians, patients with schizophrenia, and their caregivers have been associated with impaired functional abilities in patients; medication side effects might worsen both cognition and daily functioning. We assessed daily/social functioning and cognition in stable patients with schizophrenia who switched to the long-acting injectable (LAI) antipsychotic aripiprazole lauroxil (AL).
Methods: Clinically stable adults with residual symptoms of schizophrenia or intolerance following three or more doses of paliperidone palmitate or risperidone LAI were switched to flexibly dosed open-label AL treatment (441mg, 662mg, or 882mg every 4 weeks or 882mg every 6 weeks) for six months (ClinicalTrials.gov identifier: NCT02634320). Daily/social functioning was assessed using the Personal and Social Performance Scale (PSP); total and subscale scores were summarized using descriptive statistics. The cognitive status of patients was assessed using the New York Assessment of Adverse Cognitive Effects of Neuropsychiatric Treatment (NY-AACENT) at baseline and Month 6 or early termination, providing patient, caregiver, and clinician perspectives. A post hoc analysis assessed level of agreement in ratings of cognitive status among respondents, evaluated at baseline and last assessment, using weighted kappa coefficients (0.01–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement.).
Results: All 51 enrolled patients received one or more AL doses; 35 completed the study, and 45 contributed data at last assessment. Mean age was 40.6 years; 72.5 percent of patients were male. Based on PSP total score, functioning was maintained from baseline (mean [standard deviation (SD)]: 55.1 [10.5]) through six months of AL treatment (mean [SD]: 57.7 [13.2]). Proportions of patients rating personal and social functioning issues as “not present” or “mild” remained stable between baseline and Month 6 for each PSP subscale. At baseline (n=50), cognitive difficulties were most commonly rated “not present” or “mild” in all NY-AACENT domains by patients (58–86% across domains), clinicians (62–94%), and caregivers (50–92%), and these rates were maintained or increased at last assessment for all reporters. Weighted kappa coefficients indicated fair-to-substantial agreement between patients and clinicians across domains at last assessment (0.32–0.64; baseline: 0.14–0.55); patient–caregiver agreement ranged from 0.07 to 0.50 at last assessment (baseline: 0.25–0.60).
Conclusion: In clinically stable patients with schizophrenia who initiated AL, self-reported functioning was maintained over six months of treatment. Clinician-, caregiver-, and patient-reported cognitive function was stable at baseline and maintained in all NY-AACENT domains; patient–clinician agreement on level of cognitive impairment increased over six months of treatment with AL.
Keywords: Aripiprazole lauroxil, switch, cognition, function, schizophrenia
Clinical practice guidelines recommend long-term, continuous pharmacologic treatment in combination with psychosocial interventions to optimize symptomatic and functional recovery in patients with schizophrenia.1–3 For clinically stable patients, treatment goals include relapse prevention and maintenance or improvement of functioning and quality of life.3 Antipsychotic treatment is associated with significant improvement in clinical symptoms of schizophrenia and reduced risk of relapse.4–6 However, functional improvement does not necessarily follow reductions in symptoms,7 and patients who achieve symptomatic remission nonetheless might have poor work and social functioning.8 Furthermore, impaired cognition is associated with poor overall functionality in patients with schizophrenia.9,10 Individuals with schizophrenia might have deficits across multiple cognitive domains,11,12 and these impairments could be exacerbated by side effects associated with antipsychotic medication.13,14
Many patients with schizophrenia require changes in their antipsychotic medication, most often because of inadequate efficacy or intolerable adverse effects.15–17 When considering a medication switch in patients with stable symptoms, clinicians should weigh the possible benefits of the new antipsychotic against the potential for clinical deterioration and worsening function.18 A Phase IV study assessed the treatment effectiveness, safety, and tolerability of the long-acting injectable (LAI) antipsychotic aripiprazole lauroxil (AL; Aristada, Alkermes, Inc.; Waltham, MA) in clinically stable patients with schizophrenia who switched from their prior LAI antipsychotic because of persistent symptoms or poor tolerability.19 In that study, improvement in clinical symptoms was observed over six months of open-label AL treatment.19 Here, patient-reported functioning, caregiver burden, and medication satisfaction in patients with schizophrenia who switched to AL are reported. In a post hoc analysis of data from this same study, patient, clinician, and caregiver ratings of cognitive impairment were examined after the switch to AL treatment, and the level of agreement between these respective ratings was assessed.
Methods
This six-month, open-label, prospective, Phase IV study (ClinicalTrials.gov identifier: NCT02634320)19 was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and its amendments20 and with local regulations and International Council for Harmonisation guidelines.21 The protocol and amendment were approved by site-specific independent ethics committees/institutional review boards. All patients and their caregivers provided written informed consent before any study procedures were performed.
Participants. The study enrolled adult outpatients aged 18 to 65 years with a diagnosis of schizophrenia in accordance with Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) criteria.22 Eligible patients were clinically stable (defined as no hospitalizations for acute psychiatric exacerbation within the 2 months prior to screening and prior to initiation on Day 1), had a Brief Psychiatric Rating Scale (BPRS)23 score of 30 to 45 at screening, and resided in a stable living situation with a reliable caregiver in the opinion of the investigator.
Enrolled patients had received at least three doses of a prior LAI antipsychotic (paliperidone palmitate [PP] or risperidone LAI [RLAI]) before screening, with no antipsychotic medication regimen change in the four weeks before study Day 1. The option to switch to AL was provided for patients with an inadequate response or intolerance to PP or RLAI and based on the investigator’s opinion that the patient was likely to benefit from the switch. Eligible patients must also have demonstrated tolerability to oral aripiprazole, either with prior treatment or with two test doses of 5mg/day on two consecutive days during the screening period. Patients were ineligible if they had received AL, intramuscular (IM) depot aripiprazole, or the three-month formulation of PP within six months of screening; had a history of poor response to aripiprazole; or had a primary diagnosis other than schizophrenia per the DSM-5 criteria.
Study design. Patients were switched from their prior LAI antipsychotic to six months of open-label treatment with AL (441mg, 662mg, or 882mg every 4 weeks or 882mg every 6 weeks). The initial AL dose selection and any subsequent dose adjustments were made based on the investigator’s clinical judgment. Augmentation with oral antipsychotics that were started prior to study entry was permitted at the investigator’s discretion.19
Study visits were scheduled at screening, study Day 1, and monthly intervals for six months or at early termination. For patients administered AL 882mg every six weeks, visits were scheduled at six-week intervals after study Day 1.
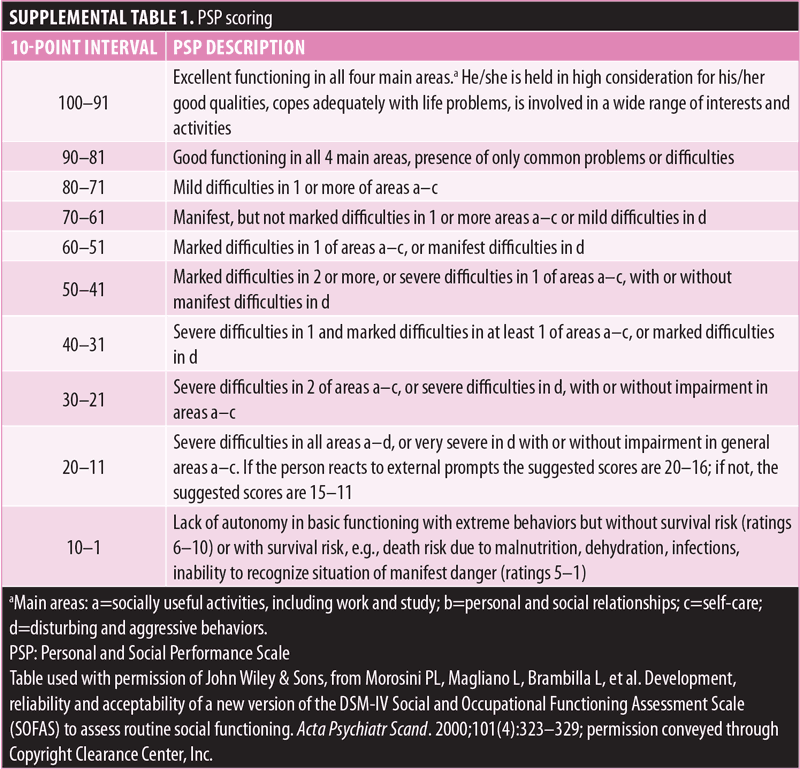
Outcome measures and assessments. Daily and social functioning were assessed using the patient-reported Personal and Social Performance Scale (PSP)24 at baseline (screening visit), three months, and six months or early termination. The PSP is scored based on four subscales—Socially Useful Activities (including work and study), Personal and Social Relationships, Self-care, and Disturbing and Aggressive Behaviors—with problems in each rated as “absent,” “mild,” “manifest,” “marked,” “severe,” or “very severe.” PSP total score ranges from 0 to 100; higher scores indicate better functioning (Supplemental Table 1).
A modified three-question Medication Satisfaction Questionnaire (MSQ)25 was used to assess satisfaction with current LAI medication (scored 1 [“very dissatisfied”]–5 [“very satisfied”]), preference for current versus previous LAI medication (scored 1 [“much prefer previous LAI”]–5 [“much prefer current LAI”]), and side effects of current versus previous LAI medication (scored 1 [“much less side effects”]–5 [“much more side effects”]). Patients completed Question #1 of the modified MSQ at screening (prior to switch) and all three questions at six months after switch or early termination.
Burden on caregivers was measured using the Burden Assessment Scale (BAS),26 a 19-item scale that focuses on subjective and objective consequences of caring for persons with severe mental disorders. The BAS was completed at baseline (screening visit) and at six months or early termination by the patient’s designated caregiver. Respondents rated subjective burden over the past four weeks for the 19 items, scoring each item as 1 (“not at all”) to 4 (“a lot”) or not applicable. BAS total score was calculated as the sum of the 19 item scores, with higher scores indicating greater caregiver burden (range: 19 [lowest possible burden]–76 [highest possible burden]); missing item scores were imputed based on the average of nonmissing items at that visit.
The New York Assessment of Adverse Cognitive Effects of Neuropsychiatric Treatment (NY-AACENT)27 was designed to evaluate the presence and severity of adverse cognitive effects in patients treated with medications, including antipsychotic agents. The instrument, administered at baseline (screening visit) and six months or early termination, includes a patient form, clinician form, and caregiver form for assessing patient cognitive impairment from each perspective. The NY-AACENT includes seven domains: Working Memory, Attention/Vigilance, Verbal Learning/Memory, Visual Learning/Memory, Reasoning and Problem Solving, Speed of Processing, and Social Cognition. Cognitive difficulty in each domain was rated as present or not present over the past week; if present, the severity of difficulty in that domain was rated as “mild,” “moderate,” “severe,” or “extreme.”
Statistical analysis. The PSP, MSQ, and BAS were summarized using descriptive statistics for all patients who received one or more doses of AL. For the PSP and BAS, mean (standard deviation [SD]) scores and change from baseline (screening visit) at six months and last assessment (last on-treatment visit at 6 months or early termination) were calculated. On the PSP, the proportion of patients reporting functioning issues as “absent,” “mild,” “manifest,” “marked,” “severe,” or “very severe” on each subscale were also summarized. MSQ responses at six months and last assessment were summarized as proportions of patients endorsing each response level.
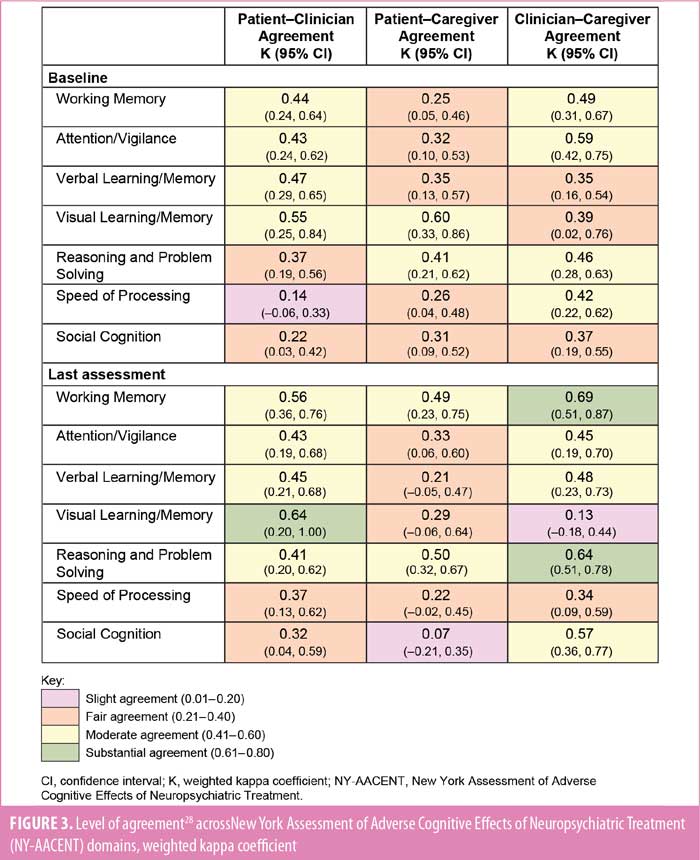
A post hoc analysis of the NY-AACENT was conducted using data from all patients previously administered PP prior to study entry. The proportion of patients, clinicians, and caregivers who endorsed each categorical response (“not present,” “mild,” “moderate,” “severe,” “extreme”) were summarized for each NY-AACENT domain at baseline and last assessment. The level of agreement between respondents in ratings of cognitive difficulty was then evaluated using weighted kappa coefficients for each NY-AACENT domain at baseline and last assessment. Kappa coefficient values were interpreted as follows: 0 or less, no agreement; 0.01 to 0.20, slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.00, almost perfect agreement.28
Results
Participants. All enrolled patients (N=51) received at least one AL dose. Thirty-five patients completed the study, and 45 contributed data at last assessment; 50 of 51 patients were being treated with PP before the switch to AL. Among the 16 patients who discontinued early, the day of last visit ranged from study Day 51 to study Day 237; the mean time between last AL injection and last on-treatment assessment was 39.9 days (SD: 21.7; median: 36 days).19 Participants were predominantly male (72.5%), and most were either Black (49.0%) or White (43.1%). Mean (SD) age at baseline was 40.6 (11.7) years.
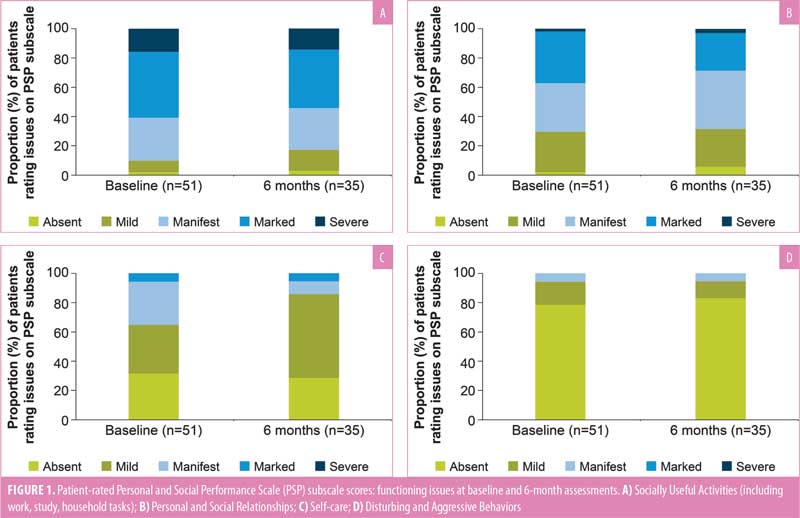
Personal and social performance. At baseline, mean (SD) PSP total score was 55.1 (10.5). On the PSP subscales, the most frequent responses reported by patients indicated an absence of difficulties related to Disturbing and Aggressive Behaviors (78.4%), mild difficulties with Self-care (33.3%), and marked difficulties with Socially Useful Activities (45.1%) and Personal and Social Relationships (35.3%; Figure 1). Mean PSP total score remained stable over six months of AL treatment (Table 1). The proportion of patients who rated functioning issues as either “absent” or “mild” was 9.8 percent at baseline and 17.1 percent at six months for Socially Useful Activities and 64.7 percent at baseline and 85.7 percent at six months for Self-care (Figure 1). The proportion of patients who rated functioning issues as “absent” or “mild” was maintained from baseline to Month 6 for Personal and Social Relationships (29.4–31.4%) and Disturbing and Aggressive Behaviors (94.1–94.3%).
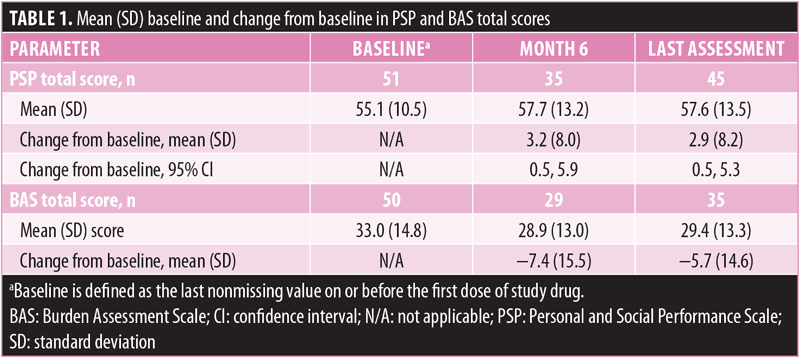
Caregiver burden. Mean (SD) baseline BAS total score was 33.0 (14.8). Perceived caregiver burden was generally stable over six months of AL treatment. A small decrease was observed in mean BAS total score at Month 6 (−7.4) and last assessment (−5.7), but variability was large (Table 1).
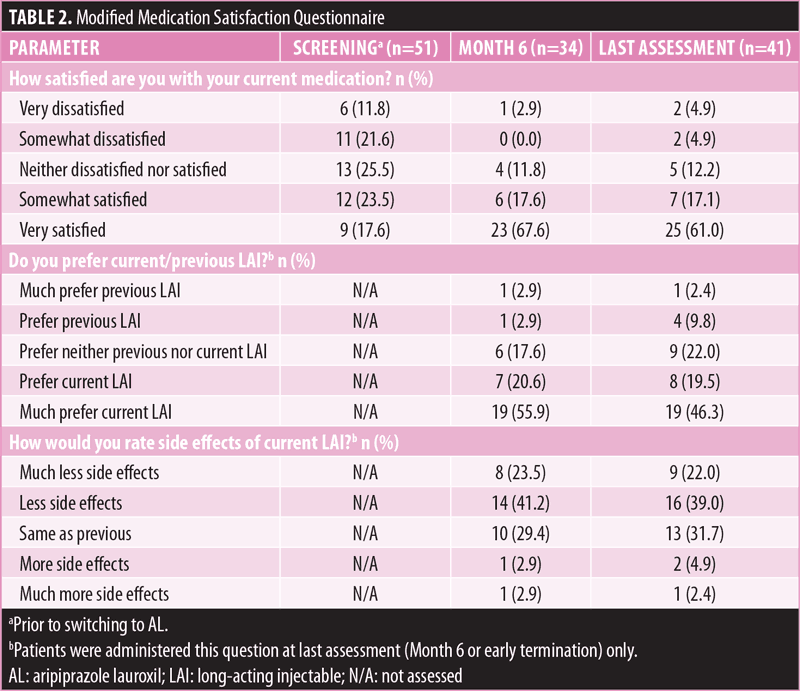
Medication satisfaction. Patient satisfaction with medication at screening (i.e., satisfaction with prior LAI medication) was distributed across response categories, from “very dissatisfied” to “very satisfied;” the largest proportion of patients indicated they were “neither dissatisfied nor satisfied” with their prior LAI antipsychotic (25.5%; Table 2). After up to six months of AL treatment, the majority of patients indicated that they were “very satisfied” with their current medication (Month 6: 67.6%; last assessment: 61.0%). Overall, 76.5 percent of patients who completed the study and 65.9 percent of patients who discontinued the study early “preferred” or “much preferred” AL over their previous LAI antipsychotic at Month 6. When asked to rate the side effects of AL versus their prior LAI antipsychotic at six months, 41.2 percent of patients who completed the study selected “less side effects,” and 29.4 percent selected “same as previous.” Responses were similar when patients who discontinued early were included (last assessment: “less side effects,” 39.0%; “same as previous,” 31.7%).
Cognitive symptoms. All 50 patients who switched from PP to AL completed the NY-AACENT at baseline and were included in the post hoc analysis; 39 of those patients also completed the NY-AACENT at six months or early termination (last assessment). Clinician and caregiver NY-AACENT data were available for 50 patients at baseline and 38 patients at last assessment.
At baseline, cognitive difficulties were most commonly reported as “not present” or “mild” across all NY-AACENT domains by patients, clinicians, and caregivers; however, there was substantial variation across domains. Clinician ratings of cognitive impairment that was “not present” included Social Cognition (32%), Speed of Processing (34%), Reasoning and Problem Solving (38%), Working Memory (46%), Attention/Vigilance (48%), Verbal Learning/Memory (52%), and Visual Learning/Memory (88%; Figure 2). A greater proportion of clinicians, compared to patients, reported cognitive impairment was “not present” or “mild” at baseline for all NY-AACENT domains, except Reasoning and Problem Solving. The proportion of patients, clinicians, or caregivers reporting moderate cognitive impairment at baseline ranged from 6 to 30 percent across NY-AACENT domains. From baseline to last assessment, there was a shift in the distribution of responses toward “not present” or “mild,” with a decrease in “moderate,” “severe,” and “extreme” responses for all reporters and across all domains. This pattern is illustrated by the shift from blue to green bars in Figures 2A–G.
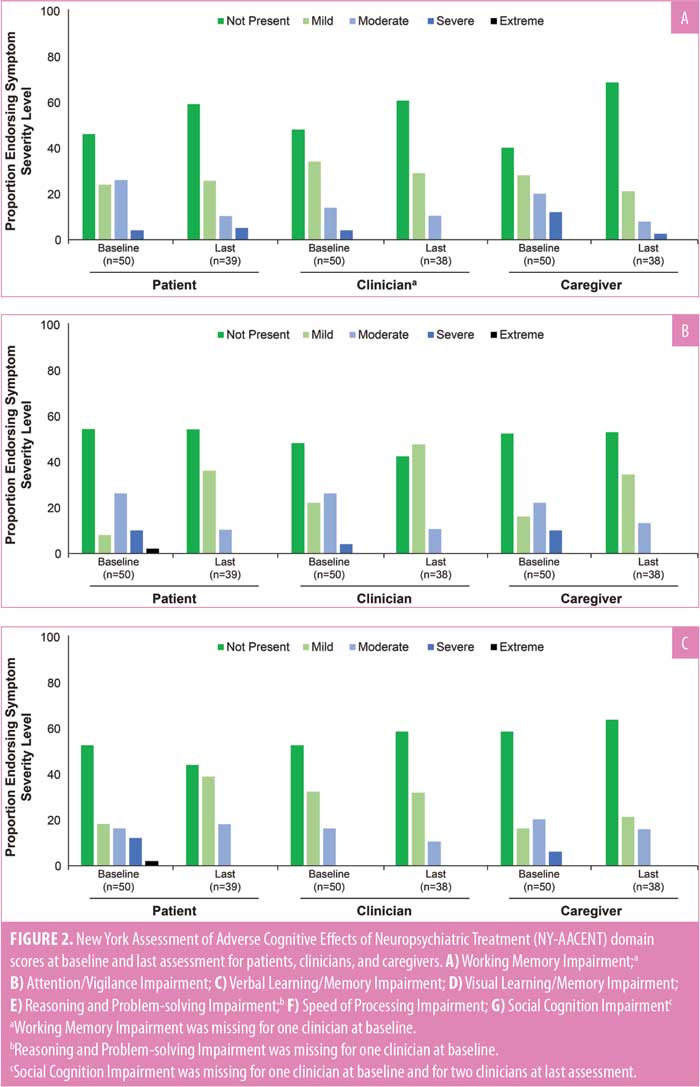
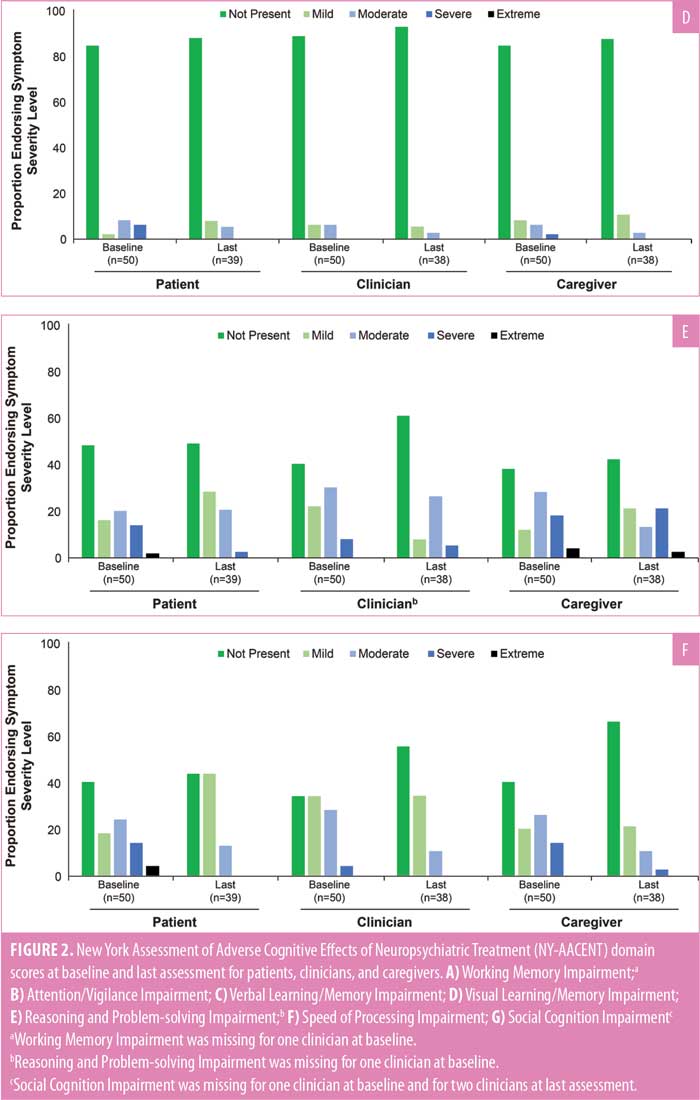
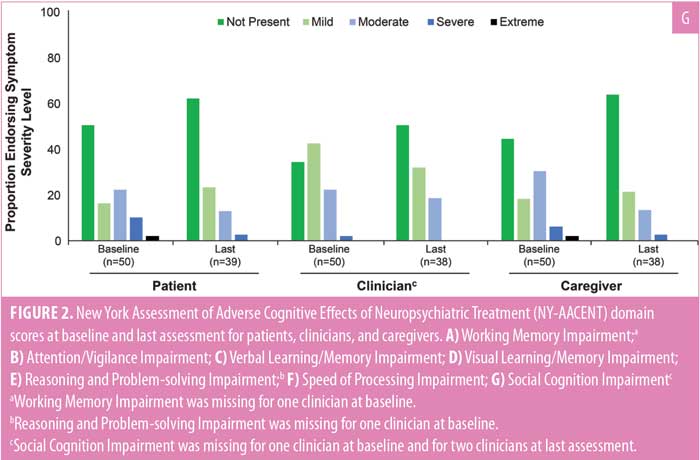
There was some variation in agreement across NY-AACENT domains at both assessments. Weighted kappa coefficients indicated fair-to-moderate patient–clinician, patient–caregiver, and caregiver–clinician agreement for most domains at baseline (Figure 3). The level of agreement was generally greater between patients and clinicians and between clinicians and caregivers than between patients and caregivers; however, the domains of greatest agreement varied. Agreement between patients and clinicians was stable or increased between baseline and last assessment across most domains; agreement was lowest for Speed of Processing and Social Cognition at both timepoints. The change in level of agreement between patients and caregivers and between clinicians and caregivers was less consistent across domains (Figure 3).
Discussion
Based on the results from these analyses, maintenance of patient-reported daily and social functioning based on PSP total score was consistent with the statistically significant improvement in BPRS and Clinical Global Improvement-Severity Scale scores previously reported for these patients.19 Proportions of patients with “absent” or “mild” functional issues were maintained from baseline to six months for each domain. In the current analysis, caregivers noted no increase in the magnitude of burden of care for these patients over the duration of the study. Overall, patients in this study indicated they preferred or much preferred AL over their prior LAI antipsychotic and were satisfied or very satisfied with AL treatment six months after the switch. Similarly, in a previous study in which AL was administered as a two-month formulation, the majority of patients (80.7%) reported they were satisfied or very satisfied with AL at 25 weeks.29
Poor cognitive functioning is a common and burdensome impairment in patients with schizophrenia,9–12,30,31 underscoring the need for assessment of cognition in clinical trials of therapies that might address cognitive deficits.32,33 In clinical trials, improvement in cognitive symptoms has been reported in patients with schizophrenia treated with antipsychotic medications.34 However, effects on cognition vary among medications,34 and worsening of cognition has been associated with the anticholinergic burden of antipsychotic treatment in schizophrenia.13,14,35 In the current study, although most patients reported cognitive impairment as “not present” or “mild” at baseline, the proportion of patients reporting “moderate,” “severe,” or “extreme” cognitive impairment after up to six months of AL treatment did not increase in any domain. Impairment in this patient population might have been milder than in previously reported study populations because the study enrolled patients who were clinically stable, with BPRS scores ranging from 30 to 45 (corresponding to mildly to moderately ill23). Additionally, patients in the current study were receiving LAI antipsychotic treatment at screening, and long-term atypical antipsychotic treatment can provide substantial cognitive benefits to patients with schizophrenia.36,37
In previously published studies, patients with schizophrenia have generally underestimated the severity of their own cognitive impairment, such that patient-reported deficits have shown little correlation with objective cognitive assessments or judgments of day-to-day cognitive functioning from reliable informants.38–44 In the current study, however, patient perceptions of cognition were consistent with clinician and caregiver reports, with similar proportions of patients and clinicians reporting “moderate,” “severe,” or “extreme” impairment in most domains. The analysis of interrater agreement indicated that patients and clinicians were more aligned on their assessments of cognitive impairment in some domains than others (with greatest agreement on Visual Learning/Memory), and kappa values generally increased in most domains over the course of the study (Figure 3). At last assessment, patient–clinician agreement was greater than patient–caregiver agreement for most, but not all, domains. While the observed level of agreement was somewhat unexpected, correlations of a similar magnitude have previously been reported between self- and informant-reported cognitive functioning in clinically stable outpatients with schizophrenia (r-values: 0.29–0.42).45
Among individual patients with schizophrenia, agreement between patient-reported deficits and clinician assessments or objective functioning can vary with the patient’s level of insight into illness.46,47 In an analysis of data from patients enrolled in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, assessments of schizophrenia severity were generally consistent between patients and clinicians, except in the 10 percent of patients with a severe lack of insight, based on the Positive and Negative Syndrome Scale insight item.47 On average, patients with very poor insight into illness rated their schizophrenia symptoms as less severe, compared to clinicians’ ratings.47 In a second analysis using the CATIE dataset, moderate-to-severe insight impairment was associated with poor performance on objective cognitive assessments and lower daily functioning scores, compared to mild insight impairment, most notably in patients with severe insight impairment,48 and lower levels of cognition have been associated with overestimation of functioning in patients with schizophrenia.48–50 Insight into illness was not assessed in the current analysis. However, the number of patients in this study population with severely impaired insight would likely be low, based on the 10 percent subgroup size in the CATIE analysis, thereby minimizing the effect of insight on the observed correlations. Additional analysis of clinical characteristics associated with degree of alignment between raters for different cognitive domains might be valuable to further the understanding of patient perspectives of their level of functioning and insight into illness and to facilitate better incorporation of patient input into treatment goals.47,51 In particular, further analysis is needed to examine associations between cognitive domains with low patient–clinician agreement and patient functioning during treatment with LAI antipsychotics.
Limitations. Interpretation of study results is limited by the open-label study design, as all patients were treated with AL without another treatment for comparison; therefore, PSP, MSQ, and BAS scores were only summarized descriptively in this analysis. Concomitant oral antipsychotics were permitted during the study and could have affected outcomes in some patients. Also, because the sample size was small and the study population was limited to those who met inclusion and exclusion criteria, these results might not be generalizable to all patients with schizophrenia who are treated with antipsychotic medications. Furthermore, the effects of patient attrition on study population composition over time might have introduced bias at later assessments.
The post hoc NY-AACENT analysis had several additional limitations. That analysis was based on available data from a clinical trial that was not designed and powered to assess the agreement between patient, clinician, and caregiver responses. The NY-AACENT was developed to evaluate cognitive impairment due to treatment, not to evaluate overall fluctuations in status, and observers may not always be readily able to distinguish between disease- and drug-related effects.52 Furthermore, cognition was not measured objectively, as the NY-AACENT is based on subjective impression, rather than performance-based testing, and is therefore potentially prone to various subjective biases. Finally, patients, clinicians, and caregivers might understand or value aspects of cognition differently.
Conclusion
In this post hoc analysis of data from clinically stable patients with schizophrenia who switched to treatment with AL, self-reported daily and social functioning was maintained over up to six months of treatment. Caregivers for the enrolled patients reported no increase in burden associated with the switch to AL. After up to six months of open-label AL treatment, patients generally preferred AL over their prior medication, and most reported fewer side effects with AL. For patients, clinicians, and caregivers in this study, there was a shift in the distribution of ratings of cognitive impairment toward “not present” or “mild” at last assessment and across all seven NY-AACENT domains. These results suggest that up to six months of open-label treatment with AL was not associated with negative effects on cognitive functioning in patients with schizophrenia who switched from their prior LAI antipsychotic. Levels of agreement between patient and clinician assessments of cognitive difficulties were maintained or increased between baseline and six months across most cognitive domains.
Acknowledgments
The authors thank Mark S. Todtenkopf, PhD, of Alkermes, Inc., who assisted in the preparation and proofreading of the manuscript.
Data Sharing Statement
The data collected in this study are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws, and requests can be submitted to the corresponding author.
References
- Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Third edition. American Psychiatric Association; 2021.
- National Collaborating Centre for Mental Health (UK). Psychosis and Schizophrenia in Adults: Prevention and Management. National Institute for Health and Care Excellence; 2014.
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44.
- Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071.
- Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942.
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(Suppl 3):1–24.
- Fleischhacker WW, Rabinowitz J, Kemmler G, et al. Perceived functioning, well-being and psychiatric symptoms in patients with stable schizophrenia treated with long-acting risperidone for 1 year. Br J Psychiatry. 2005;187:131–136.
- Ventura J, Subotnik KL, Guzik LH, et al. Remission and recovery during the first outpatient year of the early course of schizophrenia. Schizophr Res. 2011;132(1):18–23.
- Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418–425.
- Nuechterlein KH, Ventura J, Subotnik KL, Bartzokis G. The early longitudinal course of cognitive deficits in schizophrenia. J Clin Psychiatry. 2014;75(Suppl 2):25–29.
- Hoff AL, Sakuma M, Wieneke M, et al. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156(9):1336–1341.
- Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033–2046.
- Eum S, Hill SK, Rubin LH, et al. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res. 2017;190:129–135.
- Ang MS, Abdul Rashid NA, Lam M, et al. The impact of medication anticholinergic burden on cognitive performance in people with schizophrenia. J Clin Psychopharmacol. 2017;37(6):651–656.
- Edlinger M, Baumgartner S, Eltanaihi-Furtmüller N, et al. Switching between second-generation antipsychotics: why and how? CNS Drugs. 2005;19(1):27–42.
- Nyhuis AW, Faries DE, Ascher-Svanum H, et al. Predictors of switching antipsychotic medications in the treatment of schizophrenia. BMC Psychiatry. 2010;10:75.
- Guinart D, Varma K, Segal Y, et al. Reasons for antipsychotic treatment switch: a systematic retrospective review of prescription records and prescriber notes. J Clin Psychiatry. 2022;83(5):21m14272.
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93.
- Miller BJ, Claxton A, Du Y, et al. Switching patients with schizophrenia from paliperidone palmitate to aripiprazole lauroxil: a 6-month, prospective, open-label study. Schizophr Res. 2019;208:44–48.
- World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. 6 Sep 2022. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 13 Feb 2023.
- European Medicines Agency. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Guideline for good clinical practice E6(R2). 1 Dec 2016. https://www.gmp-compliance.org/files/guidemgr/WC500002874.pdf. Accessed 13 Feb 2023.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. American Psychiatric Publishing; 2013.
- Leucht S, Kane JM, Kissling W, et al. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005;187:366–371.
- Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329.
- Kalali A. Patient satisfaction with, and acceptability of, atypical antipsychotics. Curr Med Res Opin. 1999;15(2):135–137.
- Reinhard SC, Gubman GD, Horwitz AV, Minsky S. Burden Assessment Scale for families of the seriously mentally ill. Eval Program Plann. 1994;17(3):261–269.
- Correll C, Kohegyi E, Baker R, et al. T45. The New York Assessment of Adverse Cognitive Effects of Neuropsychiatric Treatment (NY-AACENT): initial validation findings. Schizophr Bull. 2019;45(Suppl 2):S221.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–282.
- Nasrallah HA, Weiden PJ, Walling DP, et al. Aripiprazole lauroxil 2-month formulation with 1-day initiation in patients hospitalized for an acute exacerbation of schizophrenia: exploratory efficacy and patient-reported outcomes in the randomized controlled ALPINE study. BMC Psychiatry. 2021;21(1):492.
- Fatouros-Bergman H, Cervenka S, Flyckt L, et al. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res. 2014;158(1–3):156–162.
- Kitchen H, Rofail D, Heron L, Sacco P. Cognitive impairment associated with schizophrenia: a review of the humanistic burden. Adv Ther. 2012;29(2):148–162.
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213.
- Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220.
- Désaméricq G, Schurhoff F, Meary A, et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol. 2014;70(2):127–134.
- Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838–847.
- Harvey PD, Bowie CR, Loebel A. Neuropsychological normalization with long-term atypical antipsychotic treatment: results of a six-month randomized, double-blind comparison of ziprasidone vs. olanzapine. J Neuropsychiatry Clin Neurosci. 2006;18(1):54–63.
- de Filippis R, Staltari FA, Aloi M, et al. Effectiveness of SGA-LAIs on clinical, cognitive, and social domains in schizophrenia: results from a prospective naturalistic study. Brain Sci. 2023;13(4):577.
- Keefe RS, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426–432.
- Medalia A, Thysen J, Freilich B. Do people with schizophrenia who have objective cognitive impairment identify cognitive deficits on a self report measure? Schizophr Res. 2008;105(1–3):156–164.
- Poletti S, Anselmetti S, Riccaboni R, et al. Self-awareness of cognitive functioning in schizophrenia: patients and their relatives. Psychiatry Res. 2012;198(2):207–211.
- Durand D, Strassnig M, Sabbag S, et al. Factors influencing self-assessment of cognition and functioning in schizophrenia: implications for treatment studies. Eur Neuropsychopharmacol. 2015;25(2):185–191.
- Gould F, McGuire LS, Durand D, et al. Self-assessment in schizophrenia: accuracy of evaluation of cognition and everyday functioning. Neuropsychology. 2015;29(5):675–682.
- Burton CZ, Harvey PD, Patterson TL, Twamley EW. Neurocognitive insight and objective cognitive functioning in schizophrenia. Schizophr Res. 2016;171(1–3):131–136.
- Durand D, Strassnig MT, Moore RC, et al. Self-reported social functioning and social cognition in schizophrenia and bipolar disorder: using ecological momentary assessment to identify the origin of bias. Schizophr Res. 2021;230:17–23.
- Halverson TF, Hajdúk M, Pinkham AE, et al. Psychometric properties of the Observable Social Cognition Rating Scale (OSCARS): self-report and informant-rated social cognitive abilities in schizophrenia. Psychiatry Res. 2020;286:112891.
- Chuang SP, Wu JYW, Wang CS. Self-perception of mental illness, and subjective and objective cognitive functioning in people with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:967–976.
- Lysaker PH, Weiden PJ, Sun X, et al. Impaired insight in schizophrenia: impact on patient-reported and physician-reported outcome measures in a randomized controlled trial. BMC Psychiatry. 2022;22(1):574.
- Siu CO, Harvey PD, Agid O, et al. Insight and subjective measures of quality of life in chronic schizophrenia. Schizophr Res Cogn. 2015;2(3):127–132.
- Ermel J, Carter CS, Gold JM, et al. Self versus informant reports on the specific levels of functioning scale: relationships to depression and cognition in schizophrenia and schizoaffective disorder. Schizophr Res Cogn. 2017;9:1–7.
- Kim SJ, Jung DU, Moon JJ, et al. Relationship between disability self-awareness and cognitive and daily living function in schizophrenia. Schizophr Res Cogn. 2021;23:100192.
- Correll CU, Ismail Z, McIntyre RS, et al. Patient functioning, life engagement, and treatment goals in schizophrenia. J Clin Psychiatry. 2022;83(5):LU21112AH21112.
- Correll CU, Kohegyi E, Zhao C, et al. Oral aripiprazole as maintenance treatment in adolescent schizophrenia: results from a 52-week, randomized, placebo-controlled withdrawal study. J Am Acad Child Adolesc Psychiatry. 2017;56(9):784–792.




