 by Jessica Robinson-Papp, MD, MS; Mary Catherine George, MM, PhD; Alexandra Nmashie, MD; Donald Weisz, PhD; and David M. Simpson, MD
by Jessica Robinson-Papp, MD, MS; Mary Catherine George, MM, PhD; Alexandra Nmashie, MD; Donald Weisz, PhD; and David M. Simpson, MD
Drs. Robinson-Papp, George, Nmashie and Simpson are with the Department of Neurology and Dr. Weisz is with the Department of Neurosurgery—all from the Icahn School of Medicine at Mount Sinai in New York, New York.
Innov Clin Neurosci. 2017;15(1–2):28–32
FUNDING: This investigator-initiated project was supported by a grant from CSL Behring. The project was also supported by Grant Number #UL1TR000067 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NCATS or NIH.
DISCLOSURES: The authors report no financial arrangements between any author and any company whose product or competing product plays a role in the manuscript. This manuscript discusses an unlabeled use of a commercial product: IVIG is not labeled for the treatment of HIV-associated myelopathy.
ABSTRACT: Objective: Open-label data suggest that intravenous immunoglobulin (IVIG) might improve lower-extremity strength in human immunodeficiency virus (HIV)-associated myelopathy (HIVM), a rare but debilitating neurologic complication of HIV. We sought to determine the feasibility of testing the efficacy of IVIG for HIVM more rigorously. Design: We conducted a randomized, double-blind, placebo-controlled feasibility trial of IVIG for HIVM, using dynamometry as an outcome measure (Clinical Trial No. NCT01561755). Setting: The study took place in an academic medical center in New York, New York Participants: Only 12 participants were enrolled in four years; critical impediments to the study were the rarity of patients with new HIVM diagnoses and prior exposure to IVIG in patients with an established diagnosis. Measurements: Dynamometry of hip flexion, knee flexion, and ankle dorsiflexion were measured; the HIV Dementia Motor Score (HDMS); and the two-minute timed walk test were utilized. Results: Recruitment was the major feasibility issue. Dynamometry was generally well-tolerated, had good test-retest reliability (r=0.71–0.86, p<0.02 for all muscle groups), and good inter-item reliability as judged by the correlations between the muscle groups (r=0.76-0.81, p=0.001–0.005). Dynamometry was valid and clinically meaningful based on its correlations with the HDMS and the two-minute timed walk test. Conclusion: We conclude that an adequately powered clinical trial of IVIG for HIVM would likely require a prolonged recruitment period and multiple participating sites. Lower limb dynamometry is a useful outcome measure for HIVM, which might also be useful in other HIV-related gait disorders.
KEYWORDS: Dynamometry, intravenous immunoglobulin (IVIG), human immunodeficiency virus (HIV), myelopathy
INTRODUCTION
Human immunodeficiency virus (HIV)-associated myelopathy (HIVM) is a rare but well-described neurologic complication of HIV; it was first described early in the acquired immunodeficiency syndrome (AIDS) epidemic, and is still observed today despite the widespread use of combination antiretroviral therapy (CART).[1–3] HIVM manifests clinically at any stage of HIV infection, with slowly progressive weakness in the lower extremities, gait disorder, sensory abnormalities in the legs, sexual dysfunction, and urinary and bowel control abnormalities.[4] There is currently no proven effective treatment for HIVM, and treatment focuses on symptomatic therapies, including antispasticity agents, management of sphincter dysfunction, and physical therapy.
The pathogenesis of HIVM is unknown. HIV itself has been detected in the spinal cord only rarely, and there is no correlation between the presence or severity of HIVM and the presence of HIV in the spinal cord, CD4+ lymphocyte cell count, or HIV viral load in plasma or cerebrospinal fluid.[5,6] The histopathology of HIVM bears some resemblance to that of subacute combined degeneration due to vitamin B12 deficiency, where vacuolization is attributed to diminished production of S-adenosyl-methionine. Given these similarities, prior studies have sought to demonstrate whether high doses of oral L-methionine supplementation would be beneficial for the treatment of HIVM. However although a preliminary open-label trial was promising—demonstrating clinical and electrophysiological improvement of HIVM, a larger double-blind, placebo-controlled study showed no effect.[7,8]
Since neither treatment of metabolic pathways with L-methionine nor treatment of HIV itself with CART has been effective in ameliorating the symptoms of HIVM, there has been interest in the possibility of secondary immunologically mediated mechanisms. This concept is supported by certain pathological findings in HIVM, which, in addition to vacuolization, include inflammatory infiltrates.[9] Furthermore, immunologic mechanisms appear to be important in another retroviral myelopathy, namely human T-leukemia virus type I (HTLV-I), in which the immunomodulatory therapy intravenous immunoglobulin (IVIG) has shown some benefit. A small Japanese open-label clinical trial showed marked improvement with infusion of IVIG in 10 out of 14 patients with HTLV-I myelopathy.[10] The therapeutic effects were seen within one week after IVIG infusion, and the effects were sustained for more than three weeks in some patients.
Inspired by these results, we sought to obtain preliminary evidence for the efficacy and safety of IVIG in an open-label pilot study of 17 patients with HIVM.[11] Following IVIG infusion, there was a trend toward improved lower-extremity strength scores at 14 days post-infusion, which became statistically significant by Day 28. However, given that time and labor-intensive treatments, such as intravenous (IV) infusions, can be associated with a strong placebo effect, we sought to investigate these findings further in the current study. This randomized, double-blind, placebo-controlled feasibility study was designed to evaluate the safety and tolerability of IVIG (2g/kg) infused over two days for the treatment of HIVM, as well as provide preliminary evidence of its efficacy. The specific issues of feasibility on which we focused were recruitment and randomization (given that IVIG is commercially available and may be obtained outside the study), retention for follow-up assessments after the infusion, and the suitability of muscle dynamometry as a primary outcome measure for the study of HIVM. Although muscle dynamometry has not been used as an outcome in prior studies of HIVM, it has been used in other neurologic conditions, including other forms of myelopathy (spinal cord injury [SCI] in particular), stroke, motor neuron disease, and cerebral palsy.[12–14] Muscle dynamometry has also been used in the assessment of non-neurologic conditions, such as frailty in older adults.[15] In the case of SCI, lower-extremity dynamometry has been validated based on its association with clinically meaningful outcomes, such as walking function.[16]
METHODS
Participants and recruitment. This was a single-center study conducted at a large academic medical center in New York City, which is the center of a large healthcare system that provides services for approximately 10,000 HIV-infected patients in a network of five primary care HIV clinics. Participants were recruited from three main sources: the clinical NeuroAIDS practices of the investigators, pre-existing observational research studies (Manhattan HIV Brain Bank and the CNS HIV Antiretroviral Effects Research [CHARTER]); and a referral network of regional and national HIV care providers.
Included participants were HIV-infected adults (age ?18 years) who were diagnosed with HIVM by a neurologist using the criteria of at least two symptoms and at least two signs. Eligible symptoms (as reported by the participant) included paresthesias and/or numbness in the lower extremities or in all four limbs; weakness of the limbs, with predominance in the lower extremities; unsteady, stiff, or uncoordinated gait; sensation of electrical shock through the back or the legs upon flexion of the neck (L’Hermitte’s sign); stiffness or spasm in the lower extremities; urinary frequency, urgency, incontinence, or retention; fecal incontinence or retention; and sexual dysfunction, with erectile impairment in men. Eligible signs (as documented on neurologic examination) were reduction in vibratory or position sensation in the lower extremities, hyperactive deep tendon reflexes in the lower limbs, abnormal response to plantar stimulation (Babinski sign), weakness in the lower extremities or in all four limbs, and spastic or ataxic gait. In addition, it was required that participants be on a stable CART regimen for at least two months prior to entry of the study; women of child-bearing potential have a negative urine pregnancy within 14 days prior to study entry; and all subjects agree to practice abstinence or a highly effective method of birth control. Finally, we required the following laboratory parameters (within 14 days of study entry): Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase less than five times the upper limit of normal (ULN); total bilirubin 2.5mg/dL or less; creatinine 2.3 or less; and serum vitamin B12 level 200pg/mL or greater.
1The following conditions were exclusionary: presence of active opportunistic infection within two weeks before randomization; evidence of another contributing cause for myelopathy; pregnancy, breast-feeding or planning a pregnancy; any medical, psychiatric or substance use condition that in the opinion of the investigators would interfere with the subject’s ability to adhere to the protocol; contraindication to IVIG (e.g., cardiac, pulmonary, or renal disease that would place the subject at risk for fluid overload; known hypersensitivity to immunoglobulin, or IgA, deficiency); vaccination with live viruses within the past 90 days; or receipt of IVIG or other immunomodulatory agent within the past 90 days.
Study procedures. Participants were seen for a total of eight visits. Visit 1 was the screening visit, which began with the informed consent process followed by assessment of eligibility. A comprehensive medical history was recorded, physical and neurological examinations were performed, and the participant’s most recent spinal magnetic resonance imaging (MRI) (within the prior six months) was reviewed to confirm the absence of a visible alternative cause of myelopathy. Blood was collected for the following tests (unless available from clinical records within the prior 14 days): CD4+ cell count, plasma HIV viral load, HTLV-I and II, venereal disease research laboratory (VDRL), complete blood count (CBC), blood urea nitrogen (BUN), creatinine, liver function tests (LFTs), urinalysis, quantitative immunoglobulins, and serum pregnancy test in women of childbearing potential.
The screening visit was also used as a training session for muscle dynamometry in order to accustom the participants to the procedures involved. Quantitative lower-extremity muscle dynamometry was performed using a Microfet 2 dynamometer (Pro Healthcare Products, Park City, Utah), a load-cell device that records the force and the duration of muscle contraction. The dynamometer was mounted on an adjustable arm, permitting its placement in an ideal location for each of the muscle groups to be tested and allowing adjustment for differing lower limb lengths of the participants. A similar apparatus was shown to be reliable in a test-retest study of lower-limb muscle groups in an elderly population.15 We tested bilateral hip flexors, knee flexors, and ankle dorsiflexors. Participants were tested in a seated position with the hip and knee flexed to 90 degrees. For hip flexion, the pad of the dynamometer was placed 2cm proximal to the femoral condyle; for knee flexion, the dynamometer pad was 4cm proximal to the lateral malleolus; and for ankle dorsiflexion, the dynamometer pad was placed on the dorsal surface of the metatarsal heads and the ankle was placed at 10 degrees of the plantarflexion prior to testing. For each muscle group, the participant was asked to exert maximal force for eight seconds against the dynamometer pad for four trials. The trial in which the most force was exerted (measured in pounds-force) was then used for analyses. This value was averaged for the right and left sides and is referred to hereafter as the “composite dynamometry score.”
Visit 2 was the baseline visit in which all baseline measures were established prior to treatment. This visit occurred the day before the treatment. The neurologic examination was repeated (by a neurologist who was blind to the results of muscle dynamometry) and the HIV-dementia Motor Score (HDMS) was calculated.[17] The HDMS is a validated instrument that is used to summarize the abnormal neurologic exam findings typical of NeuroAIDS conditions and includes measures of strength, tone, deep tendon reflexes, plantar responses, coordination, and gait and frontal release signs. In addition, we assessed gait with the two minute timed walking test.[18]
For Visit 3, the participant was admitted to the Clinical Research Unit (CRU) and assigned a randomization code according to standardized procedures. This code was delivered to and maintained by the Mount Sinai Research Pharmacy. The research pharmacist masked all study drugs by wrapping all tubing and treatment materials in opaque material prior to delivery to the CRU. All study team members interacting with participants were blinded to treatment assignment. Each participant was then infused with either placebo or
IVIG (1g/kg) over approximately five hours. To ensure safety, electrocardiogram, vital signs, and adverse event monitoring took place during the infusion and for 90 minutes afterward. Physical examination and appropriate monitoring laboratory tests (CBC and metabolic panel) were also performed on treatment days. Visit 4 occurred the following day and consisted of a second infusion, which was identical to the first. Participants were then evaluated at 14, 28, 42, and 56 days post-infusion (Visits 5–8). These visits were identical and consisted of repetition of the neurological examination and dynamometry.
Regulatory considerations. All study procedures were conducted according to a protocol that was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai, in accordance with the ethical principles stated in the United States Title 21 of CFR Parts 50, 54, 56, and 312 and applicable guidelines in Good Clinical Practices (GCP). All members of the research team received appropriate training in the protocol and certification for the Education on the Protection of Human Subjects in Research, the Health Insurance and Portability and Accountability Act of 1996 (HIPAA), and GCP, as required by the United States Food and Drug Administration (FDA) for all research involving human subjects. Additionally, the investigators submitted Forms 1571 and 1572 and the study protocol to the FDA for an investigational new drug (IND) determination. The FDA provided an exemption from the IND regulations for this study. The study was registered with ClinicalTrials.gov (NCT01561755). All participants provided written informed consent.
Statistical considerations. In the initial design of the study, both the rarity of HIV-associated myelopathy and the cost of IVIG were taken into account as constraints on the projected sample size. Our goal was to recruit 30 participants (i.e., 15 per treatment group), based on projected feasibility. However, recruitment proved more difficult than anticipated, and we ultimately randomized only 12 participants before determining that attempts at further recruitment were futile (as described further in the Results section). We had originally planned to test the hip flexion composite dynamometry score (HFCDS) as the primary outcome measure using an analysis of covariance (ANCOVA) with Visit 8 (56 days post-infusion) HFCDS as the dependent variable, treatment group as the independent variable, and baseline HFCDS as the covariate. We chose hip flexion because it was deemed the most clinically relevant muscle in terms of walking ability, and dynamometry because it is more quantitative and thus potentially more sensitive to change than other purely clinically based measures, such as the Medical Research Council (MRC) Grading Scale for Strength. However, given the unexpectedly small sample size and the resultant low power to detect between group differences, it was not appropriate to carry out this intended analytic plan. Instead, we limited ourselves to description of the dynamometry and other results for the placebo and IVIG groups over the course of the study, with continuous variables presented as means with standard deviations (SD) or medians with interquartile ranges and categorical variables as frequencies with proportions in descriptive summaries.
We had also planned to perform baseline correlational analyses to understand the performance of dynamometry as an outcome measure in this patient population. Despite the smaller than anticipated sample size, these analyses were still possible and included Spearman rank correlations between the CDSs at the screening and baseline visits to assess test-retest reliability and between the CDSs and other validated measures (e.g. HDMS, 2-minute timed walk) to assess validity. Finally, for safety analyses, we described any adverse events experienced by the treatment groups.
RESULTS
Recruitment and participants. The study was open to enrollment for a total of four years from 2012 to 2016. Nineteen patients provided informed consent to participate, and, of these, 13 were found to be eligible. One participant was lost to follow-up prior to randomization, and 12 participants were randomized and treated. Numerous barriers to recruitment were encountered. Chief among these was the low incidence of new diagnoses of HIVM. Most of the HIVM patients of which we and our referral sources were aware had longstanding disease and had already received IVIG as part of clinical care. While we had originally intended to exclude these patients, we eventually loosened the criteria to allow their participation. However, this did not help significantly, since patients who had found IVIG effective were often still receiving it and were therefore not interested in a placebo-controlled study, and those who had not found IVIG effective saw little purpose in joining the study. Ultimately only one participant who had previously received IVIG was enrolled; this participant was randomized to the placebo group.
Of the 12 participants enrolled, all were successfully randomized, six to IVIG and six to placebo, and all were treated. One member of the IVIG group attended only one assessment following treatment (Visit 6, ~28 days after treatment), and so this participant’s data were included only in the safety analyses. The 11 remaining participants attended all subsequent visits. Participants were predominantly male (82%) with a mean age of 48 years. Most had longstanding HIV with a mean duration of known infection (by self-report) of 15.5 years (SD=9.4). HIVM had been present for 4.2 (SD=5.4) years on average. HIV was well controlled and undetectable in all patients except for one (in the IVIG group) with a viral load of 896 copies/mL. CD4+ counts ranged from 218 to 850 cells/mm3 with a mean of 592 (SD=212).
Muscle dynamometry. Muscle dynamometry was generally well tolerated by participants, and they were all able to complete all the dynamometry procedures, with the exception of two participants who experienced discomfort with the ankle dorsiflexion related to co-existing peripheral neuropathy. Due to this discomfort, it was necessary to modify the dynamometry procedure for one of these participants, using two attempts to generate maximal force instead of the usual four trials.
Overall, the dynamometry showed good test-retest reliability as demonstrated by significant correlation between the CDS obtained at the screening and baseline visits for hip flexion (r=0.86, p=0.001), knee flexion (r=0.85, p=0.001), and ankle dorsiflexion (r=0.71, p=0.015). In addition, the CDSs for each anatomic location were highly correlated with one another (hip flexion and knee flexion: r=0.80, p=0.002; hip flexion and ankle dorsiflexion: r=0.81, p=0.001; knee flexion and ankle dorsiflexion r=0.76, p=0.005). The hip flexion and knee flexion CDS were also associated with the HDMS in the expected direction (r= -0.69, p=0.013 and r= -0.634, p=0.027 respectively) although the ankle dorsiflexion score was not. The hip flexion CDS was also correlated with the two-minute timed walk (r=0.78, p=0.004), and there was a trend for correlation between the two-minute timed walk and knee flexion (r=0.60, p=0.051) and ankle dorsiflexion (r=0.56, p=0.71) CDSs.
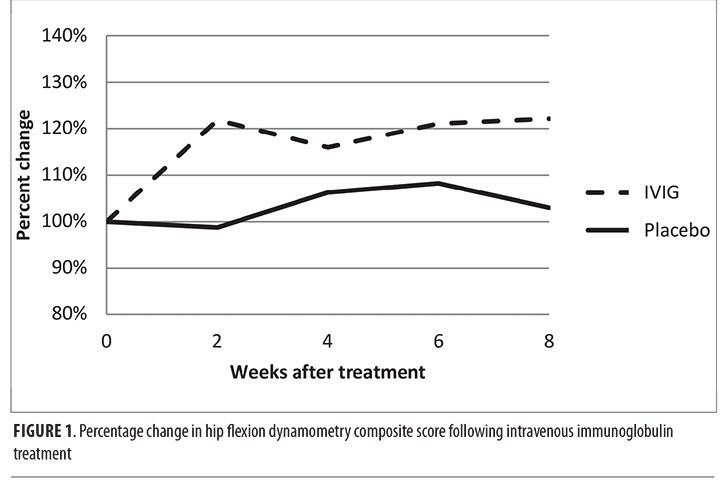
Examination of the dynamometry results over the course of the study revealed that, in general, proximal lower limb (hip flexion and knee flexion) force increased over time, whereas dorsiflexion force declined slightly. When these data were examined according to group assignment (Table 1), the IVIG group had larger increases in force generated on dynamometry. For example, the IVIG group experienced a 22-percent increase in the HFCDS, whereas the placebo group increase was only three percent. As shown in Figure 1, this effect appeared at Week 2 and was maintained until the end-of-study visit. However, we reiterate that these analyses are merely descriptive, given the small sample size, so we cannot exclude the possibility that any perceived between-group differences occurred purely by chance alone.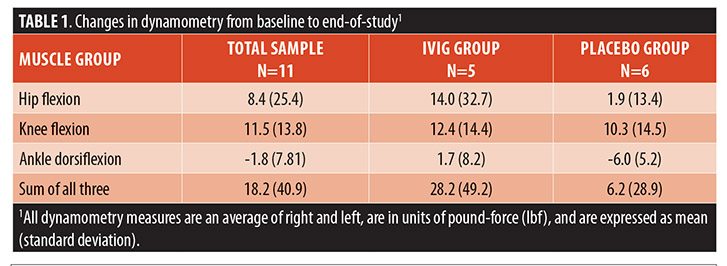
Adverse events and safety. In terms of safety, the infusions were well tolerated. One participant in the IVIG group developed a mild headache toward the end of the second infusion, which resolved spontaneously without specific treatment. There were no significant changes in the monitoring laboratory parameters.
DISCUSSION
HIVM is a disabling neurological disorder associated with HIV infection for which there is no known effective treatment. There are open-label data to suggest that IVIG might be effective in improving lower-extremity strength in HIVM, and data from other disorders, such as HTLV-1-associated myelopathy, and pathologic data implicating immune mechanisms in the pathogenesis of HIVM, support IVIG as a rational choice.[9–11] However given the expense and inconvenience of IVIG, it is desirable to have more definitive evidence as to its efficacy in HIVM. Thus, we designed the randomized, placebo-controlled feasibility trial described herein. The main conclusion of our study, which sought to enroll 30 participants but succeeded in enrolling only 12 over the course of four years, is that unfortunately a randomized, placebo-controlled trial of IVIG for HIVM that is large enough to provide data adequate to confidently guide clinical decision making is not feasible, at least not as a single-center study. We were unable to do so from within a large healthcare system in New York City, which services approximately 10,000 HIV-infected adults, and so a costly multicenter approach would likely be necessary for successful recruitment large enough to adequately power an IVIG myelopathy study. We believe that the main causes of this recruitment difficulty are the widespread availability of IVIG, which can be prescribed in an “off-label” fashion; the low incidence of new HIVM cases; and a reluctance of this patient population to undergo randomization.
While this trial did not meet its recruitment goals, other useful information can be gleaned from our experience with this study. Muscle dynamometry had not previously been used as an outcome measure for HIVM. Our data demonstrate that it is feasible based on its tolerability to most participants, reliable based on test-retest correlations, and valid based on its internal consistency between muscle groups and its correlation with other measures, namely the HDMS and the two-minute timed walk test.
Although HIVM is a rare disorder, functional gait limitation due to frailty and sarcopenia is common in HIV, particularly in aging populations.[19] Hand-grip dynamometry has been employed to study these conditions, but it is not a direct measure of lower-limb strength, so is only weakly correlated with function.[20,21] It is possible that lower-limb dynamometry would provide a more accurate representation of the functional consequences of frailty and sarcopenia on gait and could be used more widely as an outcome measure in HIV-infected populations. Lower-limb dynamometry might also prove useful in commoner forms of myelopathy, and indeed other studies have begun to examine its utility in this setting.[16] Of the three muscle groups tested (hip flexion, knee flexion, and ankle dorsiflexion), we found that hip flexor dynamometry was most strongly correlated with other measures, in keeping with our original supposition that hip flexion would be the most functionally important measure. Thus, if it were necessary to use one lower-limb dynamometry measure in isolation, hip flexion would likely be the best choice.
CONCLUSION
In summary, while the data produced by this study cannot be used to support or refute the efficacy of IVIG for HIVM due to low power related to small sample size, the treatment was generally well tolerated without significant adverse events in this population, and there was a suggestion of improvement in some patients. Thus, we recommend that clinical use of IVIG for the treatment of HIVM be based on an informed discussion and open communication between patient and provider.
References
- Simpson DM, Tagliati M. Neurologic manifestations of HIV infection. Ann Intern Med. 1994;121(10):769–875.
- Artigas J, Grosse G, Niedobitek F. Vacuolar myelopathy in AIDS. A morphological analysis. Pathol Res Pract. 1990;186(2): 228–237.
- Henin D, Smith TW, De Girolami U, et al. Neuropathology of the spinal cord in the acquired immunodeficiency syndrome. Hum Pathol. 1992;23(10):1106–1114.
- Dal Pan GJ, Glass JD, McArthur JC. Clinicopathologic correlations of HIV-1-associated vacuolar myelopathy: an autopsy-based case-control study. Neurology. 1994;44(11):2159–2164.
- Geraci A, Di Rocco A, Liu M, et al. AIDS myelopathy is not associated with elevated HIV viral load in cerebrospinal fluid. Neurology. 2000;55(3):440–2.
- Petito CK, Vecchio D, Chen YT. HIV antigen and DNA in AIDS spinal cords correlate with macrophage infiltration but not with vacuolar myelopathy. J Neuropathol Exp Neurol. 1994;53(1):86–94.
- Di Rocco A, Tagliati M, Danisi F, et al. A pilot study of L-methionine for the treatment of AIDS-associated myelopathy. Neurology. 1998;51(1):266–268.
- Di Rocco A, Werner P, Bottiglieri T, et al. Treatment of AIDS-associated myelopathy with L-methionine: a placebo-controlled study. Neurology. 2004;63(7):1270–1275.
- Tyor WR, Glass JD, Baumrind N, et al. Cytokine expression of macrophages in HIV-1-associated vacuolar myelopathy. Neurology. 1993;43(5):1002–1009.
- Kuroda Y, Takashima H, Ikeda A, et al. Treatment of HTLV-I-associated myelopathy with high-dose intravenous gammaglobulin. J Neurol. 1991;238(6):309–314.
- Cikurel K, Schiff L, Simpson DM. Pilot study of intravenous immunoglobulin in HIV-associated myelopathy. AIDS Patient Care STDS. 2009;23(2):75–78.
- Shefner JM. Strength testing in motor neuron diseases. Neurotherapeutics. 2017;14(1):154–160.
- Mulder-Brouwer AN, Rameckers EA, Bastiaenen CH. Lower extremity handheld dynamometry strength measurement in children with cerebral palsy. Pediatr Phys Ther. 2016;28(2):136–153.
- Kristensen OH, Stenager E, Dalgas U. Muscle strength and poststroke hemiplegia: a systematic review of muscle strength assessment and muscle strength impairment. Arch Phys Med Rehabil. 2017;98(2):368–380.
- Ford-Smith CD, Wyman JF, Elswick RK Jr, Fernandez T. Reliability of stationary dynamometer muscle strength testing in community-dwelling older adults. Arch Phys Med Rehabil. 2001;82(8):1128–1132.
- DiPiro ND, Holthaus KD, Morgan PJ, et al. Lower extremity strength is correlated with walking function after incomplete SCI. Top Spinal Cord Inj Rehabil. 2015;21(2):133–139.
- Robinson-Papp J, Byrd D, Mindt MR, et al. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol. 2008;65(8):1096–1101.
- Rossier P, Wade DT. Validity and reliability comparison of four mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82(1):9–13.
- Greene M, Justice AC, Covinsky KE. Assessment of geriatric syndromes and physical function in people living with HIV. Virulence. 2016;07:1–13.
- Richert L, Brault M, Mercie P, et al. Handgrip strength is only weakly correlated with physical function in well-controlled HIV infection: ANRS CO3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2014;65(1): e25–e27.
- Raso V, Shephard RJ, do Rosario Casseb JS, et al. Handgrip force offers a measure of physical function in individuals living with HIV/AIDS. J Acquir Immune Defic Syndr. 2013;63(1):e30–e32.





