 by Takahiko Nagamine, MD, PhD
by Takahiko Nagamine, MD, PhD
Dr. Nagamine is with the Sunlight Brain Research Center in Yamaguchi, Japan.
FUNDING: No funding was provided for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Lithium intoxication might be lethal particularly in elderly persons who have multiple drugs. We present an aged bipolar patient with lithium intoxication probably caused by the interaction between fluvoxamine and azilsartan. Fluvoxamine inhibits many cytochrome P450 enzyme (CYP) including CYP2C9, which metabolizes azilsartan. Thus, azilsartan could cause more negative effects for sodium reabsorption at renal tubules when co-administered with fluvoxamine, resulted in a decrease in lithium excretion.
Keywords: lithium intoxication, elderly, drug interaction
Innov Clin Neurosci. 2020;17(4–6):45–46
Lithium continues to be considered a first-line maintenance treatment of bipolar affective disorder.1 However, lithium has a risk of intoxication because of its narrow therapeutic index. Lithium intoxication could be lethal particularly in elderly persons who have multiple drugs and altered pharmacokinetics. Here, we present an older patient with bipolar affective disorder with lithium intoxication after initiation of fluvoxamine. Informed consent was obtained from the patient for publication.
Case Presentation
An 82-year-old male patient with a 25-year history of bipolar affective disorder and hypertension presented to our emergency department because of hand tremor and consciousness disturbance. He had been maintained with lithium carbonate 600mg/day and azilsartan 20mg/day with his serum lithium concentration ranged from 0.7 to 0.9mEq/L for the last few years. Since he seemed to be slightly depressive, his attending psychiatrist added fluvoxamine 50mg/day a week before admission. Severe tremor in both hands began two days after initiation of fluvoxamine. On arrival, the patient was drowsy and disoriented with a blood pressure of 110/84mmHg, a heart rate of 66/minute, a respiratory rate of 18 breaths/minute, a saturation level of 99 percent on room air, and a body temperature of 36.4 degrees Celsius. Neurological examination revealed severe tremor in both hands and myoclonic jerks of the upper extremities. Serum lithium concentration was 3.2mEq/L. Other laboratory results were as follows: fasting blood glucose, 88mg/dL; blood urea nitrogen, 47.6mg/dL; creatinine, 2.16mg/dl; sodium, 143mEq/L; potassium, 5.3mEq/L; calcium, 9.2mg/dL; alanine aminotransferase, 20U/L; aspartate aminotransferase, 17U/L; creatine kinase, 43U/L; white blood cell, 8.000×103/μL; red blood cell, 4.31×106/μL; hemoglobin, 14.1g/dL; and hematocrit, 39 percent. Neither his cranial magnetic resonance imaging (MRI) nor electrocardiography showed any abnormalities.
All prescribed drugs, including lithium, were immediately discontinued. We started fluid resuscitation with 0.9% saline solution. On the second day, drowsiness, disorientation, and bilateral hand tremors were still evident. However, a week later, his consciousness gradually became clear as serum lithium concentration decreased to 0.35mEq/L by providing continuous fluid resuscitation. The remaining neurological signs, including tremors and myoclonic jerks, resolved with serum lithium dropped to 0.05mEq/L, and his renal function completely recovered to almost normal two weeks later (Figure 1).
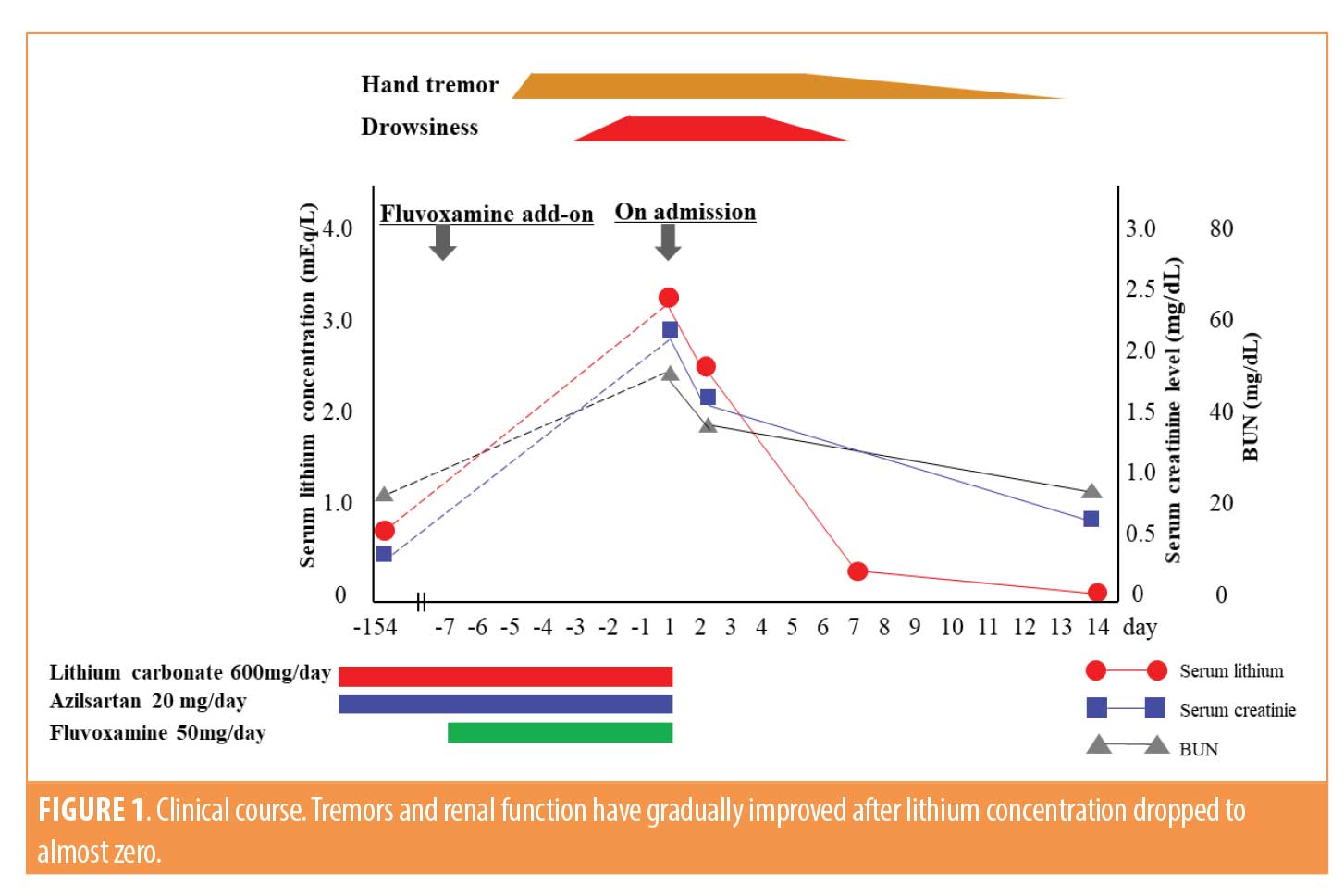
Discussion
We diagnosed the patient as having lithium intoxication after ruling out hypoxia, hypoglycemia, electrolyte disorders, intracranial bleeding, and neuroleptic malignant syndrome. Adverse events of lithium might be life-threatening situations, particularly prevalent in elderly patients due to multiple drug use.2 Our patient was 82 years of age and using multiple drugs that might facilitate lithium retention. Azilsartan is an angiotensin II receptor blocker (ARB) indicated for the treatment of mild-to-moderate hypertension. It is well known that angiotensin converting enzyme inhibitors (ACEIs) have a risk of lithium intoxication. A new prescription of ACEIs, for example, caused a substantial increase in the risk of admission to hospital with lithium toxicity (relative risk=7.6, 95% confidence interval [CI]=2.6–22.0).3 However, interaction between lithium and ARBs is rarely documented. The exact mechanism of lithium intoxication by ARBs remains unclear, but natriuresis induced by ARBs might facilitate lithium retention. Angiotensin II leads to stimulation of sodium reabsorption in the proximal tubules and secretion of aldosterone by the adrenal cortex. Therefore, ARBs have resulted in a decrease in sodium reabsorption in the proximal tubules and the decrease of aldosterone has a similar effect in distal tubules. Subsequently, the depletion of sodium might contribute to lithium reabsorption, eventually leading to lithium intoxication.4 However, our patient has used azilsartan over the past few years. The patient might have been nonadherent with azilsartan and then azilsartan was reintroduced. But this possibility is extremely low because the patient was administered all drugs by his wife routinely. He did not have any neurotoxicity symptoms until the fluvoxamine add-on. Thus, we next focused on the interaction between azilsartan and fluvoxamine.
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), does not affect lithium disposition because serum lithium levels could maintain in therapeutic concentrations in pharmacokinetic studies of concomitant use of fluvoxamine with lithium.5 The interaction between lithium and fluvoxamine is less well-known. Since both lithium and fluvoxamine can impact serotonin levels, serotonin syndrome should be considered. However, the patient did not reveal the symptoms of serotonin syndrome including increased blood pressure and/or increased heart rate. Fluvoxamine inhibits many cytochrome P450 enzyme (CYP), such as CYP1A2, CYP3A4, CYP2B6, CYP2C9, CYP2C19, and CYP2D6. In the first place, metabolic clearance by CYP is approximately 30-to-50 percent lower in older compared with younger people.6 On top of that, azilsartan is metabolized by CYP2C9, which fluvoxamine inhibits.7 Azilsartan could cause more negative effects for sodium reabsorption at renal tubules when coadministered with fluvoxamine, resulting in decreased lithium excretion. But serum electrolyte levels in our patient appeared to indicate normal sodium levels so that the patient did not seem to be excreting sodium excessively and retaining lithium. However, normal serum level does not always indicate normal sodium excretion rate because homeostasis keeping a normal serum sodium rage would work. Normal serum sodium level was reported in a case of lithium intoxication caused by telmisartan, a kind of ARB.4 Clearance is the most important pharmacokinetic parameter to consider in clinical practice because it determines steady-state drug concentration at a given maintenance dose. Decreased lithium clearance due to drug interaction is a major cause of lithium intoxication.
Conclusion
Despite many reports of lithium toxicity, there are no case reports of interaction between azilsartan, fluvoxamine, and lithium in the literature. Considering possible drug interaction in elderly patients receiving lithium is essential. Although evidence for both the safety and the efficacy of lithium has been established, it is easy to exceed therapeutic serum lithium concentration because of its narrow therapeutic range, which might lead to serious physical complications.8 When prescribing lithium, clinicians need to be cautious about adequate monitoring of serum lithium level to prevent excess death in psychiatric patients, especially when a new medication is added to a person’s regimen.
References
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217:266–280.
- Nakamura M, Nakatsu K, Nagamine T. Sinus node dysfunction after acute lithium treatment at therapeutic levels. Innov Clin Neurosci. 2015;12(11-12):18–20.
- Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794–8.
- Nagamine T. Lithium intoxication associated with angiotensin II type 1 receptor blockers in women. Clin Neuropsychopharmacol Ther. 2013;4:23–25.
- Finley PR. Drug interactions with lithium: an update. Clin Pharmacokinet. 2016;55(8):925–941.
- Butler JA, Begg EJ. Free drug metabolic clearance in elderly people. Clin Pharmacokinet. 2008;47:297–321.
- Yang R, Luo Z, Liu Y, et al. Drug interactions with angiotensin receptor blockers: role of human cytochromes P450. Curr Drug Metab. 2016;17(7):681–691.
- Rej S, Herrmann N, Shulman K. The effects of lithium on renal function in older adults—a systematic review. J Geriatr Psychiatry Neurol. 2012;25(1):51–61.





