
By Rocco Salvatore Calabrò, MD, PhD; Simona Portaro, MD, PhD; Alfredo Manuli, MSc; Antonino Leo, MSc; Antonino Naro, MD, PhD; and Alessia Bramanti, PhD
IRCCS Centro Neurolesi “Bonino Pulejo”, Messina, Italy
Funding/financial disclosures. The author has no conflict of interest relevant to the content of this letter. No funding was received for the preparation of this letter.
Innov Clin Neurosci. 2019;16(1–2):11–12
Dear Editor:
Amyotrophic lateral sclerosis (ALS) is a progressive, neurodegenerative, multisystem disorder characterized by the loss of cortical, brain stem, and spinal motor neurons, but usually sparing cognitive, sensory, sexual, and sphincterial functions.1 The main presentations of ALS are limb-onset (70%), which is the most typical form, and bulbar-onset (25%), characterized by difficulties with speech and swallowing.1
ALS is considered a complex disorder involving multiple genetic factors and environmental exposures that combine to render a person susceptible to the disease.1 Research has identified some of the cellular processes that occur after disease onset, including mitochondrial dysfunction, protein aggregation, generation of free radicals, excitotoxicity, inflammation, and apoptosis. For most patients, however, the underlying cause is unknown.1,2
Treatments cannot reverse the damage of ALS, but they can slow the progression of symptoms, prevent complications, and potentiate independence. There are two medications that have been shown to slow the progression of ALS and extend the life of those who have been diagnosed with the disease: 1) riluzole (taken orally), which helps mitigate damage to motorneurons by reducing the amount of glutamate responsible for excitotoxicity, and 2) edaravone, which is an antioxidant administered intravenously that can prevent motorneuron damage caused toxic substances, such as free radicals.3
Despite ongoing research to develop pharmacological treatment options for ALS, insufficient attention is paid to rehabilitation therapy for patients who suffer from the diease.3 Indeed, physical activity (with regard to aerobic exercise) can reduce muscle atrophy and increase endurance by slowing neural loss, as it has been shown that regular exercise might attenuate the aging process by potentiating the cardiovascular, respiratory, endocrine, musculoskeletal, and immune systems.
Although no effect on survival was demonstrated, a recent study by Lunetta et al4 suggested that a strictly monitored exercise program might significantly reduce motor deterioration in patients with ALS. In addition, moderate-intensity exercise is beneficial in ALS and decreases the deconditioning and muscle atrophy that can result from progressive inactivity.
Robotic rehabilitation tools are typically based on the phenomenon of motor learning that results from intensive, repetitive, and task-oriented motor activities that require a patient’s effort and attention.6 Robotic devices for walk training can be classified into either stationary or overground systems.7 The former is implemented by using a fixed structure combined with a moving ground platform and, according to the types of mobile platform adopted, can be divided into treadmill gait trainers (such as the exoskeleton Lokomat® [Hocoma AG, Volketswil, Switzerland]) and programmable foot end-effector trainers (e.g., Geo-System®).6,7 The latter (Ekso-GT [Ekso Bionics, Richmond, California, USA] and Indego [Parker Hannifin, Cleveland, Ohio, USA] are the main examples) consists of a moving base robot that assists patients with gait, postural, and balance exercises in a safe, controlled manner, while allowing the patients to move under their own control rather than being moved through predetermined patterns by clinicians.6,7 Concerning upper limb, exoskeletons offer a larger range of motion (up to 7 degrees of freedom) compared to end-effector robots, with guaranteed optimal control of the arm and wrist movement.8 These systems are suitable for the early stage of rehabilitation in patients with ALS because they do not require significant the motor abilities that end-effectors require.
Many studies have shown robotic devices to be useful in treating several neurological diseases, including severe acquired brain injury, spinal cord injury, and multiple sclerosis.7 However, few studies have paid attention to rare neuromuscular disorders such ALS.4,5
Usually, patients undergoing robotic rehabilitation start the treatment in the acute/subacute phase (when the clinical condition is worse) and are more intensively trained with different devices as treatment continues, leading to improved motor outcomes (Figure 1). In patients with ALS, the robotic rehabilitation takes place the other way around: usually patients are trained more intensively using tailored protocols after diagnosis (i.e., in the first phases of the disease) when their physical condition is better, and then, as the disease progresses, their training is adjusted.
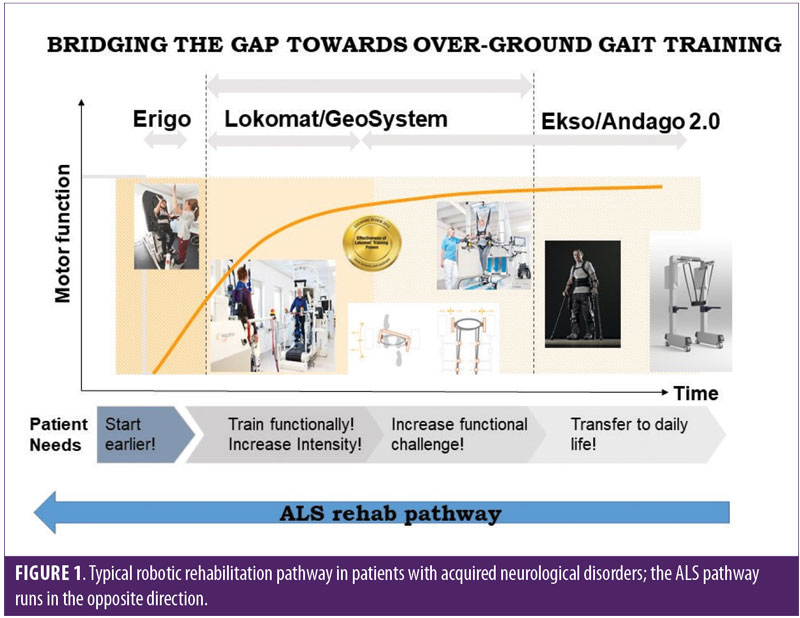
The positive effect of robotic rehabilitation on patient outcome might be related both to the intensive and repetitive aerobic exercises9 and to the task-oriented training with computerized visual feedback. A visual feedback tool is used to increase the motor output, involvement, and motivation of patients during training. Thus, it is a reasonable assumption that robotic rehabilitation also would be effective in patients with ALS, given that this can act both on brain and spinal cord neural plasticity (by boosting connectivity and reducing neural loss) and muscle strength and endurance (through measurable aerobic training).
Randomized clinical trials are needed to establish if and to what extent aerobic training by means of robotics might slow disease progression and improve quality of life in patients with ALS.
References
- Gordon PH. Amyotrophic lateral sclerosis: pathophysiology, diagnosis and management. CNS Drugs. 2011;25:1–15.
- Portaro S, Cimino V, Naro A, Calabrò RS. Discussing ANT1 role in amyotrophic lateral sclerosis pathogensis. Innov Clin Neurosci. 2018;15:11.
- Dorst J, Ludolph AC, Huebers A. Disease-modifying and symptomatic treatment of amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2017;11:1756285617734734. eCollection.
- Lunetta C, Lizio A, Sansone VA, et al. Strictly monitored exercise programs reduce motor deterioration in ALS: preliminary results of a randomized controlled trial. J Neurol. 2016; 263:52–60.
- Merico A, Cavinato M, Gregorio C, et al. Effects of combined endurance and resistance training in amyotrophic lateral sclerosis: a pilot, randomized, controlled study. Eur J Transl Myol. 2018 23;28:7278.
- Bruni MF, Melegari C, De Cola MC, et al. What does best evidence tell us about robotic gait rehabilitation in stroke patients: a systematic review and meta-analysis. J Clin Neurosci. 2018;48:11–17.
- Calabrò RS, Cacciola A, Bertè F, et al. Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now? Neurol Sci. 2016;37(4):503–514.
- Bertani R, Melegari C, De Cola MC, et al. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurol Sci. 2017;38:1561–1569.
- Calabrò RS, De Luca R, Leo A, et al. Lokomat training in vascular dementia: motor improvement and beyond! Aging Clin Exp Res. 2015;27:935–937.
- Calabrò RS, Naro A, Russo M, et al. The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial. J Neuroeng Rehabil. 2017;14:53.


