
by Eirini Beneki, MD, MSc, PhD; Kyriakos Dimitriadis, MD, PhD; Konstantinos Tsatiris, MD, MSc; Konstantina Aggeli, MD, PhD; and Konstantinos Tsioufis, MD, PhD
Drs. Beneki, Dimitriadis, Aggeli, and Tsioufis are with First Cardiology Department, School of Medicine, Hippokration General Hospital, National and Kapodistrian University of Athens in Athens, Greece. Dr. Tsatiris is with Department of Cardiology, Karditsa General Hospital in Karditsa, Greece.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2023;20(10–12):9–11.
Abstract
Transient loss of consciousness (TLOC) is a common presentation to emergency departments and may be due to syncope or epileptic seizures. The distinction between both entities can be challenging. This case illustrates the need for a multidisciplinary team approach in TLOC to avoid misdiagnosis leading to improper treatment.
Keywords: Noncompaction cardiomyopathy, epilepsy, ventricular tachycardia, seizure, loss of consciousness
Transient loss of consciousness (TLOC) represents a significant healthcare burden, affecting a large proportion of the global population. Syncope and epileptic seizures comprise the two major groups of TLOC.1 Seizures are episodes of an abnormally increased or synchronous neuronal activity in the brain, commonly resulting in epileptic spasms. Convulsive syncope resulting from cerebral hypoperfusion due to cardiac arrhythmias or vasovagal reactions may also be accompanied by tonic or myoclonic activity,2 making its distinction from seizures troublesome.
In an effort to reduce waste of economical and human resources and unneeded examinations, current guidelines have proposed a diagnostic algorithm of TLOC that could improve diagnostic accuracy and risk stratification for sudden death.1
In this case, we present the diagnostic and therapeutic approach of a female patient with TLOC admitted to our hospital. A statement of consent was obtained from the patient prior to the publication of this report.
Case Presentation
A 36-year-old female patient without previous comorbidities and no family history of sudden cardiac death or related cardiomyopathies presented to the emergency department of our institution after an unwitnessed TLOC. The patient reported no presyncope symptoms and suffered dental traumatic injuries. There was no urinary or bowel incontinence.
Physical examination and routine blood tests were unremarkable. The baseline electrocardiogram demonstrated atrial fibrillation with rapid ventricular response that was converted to sinus rhythm spontaneously without atrioventricular or intraventricular conduction abnormalities. To exclude stroke or other brain lesion, although focal neurological deficits were not detected on clinical neurological examination, the patient underwent computed tomography (CT) and magnetic resonance imaging (MRI) scans of the brain, which revealed normal findings. The carotid and vertebral ultrasound was without any pathology.
Cardiac evaluation with echocardiogram showed increased left ventricular trabeculation of the apical segments and deep intertrabecular recesses consistent with left ventricular noncompaction cardiomyopathy (Figures 1A–B), with the typical end-systolic ratio of noncompacted/compacted greater than two. Systolic function of both ventricles was normal, without evidence of myocardial scarring. A contrast study demonstrated blood flow through the trabeculated noncompact inner layer to the outer compact layer. For confirmation of the diagnosis, we performed a cardiac MRI, which also demonstrated the prominent left ventricular trabeculation pattern, thus confirming the transthoracic echocardiogram findings. However, family screening was negative for the presence of noncompaction cardiomyopathy.
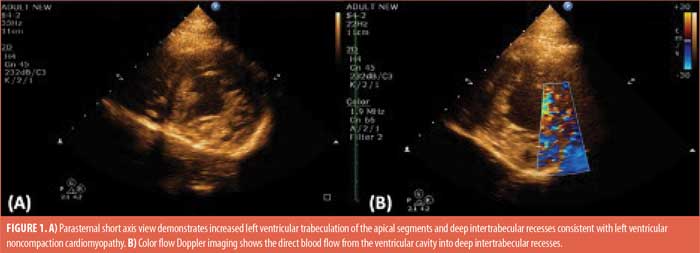
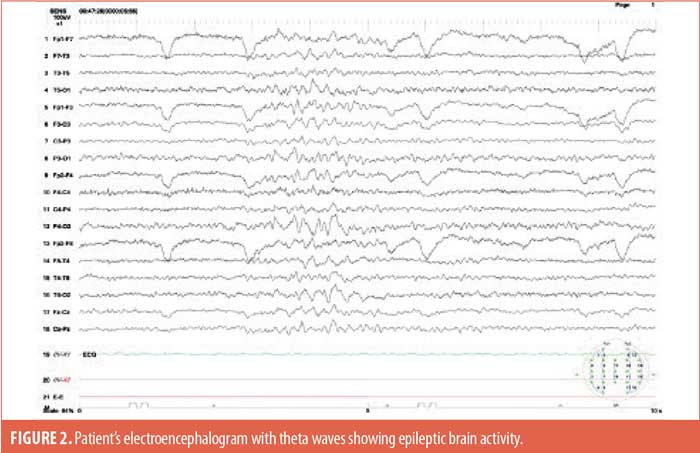
Throughout the hospitalization, the patient was asymptomatic, and continuous heart rhythm monitoring revealed sinus rhythm with no supraventricular or ventricular significant arrythmias. Given the clinical course of the case, after a multidisciplinary team discussion with a neurologist and electrophysiologist, the possibility of epilepsy was examined. An electroencephalogram (EEG) was performed, which showed bursts of slow and spike wave complexes indicative of abnormal theta wave activity (Figure 2), and epilepsy syndrome was considered the most probable diagnosis. Antiepileptic therapy with levetiracetam 1,000mg three times per day was initiated. During 10 days of hospitalization, no more TLOC episodes occurred. However, due to the structural cardiomyopathy and its related risk, we decided to implant a loop recorder in the patient with the aim of excluding an additional arrhythmogenic substrate responsible for TLOC.
The patient remained in close follow-up, and at two years following the initial hospital discharge was still on the same antiepileptic medication. During this time, there were no arrhythmias recorded on loop recorder monitoring, and the patient was without any clinically significant neurological and cardiovascular events.
Discussion
This case report highlights the importance of a complete, thorough workup and multidisciplinary team approach for patients with TLOC. The rare coexistence of two distinct diseases in the same individual, with the possibility of causing TLOC, either of neurological or cardiac nature, related to cardiomyopathy requires detailed investigation for optimal management with continuous follow-up of the clinical course. In this sense, both the short- and long-term efficacy and safety of the therapeutic strategies should be ensured.
A careful clinical history taken from patients and eyewitnesses helps physicians to differentiate syncope from nonsyncopal forms of TLOC in approximately 60 percent of cases. The clinical presentation of epilepsy and syncope can be confusingly similar, and clinical features may not always be reliable, leading to misdiagnosis and improper treatment,3 as was the risk in our case. It should be noted that in 20 to 30 percent of adults who are diagnosed with epilepsy, the diagnosis of epilepsy is incorrect, and misdiagnosis rates are even higher in patients with epilepsy refractory to therapy.3 Moreover, misdiagnosis of nonepileptic events as epilepsy might lead to unnecessary prescription of antiepileptic drugs, with the increased risk of malignant cardiac arrhythmias by PR and QT interval prolongation.4 On the other hand, missing a diagnosis of epilepsy augments the possibilities of additional seizures, injuries, and sudden death in people with epilepsy, or even status epilepticus.
The rather rare coexistence of left ventricular noncompaction cardiomyopathy with epilepsy makes correct management more complex. In this setting, the diagnostic workup by a multidisciplinary team and EEG with simultaneous cardiac recordings increases diagnostic accuracy. As brain imaging cannot rule out epileptic activity, EEG is recommended when epilepsy is the likely cause of TLOC or when clinical data are equivocal.1 Our patient presented with atrial fibrillation, and thorough cardiac evaluation was necessary. The transthoracic echocardiogram and cardiac MRI revealed left ventricular noncompaction. However, arrhythmias developing in the course of seizures are frequently reported and may increase the risk of sudden unexpected death in epilepsy.5 The co-occurrence of arrhythmias and seizures may result from the common genetic background of the two disorders, including mutation of genes encoding Na+, K+, and Ca2+ ion channels.5 Patients with epilepsy and coexisting arrhythmias should receive antiseizure and antiarrhythmic drugs simultaneously.6
Although cardiac arrhythmias and epilepsy are clinical manifestations of different organs, they share a similar pathogenesis and electrical background and may coexist in the same patient, coincidentally or by a causative pathophysiological mechanism.7 As the central nervous system and myocardium share common embryologic origins, noncompaction cardiomyopathy is frequently associated with several extracardiac abnormalities, especially neuromuscular disorders, such as epilepsy.8 In particular, an association with a truncation mutation of the KCNMB3 gene has been described, which is partly affected by the duplication on Chromosome 3 in patients with left ventricular noncompaction myocardiopathy and epilepsy. However, a causal relationship between neuromuscular disorders and left ventricular noncompaction has not been proven yet.8
Focusing on left ventricular noncompaction cardiomyopathy, it is important to underscore that this primary cardiomyopathy can often manifest as recurrent ventricular tachycardia,9 and it is rather interesting that ventricular arrhythmias, by triggering hypoxia, can result to prolonged clonic movements that can last for minutes, mimicking epileptic seizures.10 Our patient’s monitoring for arrhythmias did not detect abnormal rhythm findings during hospitalization, making the diagnosis of epileptic syndrome more possible. Hence, seizures in patients with structural or arrhythmogenic cardiomyopathy may be due to generalized cerebral hypoxia during ventricular arrhythmias or also an independent second trouble in these patients.
Our patient did not experience further seizures after the initiation of antiepileptic treatment, not only during the hospitalization but also during the two-year follow-up. The uneventful close follow-up was also supported by the data extracted from the implantable loop recorder with continuous heart monitoring. The latter showed no clinically significant arrhythmic events. However, there is a possibility for future development of a more malignant phenotype of the left ventricular noncompaction, leading to arrhythmias needing further treatment. For this reason, the patient undergoes frequent clinical, laboratory, and imaging testing.
Conclusion
This case report contributes to further understanding the value of a multidisciplinary approach to TLOC. In the rare and adverse coexistence of a “double-edged sword” of cardiomyopathy with epilepsy, diagnosis and long-term management of patients with TLOC is a challenge.
References
- Brignole M, Moya A, de Lange FJ, et al 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–1948.
- van Dijk JG, Wieling W. Pathophysiological basis of syncope and neurological conditions that mimic syncope. Prog Cardiovasc Dis. 2013;55(4):345–356.
- van Dijk N, Boer KR, Colman N, et al. High diagnostic yield and accuracy of history, physical examination, and ECG in patients with transient loss of consciousness in FAST: the Fainting Assessment study. J Cardiovasc Electrophysiol. 2008;19(1):48–55.
- Zaccara G., Lattanzi S. Comorbidity between epilepsy and cardiac arrhythmias: Implication for treatment. Epilepsy Behav. 2019;97:304–312.
- van der Lende M, Surges R, Sander JW, Thijs RD. Cardiac arrhthymias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2016;87(1):69–74.
- Bouza AA, Isom LL. Voltage-gated sodium channel β subunits and their related diseases. Handb Exp Pharmacol. 2018;246:423–450.
- Garcìa- Cosìo F, Pastor Fuentes A, Nùnez Angulo A. Arrhythmias (IV). Clinical approach to atrial tachycardia and atrial flutter from an understanding of the mechanisms. Electrophysiology based on anatomy. Rev Esp Cardiol (Engl Ed). 2012;65(4):363–375.
- Finsterer J, Stöllberger C, Fazio G. Neuromuscular disorders in left ventricular hypertrabeculation/noncompaction. Curr Pharm Des. 2010;16(26):2895–2904.
- Muser D, Liang JJ, Witschey WR, Pathak RK, et al. Ventricular arrhythmias associated with left ventricular noncompaction: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2017;14(2):166–175.
- Horrocks IA, Nechay A, Stephenson JB, Zuberi SM. Anoxic-epileptic seizures: observational study of epileptic seizures induced by syncopes. Arch Dis Child. 2005;90(12):1283–1287.


