 by Brian Chau, MD; Ivan Phelan, MSc; Phillip Ta, MD; Bradley Chi, BS; Kevin Loyola, BS; Elizabeth Yeo, BS; Justin Dunn, BS; Sarah Humbert, MD; Justin Hata, MD; Ryan Haglund, AS; Luis Luna, BS; Graham Kampmeier; and Brandon McCowan, MS
by Brian Chau, MD; Ivan Phelan, MSc; Phillip Ta, MD; Bradley Chi, BS; Kevin Loyola, BS; Elizabeth Yeo, BS; Justin Dunn, BS; Sarah Humbert, MD; Justin Hata, MD; Ryan Haglund, AS; Luis Luna, BS; Graham Kampmeier; and Brandon McCowan, MS
Drs. Chau, Ta, Humbert, and Hata and Mr. Chi are with the Department of Physical Medicine & Rehabilitation at Loma Linda University Health in Loma Linda, California. Mr. Loyola, Ms. Yeo, and Mr. Dunn are with the Loma Linda University School of Medicine in Loma Linda, California. Mr. Phelan is with the Research Institute C3RI at Sheffield Hallam University in Sheffield, England. Mr. Haglund, Mr. Luna, Mr. Kampmeier, and Mr. McCowan are with Loma Linda University’s Health Interactive Studio in Loma Linda, California.
FUNDING: Funding was provided by the Department of Physical Medicine & Rehabilitation at Loma Linda University Health.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. This pilot study explored the effects of therapeutic immersive virtual reality (VR) on pain in upper limb complex regional pain syndrome (CRPS). While acute pain relief with VR has been studied in multiple populations, there is little data on the use of this modality in treating chronic pain, especially CRPS.
Participants. Volunteer participants were recruited from outpatient rehabilitation services. Inclusion criteria required the diagnosis of CRPS in at least one upper limb and the ability to communicate in English to receive instructions from study personnel. A total of eight participants were recruited, with six fully completing the study.
Interventions. An immersive virtual three-dimensional interactive kitchen environment was designed that allowed visualization of and object manipulation with virtual hands. Participants performed tasks representative of daily activities, as well as guided visualization exercises for a total of 10 sessions.
Main Outcome Measure. Pre- and post-session pain scale measurements (Short Form McGill Pain Questionnaire, Visual Analog Scale, and Wong-Baker FACES) and subjective feedback were collected with each session.
Results. Four of the six participants that completed the study reported subjective improvement of their pain and daily function. However, objective pain scales had limited correlation to reported subjective relief.
Conclusions. Immersive virtual reality might provide subjective analgesia and functional improvement in select patients with upper limb complex regional pain syndrome, but objective data is lacking.
Keywords: Virtual reality, Complex Regional Pain Syndrome, rehabilitation, VR, chronic pain, therapy
Innov Clin Neurosci. 2019;17(4–6):47–52
Complex regional pain syndrome (CRPS) is a chronic pain disorder with classic neuropathic characteristics (e.g., burning pain, hyperalgesia, allodynia) and significant autonomic features (e.g., local edema, sweating, skin discoloration, skin temperature changes) in one or more extremity. CRPS is typically triggered by a known physical insult, such as a fracture or surgery. There might also be trophic changes to the skin, hair, and nails, as well as altered motor function (e.g., loss of strength, decreased active range of motion, tremor).1 CRPS can be classified into CRPS-1 and CRPS-2, differentiated by a known history of nerve damage in the latter.2 The exact pathophysiology of CRPS remains unknown and is likely multifactorial with components of inflammation, autoimmune factors, neuronal plasticity, and autonomic dysregulation involved.3 Patients with CRPS generally report a poorer quality of life than those with other chronic pain conditions.4,5 Conventional treatment for CRPS can be challenging and often involves physical therapy, medication management, and interventional procedures.6
Additional treatment modalities for CRPS that have been discussed in previous research include graded motor imagery, visualization techniques, and the use of mirror therapy.7 While the exact mechanism of how these modalities alleviate pain is unknown, it is proposed that there is alteration in the brain’s somatosensory representation of the body, as well as changes in motor cortical plasticity after an injury in patients with CRPS.8 McCabe et al9 hypothesized that the pain of CRPS is a result of central sensory processing dysregulation and that congruent visual feedback from the unaffected limb, such as a mirror, could restore the integrity of cortical processing leading to pain relief and improved function in the affected limb.
Immersive virtual reality (VR) might provide an engaging and customizable environment for rehabilitation exercises in CRPS. The use of VR for clinical rehabilitation has become increasingly popular over the past decade as recent developments in the entertainment industry have made VR equipment and software more accessible.10 VR might address the changes in the body scheme of CRPS patients by mimicking the effects of mirror therapy while giving patients a more interactive experience that better resembles purposeful activities of daily living. VR allows users to visualize a custom avatar that ideally would replace their sense of perception from their physical body in the physical world. We hypothesize that this dissociation would allow more intensive, engaging interventions than mirroring the unaffected limb alone.
To our knowledge, few studies have investigated treatment of CRPS through the use of VR. Jeon et al11 found participants that mentally rehearsed the virtual movements they were shown via a virtual headset, compared to those that simply observed the virtual movements, had a significant improvement in body perception disturbance but no significant difference in pain intensity. Sato et al12 combined mirror therapy with a VR feedback system where the affected arm controlled positional movement of the virtual image, and the unaffected hand, via a sensorized glove, controlled the hand grasping movements of the VR image. Four out of the five patients in their study had greater than 50-percent reduction in pain intensity after completing 3 to 8 consecutive sessions. Matamala-Gomez et al13 showed the use of a virtual arm with increased transparency decreased pain in CRPS participants by half. Won et al14 conducted a pilot study on pediatric patients with CRPS, wherein participants controlled a virtual balloon popping simulation through movements of their legs. Although they did not have enough participants to draw conclusions on the effect on pain reduction, they were able to confirm that VR was both safe for future use and did not adversely affect pain or physical function. These studies further support treatment of CRPS with neural rehabilitation with the goal of correcting cortical disruptions, such as dysfunctions of the somatosensory network, motor cortex, and body schema.
Methods
Participants. Volunteer participants were recruited from outpatient rehabilitation services. Approval from the Institutional Review Board (IRB 5150437) and participant’s consent were obtained to proceed with the investigation. Inclusion criteria required the diagnosis of CRPS in at least one upper limb and the ability to communicate in English to receive instructions from study personnel. Exclusion criteria included active history of seizures, or motion sickness with VR equipment use.
Hardware. This investigation utilized the HTC Vive VR system (HTC Corporation, Taoyuan City, Taiwan), which consists of a wired headset, two handheld motion controllers, and two base stations. The base stations, which provided the boundaries and tracking system of the virtual space, were mounted by two tripods. Both headset and controllers allow real time three-dimensional tracking. A personalized computer with VR capability, monitor, keyboard, and mouse were also essential to connect and run the VR software.
Software. The SteamVR software (HTC Corporation, Taoyuan City, Taiwan) allows real-time tracking of the headset and controllers in the space bounded by the base stations. The system permitted the user to freely walk in the premeasured space (typically 1.5m x 2.0m) to explore the environment. The therapeutic environment consisted of two software simulations of a virtual kitchen. Environments differed by interior design, size, and number of interactive objects but were otherwise similar. Both environments included many interactive features to give the user an immersive, nonmonotonous, engaging experience. Kitchen appliances (e.g., faucet, refrigerator, oven) and kitchenware (e.g., pots, pans, plates, utensils) were available for the user to manipulate (Figure 1). The controllers appeared in the virtual environment as virtual hands in a resting position with the ability to pronate, supinate, and grip objects with virtual grasp. The software also had the capability to mirror hand movements of the user if the affected limb was not strong enough to hold the controller. This feature allowed a nonpainful limb to carry out motions that would be too painful in the involved limb, but still appeared as if the painful limb were doing so in VR. Hand position and skin color of the virtual limbs were adjustable by study personnel.

Outcomes. This investigation studied quantitative and qualitative outcomes via a survey consisting of the Short-Form McGill Pain Questionnaire (SF-MPQ), the Visual Analog Scale (VAS), and the Wong-Baker FACES (WBF). Subjective comments of function and symptoms by participants were also recorded.
Procedure. All recruited participants gave their informed consent prior to the first session. Participants were approved for up to 10 sessions of VR therapy (initially five; extended upon study renewal). Most participants completed a total of 10 sessions, 1 to 3 sessions per week. Each session lasted approximately 45 minutes to an hour. At the beginning and end of each session, participants filled out a form consisting of the SF-MPQ, VAS, WBF, and self-reported subjective data about their pain. VR therapy sessions consisted of guided visualization exercises and interactions with the virtual environment using their virtual hands. Activities included washing hands, tossing a paper airplane, assembling a sandwich, sorting dishware, and arranging utensils (Figure 2). In addition to completing pre- and post- VR surveys, participants reported any subjective changes noticed between sessions at the start of each subsequent therapy visit. Participants were contacted at least six weeks later to share any persistent changes in function or symptoms.

Results
Eight participants with upper limb CRPS were recruited for this investigation. Demographics, span of full VR therapy, and previous treatments are listed below (Table 1). A single participant completed only five sessions due to the IRB approval at that time, five participants completed all 10 sessions once IRB extension was approved, and two participants withdrew from the study before completing all sessions. Figures 3, 4, and 5 show the scores for the SF-MPQ, VAS, and WBF for each participant that completed more than one VR session. VAS mean pain scores of all participants who completed the 10 sessions typically demonstrated lower pain scores after each VR session (pre- vs. post-session), but also higher total scores by completion of all visits (Figure 6). Higher average scores were also noted with SFMPQ measurements. There was little average difference from first sessions to last session with WBF scores; although, in general, post-VR sessions scores were lower than pre-session scores at individual sessions.
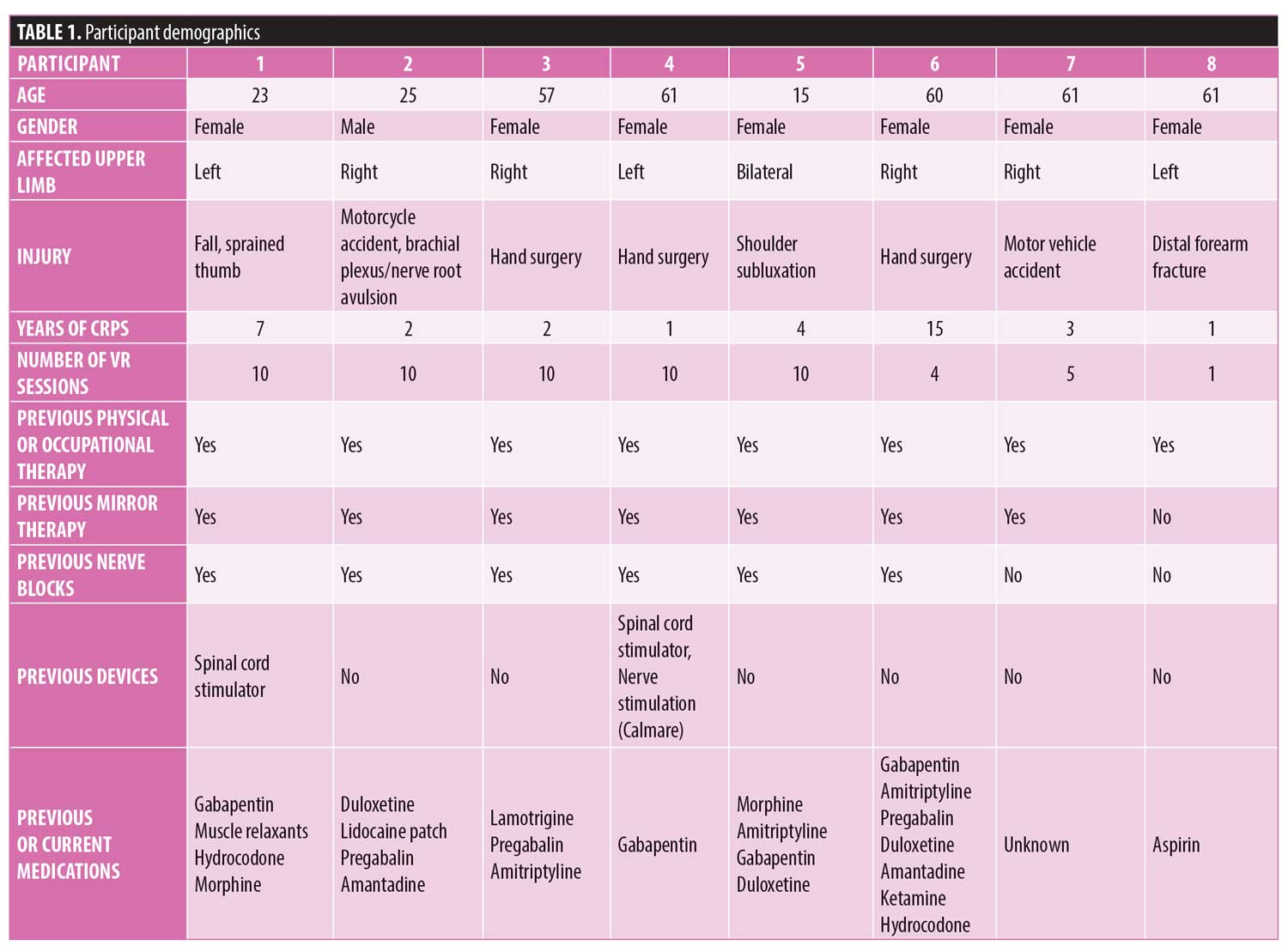
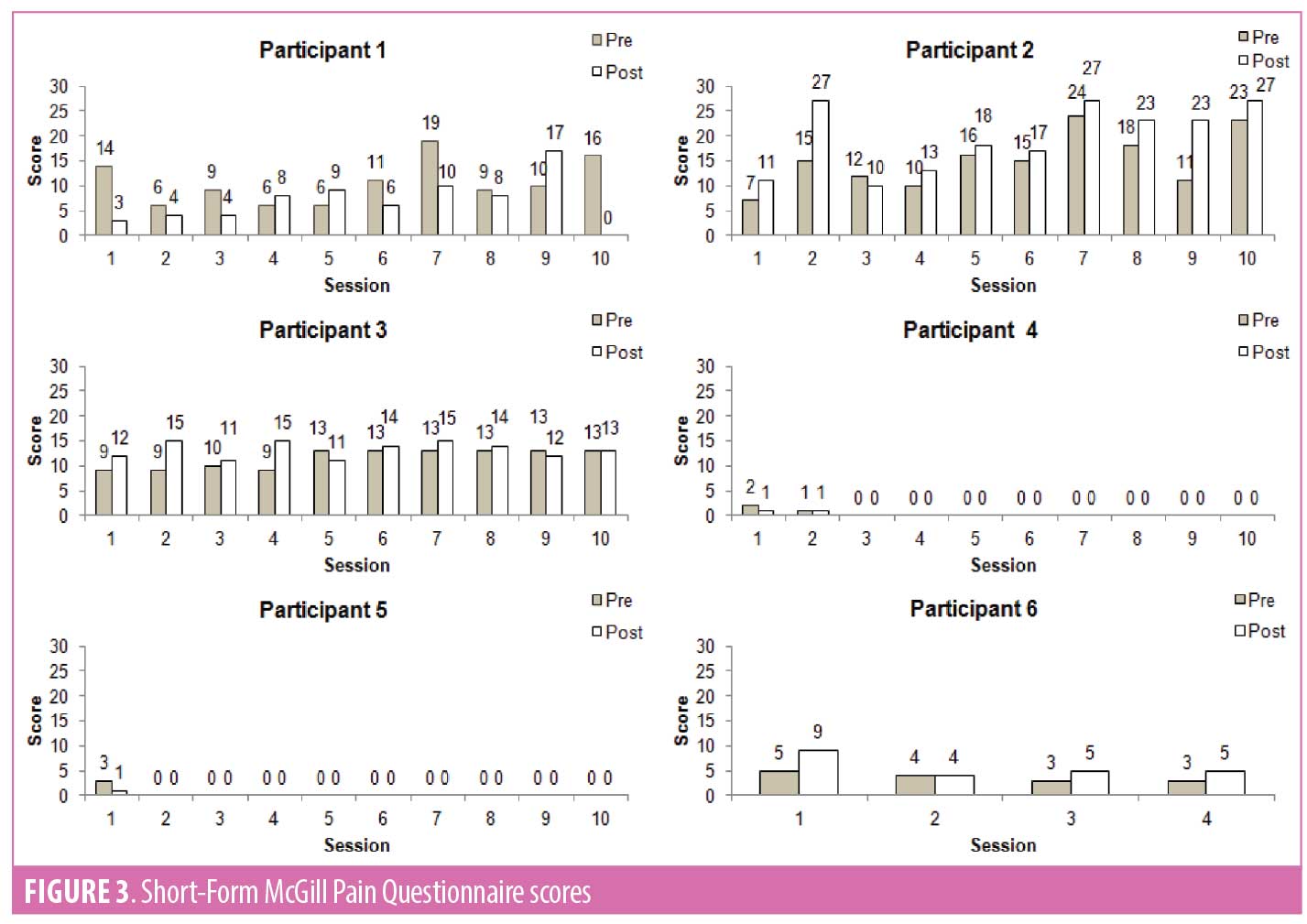
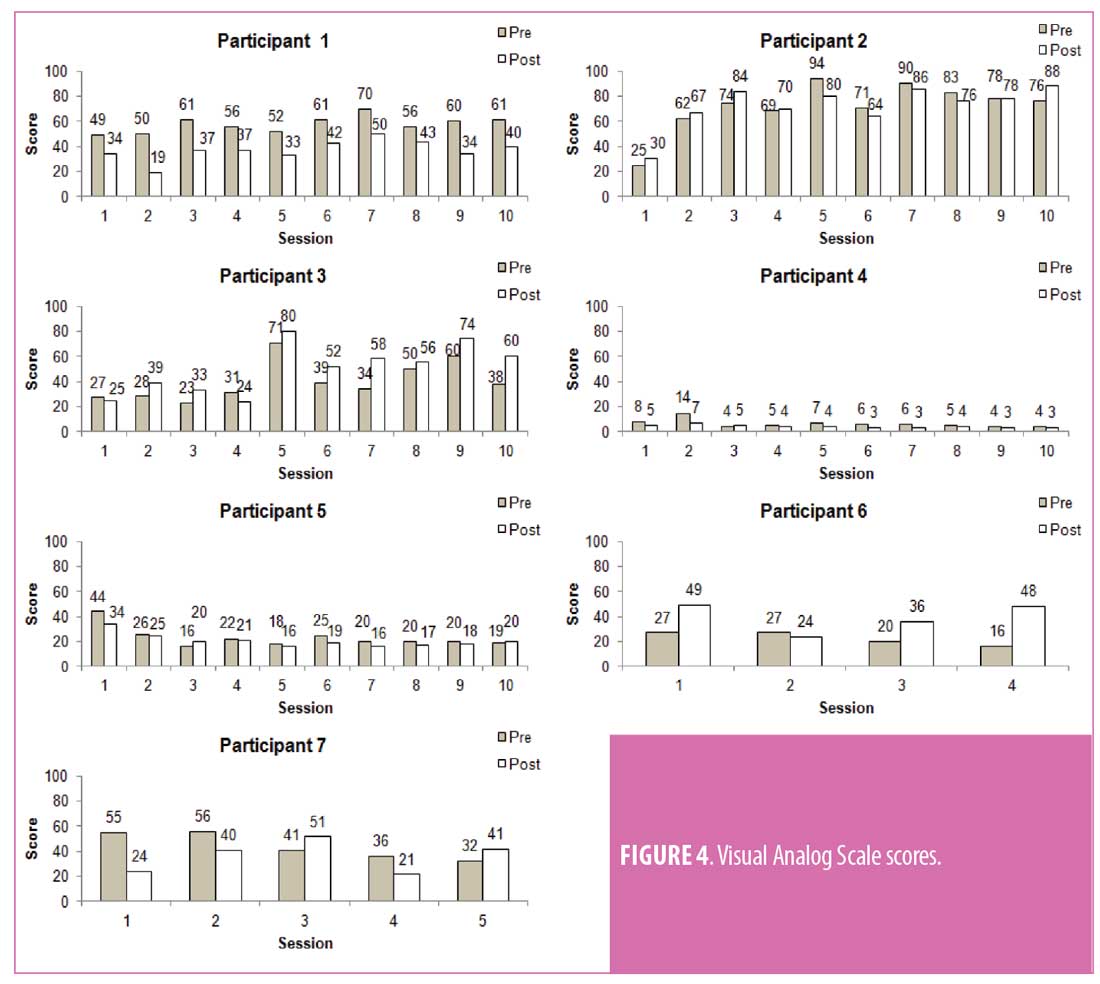
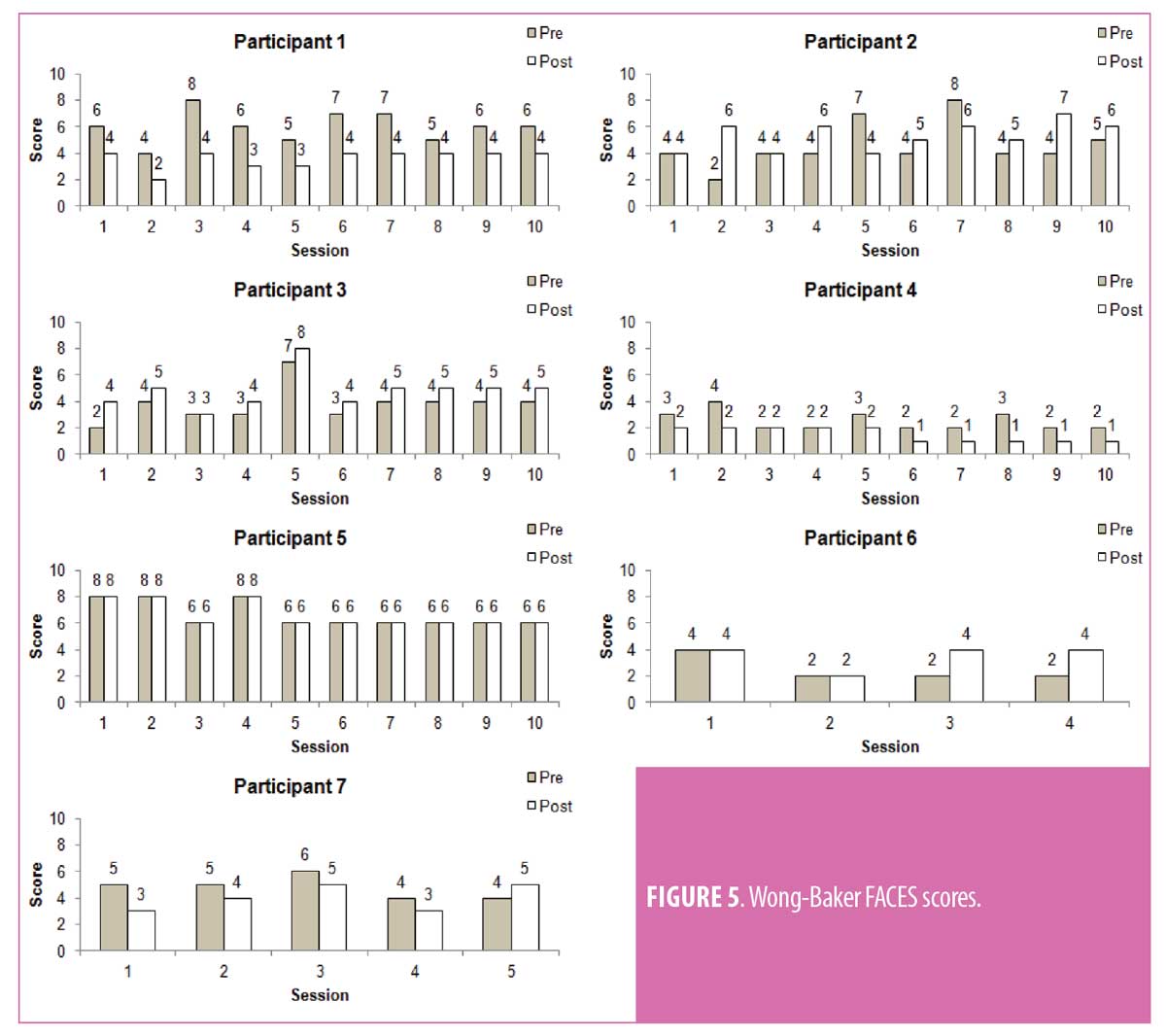
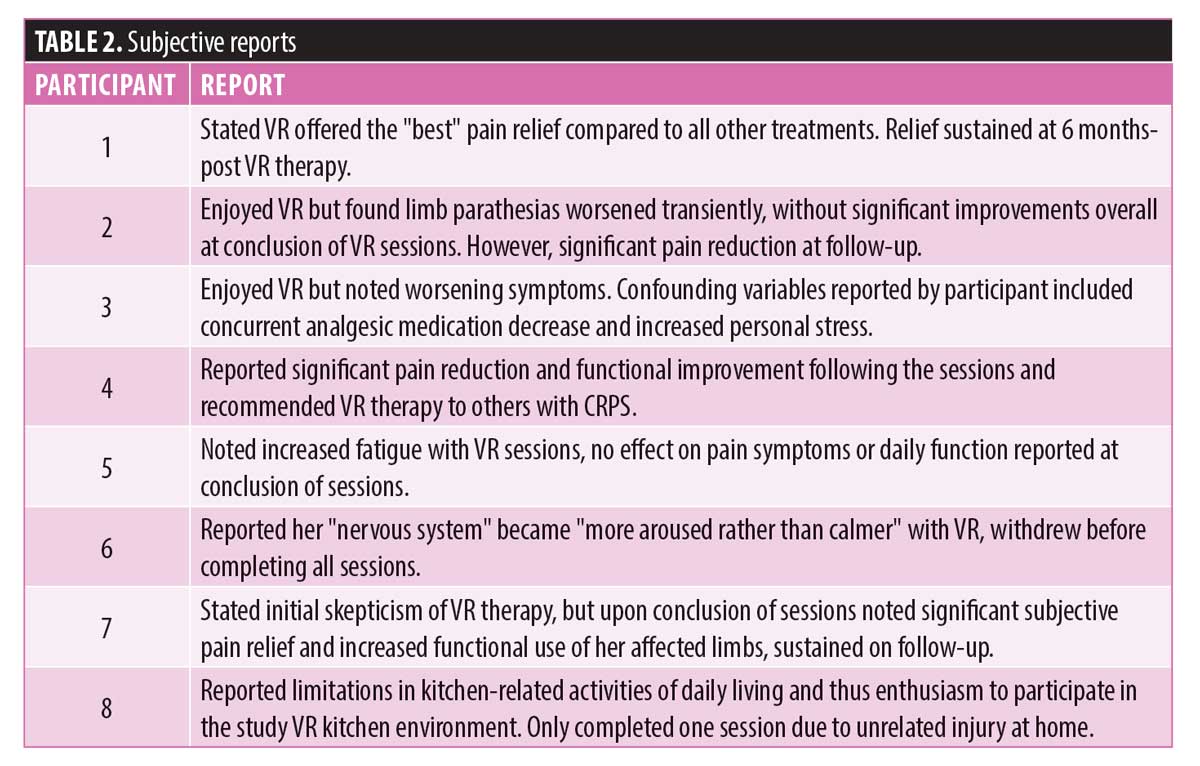
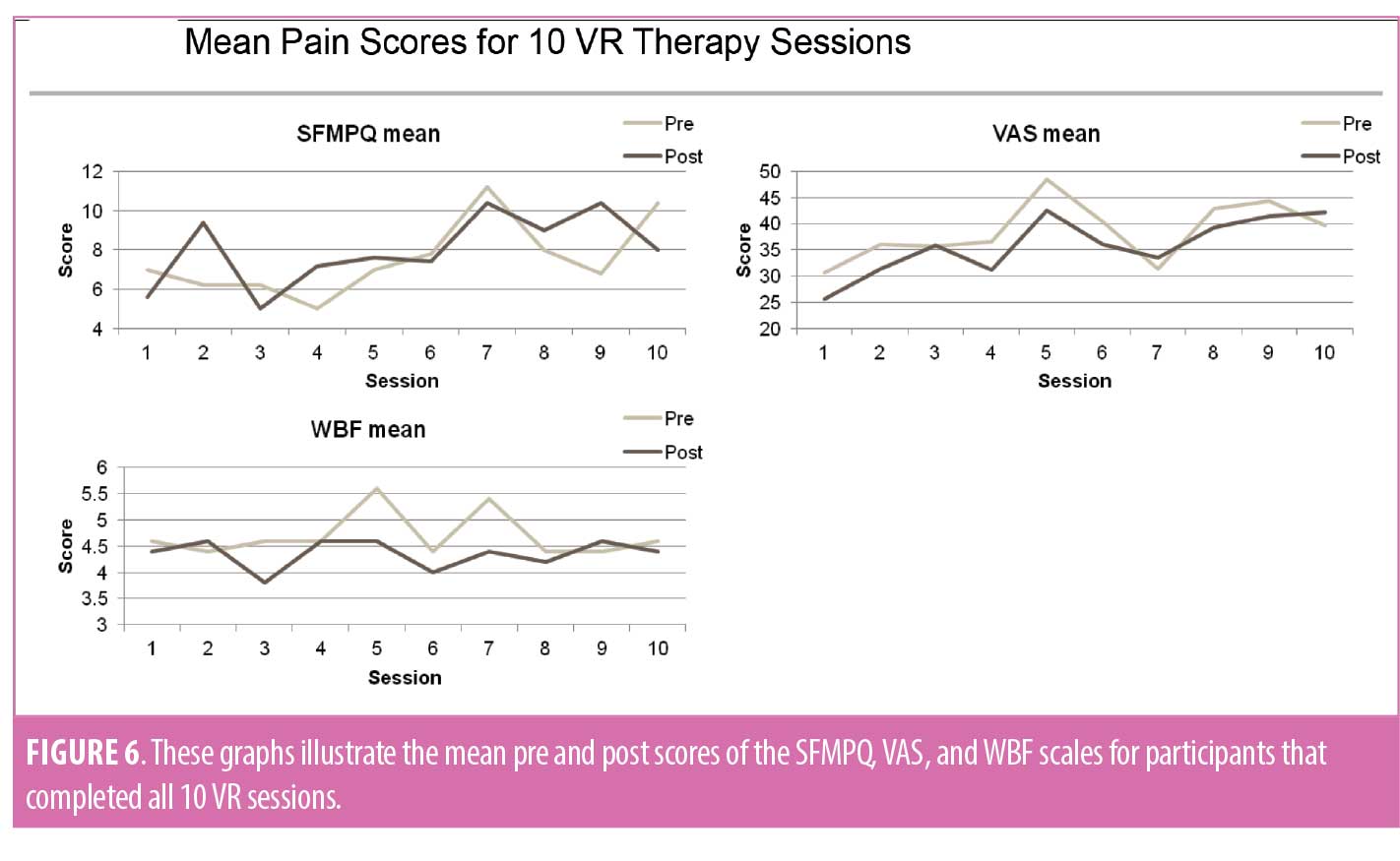 Unlike the pain scores, participant subjective feedback included clear reports of pain relief and functional improvements. Three participants noted marked relief in symptoms at the conclusion of the VR sessions, and two of these participants reported with sustained relief at long-term follow-up. One of these participants had near-resolution of chronic symptoms upon follow-up, with increased functional use of previously disused limb reported. Additionally, one participant that did not note immediate improvements was found to have significant pain relief at long-term follow-up, without additional interventions since the VR sessions. His pain relief was striking enough that he had been discharged from his pain management specialty clinic, given sustained improvements. Other themes noted in subjective participant reports included positive feedback about the design of the kitchen environment and the VR experience in general, even among those who did not report symptom improvement. Further details of such feedback are available in Table 2.
Unlike the pain scores, participant subjective feedback included clear reports of pain relief and functional improvements. Three participants noted marked relief in symptoms at the conclusion of the VR sessions, and two of these participants reported with sustained relief at long-term follow-up. One of these participants had near-resolution of chronic symptoms upon follow-up, with increased functional use of previously disused limb reported. Additionally, one participant that did not note immediate improvements was found to have significant pain relief at long-term follow-up, without additional interventions since the VR sessions. His pain relief was striking enough that he had been discharged from his pain management specialty clinic, given sustained improvements. Other themes noted in subjective participant reports included positive feedback about the design of the kitchen environment and the VR experience in general, even among those who did not report symptom improvement. Further details of such feedback are available in Table 2.
Discussion
Four of the six participants that completed the study reported subjective improvement of their symptoms and daily function, with several of these noting markedly improved symptoms. While the exact mechanism of such analgesia is unclear, a variety of theories have been postulated, including the Gate Control Theory, activation of descending inhibitory pathways, production of endogenous opioids, mirror neuron activation, and beneficial neuroplasticity.12,15 Neuroplastic change is thought to be dependent upon repetitive practice of motor skills, with VR offering the ability tailor the task for optimal engagement and learning.16 While limited in data, functional neuroimaging after focused VR training has demonstrated neuronal reorganization with associated motor changes.17 Despite these positive subjective reports, the objective measures from the SF-MPQ, VAS, and WBF did not demonstrate such changes. On the contrary, it appeared by the last session, pain scores were higher than their baseline score prior to starting VR sessions. Increased awareness of limb symptoms might explain these findings, as might the limitations in these pain scales when it comes to chronic neuropathic pain measurements. This paradoxical result—subjective relief but worsened or unchanged objective findings—prevents us from drawing definitive conclusions from this series of VR therapy.
Overall, the VR therapy was well tolerated and found to be enjoyable by most participants. Only one participant voluntarily withdrew due to intolerance, citing increased sensitivity of their “nervous system.” Moreover, during the sessions, hyperparesthesia was reported whenever the connecting cord from the headset to the computer grazed their arm. Based on this experience, a wireless VR system might be considered for future studies. Of note, this participant was diagnosed with CRPS for 15 years, the longest of all the participants recruited. No study thus far has correlated the effects of VR based on the chronicity of CRPS.
This uncontrolled pilot study had several limitations, including design, small number of participants, and the lack of objective measures of function. Additionally, heterogeneity of the participants and paradoxical findings of this investigation makes it difficult to draw a definite conclusion regarding the benefits of VR for patients with CRPS.
Conclusion
From this limited case series, immersive virtual reality could potentially offer pain relief as well as functional improvement in select patients with upper limb complex regional pain syndrome. Future studies need to take into consideration the diversity of this pain syndrome and utilize outcome measures suitable to this population (e.g., Neuropathic Pain Symptom Inventory, Pain Disability Index, and Neuropathic Pain Scales) to confidently characterize the effects of immersive virtual reality.
Acknowledgments
We thank Loma Linda University’s Health Interactive Studio, Loma Linda University Health’s Department of Physical Medicine & Rehabilitation, Michael Davidson of the Department of Orthotics and Prosthetics at Loma Linda University Health, and C3RI at Sheffield Hallam University for their contribution, collaboration, and support of this research.
References
- Bruehl S. An update on the pathophysiology of Complex Regional Pain syndrome. Anesthesiology. 2010;113(3):713–725.
- Merskey H, Bogduk N. Classification of chronic pain, 2nd edition. Seattle, WA: IASP Press;1994:1.
- Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of Complex Regional Pain syndrome. Lancet Neurol. 2011;10(7):637–648.
- Birklein F, Schlereth T. Complex Regional Pain syndrome-significant progress in understanding. Pain. 2015;156(1):S94–103.
- Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in Complex Regional Pain syndrome: a pilot survey. J Pain Symptom Manage. 2000;20(4):286–292.
- Van velzen GA, Perez RS, Van gestel MA, et al. Health-related quality of life in 975 patients with Complex Regional Pain syndrome type 1. Pain. 2014;155(3):629–34.
- Smart KM, Wand BM, O’connell NE. Physiotherapy for pain and disability in adults with Complex Regional Pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2016;2:CD010853.
- Dascal J, Reid M, Ishak WW, et al. Virtual Reality and Medical Inpatients: A systematic review of randomized, controlled trials. Innov Clin Neurosci. 2017;14(1-2):14–21.
- Mccabe CS, Haigh RC, Ring EF, et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of Complex Regional Pain syndrome (Type 1). Rheumatology (Oxford). 2003;42(1):97–101.
- Urits I, Shen AH, Jones MR, et al. Complex Regional Pain syndrome, current concepts and treatment options. Curr Pain Headache Rep. 2018;22(2):10.
- Jeon B, Cho S, Lee JH. Application of virtual body swapping to patients with Complex Regional Pain syndrome: a pilot study. Cyberpsychol Behav Soc Netw. 2014;17(6):366–370.
- Sato K, Fukumori S, Matsusaki T, et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of Complex Regional Pain syndrome: an open-label pilot study. Pain Med. 2010;11(4):622–629.
- Matamala-Gomez M, Diaz Gonzalez AM, Slater M, Sanchez-Vives MV. Decreasing pain ratings in chronic arm pain through changing a virtual body: different strategies for different pain types. J Pain. 2019;20(6):685–697.
- Won AS, Tataru CA, Cojocaru CM, et al. Two virtual reality pilot studies for the treatment of pediatric CRPS. Pain Med. 2015;16(8):1644–1647.
- Gold JI, Belmont KA, Thomas DA. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav. 2007;10(4):536–544.
- Cheung KL, Tunik E, Adamovich SV, et al. Neuroplasticity and virtual reality. Weiss P, Keshner E, Levin M. (eds) Virtual Reality for Physical and Motor Rehabilitation. Virtual Reality Technologies for Health and Clinical Applications. 2014:5–18.
- You SH, Jang SH, Kim YH, et al. Cortical reorganization induced by virtual reality therapy in a child with Hemiparetic cerebral palsy. Dev Med Child Neurol. 2005;47(09):628.





