
by Yael Marks, BS, PharmD; Tammie Lee Demler, BS, PharmD, MBA, BCGP, BCPP; and Chloe Matecki, PharmD
All authors are with Department of Pharmacy Practice, State University of New York at Buffalo, School of Pharmacy and Pharmaceutical Sciences in Buffalo, New York. Dr. Demler is additionally with Department of Pharmacy, New York State Office of Mental Health in Buffalo, New York; and Department of Psychiatry, State University of New York at Buffalo, Jacobs School of Medicine and Biomedical Sciences in Buffalo, New York.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2023;20(10–12):23–28.
Abstract
Objective: This study reviewed the cases of 13 patients in a psychiatric hospital during the 2022 lorazepam injection shortage in the United States (US). The objective was to determine if there were any patterns to the management of the medication shortage of an essential psychiatric medication at a psychiatric hospital.
Methods: A retrospective review of eligible patients who had an order for lorazepam injection prescribed as needed (PRN) between July and October 2022 were divided into, and compared between, two groups: those who had orders permitting continued PRN administration of the medication and those who were discontinued.
Results: No negative behavioral issues were seen in the patients who had their doses discontinued.
Conclusion: The absence of negative psychiatric consequences suggests that either nonpharmacotherapeutic interventions were alternatively stabilizing or that the standing PRN orders for lorazepam injection were not needed for these patients. A proactive emergency management plan to address critical medication shortages has become an increasingly necessary contingency and would have been appropriate for use during this national shortage.
Keywords: Lorazepam, shortage, behavioral issues, discontinuation
The past few years have been plagued by numerous medication shortages, impacting patients of various ages challenged by a variety of conditions, ranging from mental health providers managing their pediatric patients’ attention deficit hyperactivity disorder (ADHD) to adult psychiatric inpatients needing symptom control.1,2 The injectable formulation of lorazepam, known by its trade name Ativan, was intermittently, and often completely, unavailable from June through at least December 2022.3 The most current shortage followed years of previous lorazepam shortages, requiring health professionals to adjust treatment plans to care for patients as best they could with limited options.4 These shortages might have been caused by several factors, including supply chain issues, regulatory problems, and increased medication demand.5 Medication shortages can lead to many negative impacts for patients when their original treatment plan is changed as a consequence. One such example was seen in patients with ulcerative colitis who experienced adverse impacts from a balsalazide shortage; some patients who were not able to receive the intended treatment intervention experienced at least a one-point increase in their partial Mayo score, in addition to abdominal pain, hepatotoxicity, hypersensitivity reactions, and/or nausea from alternative agents.6 Additional serious consequences of drug shortages have resulted in increased drug resistance mutations, such as those seen in patients who experienced interrupted antiretroviral therapy in Nigeria.7 Additionally, a review of a French database revealed that medication shortages can result in patients experiencing medication errors, receiving medications that are less effective, and increasing the likelihood of adverse events.8 These impacts on patients demonstrate a need for improved planning to both reduce the occurrence of medication shortages and better manage shortages to limit patient harm.
Lorazepam, a benzodiazepine medication with anticonvulsant, anxiolytic, and sedative effects, is indicated in the setting of status epilepticus and as a pre-anesthetic medication.9 Due to both its noted clinical effects and rapid onset of action, lorazepam is used in the setting of acute episodes of psychiatric disorders, including agitation, psychosis, and anxiety.10 While most recent systematic reviews on the use of benzodiazepine usage do not prefer their use as single agents, lorazepam remains a popular choice due to its favorable side effect profile, established 40-year history of use, lower cost compared to agents in an alternative drug class, and somewhat unique availability as a parenteral injection for intramuscular (IM) administration for immediate (STAT) or PRN dosing.10,11
With many medications facing shortages, healthcare providers, including pharmacists on the treatment team, must plan proactively for alternative treatment options for patients with the greatest needs. One method of addressing a medication shortage is to allocate the existing medication to patients based on a triage process, established by the facility, that ensures the availability of the remaining supply goes to those who would most benefit from the specific medication.12 With this method, alternative treatments must be provided to patients who do not meet the threshold triage priority and thus are not eligible to receive the medication associated with the shortage. Establishing a standardized algorithm that is designed to objectively prioritize and reallocate the existing supply to those with the greatest need, as well as recommending alternative medications to those who are not eligible, is highly desirable. However, this process often does not occur.
The American Association for Emergency Psychiatry (AAEP) has sought to rectify gaps in treatment created by drug shortages and has released its own suggestions for the use of emergency medications. Notably, benzodiazepines, by the nature of their mechanism of action at the gamma-aminobutyric acid (GABA) receptor, are central nervous system (CNS) depressants. They do not treat underlying psychosis, but rather sedate and induce anxiolysis in the patient experiencing the acute event.13 In cases of agitation, lorazepam and other benzodiazepines remain among the agents of choice. They are not the agents of choice in cases of acute psychiatric illness; instead, the AAEP prefers the use of antipsychotics until the patient plateaus with no further improvement, at which point lorazepam usage is then preferred over ineffective antipsychotic dose escalation or polypharmacy using additional antipsychotics due to compounding side effect profiles.13 Despite no longer being exclusively first-line treatments, benzodiazepines continue to be considered a mainstay in therapy due to prescriber familiarity and ongoing recognition that short-term use may be appropriate based on the clinical condition of the patient.13
The facility of record in this article, a state-operated psychiatric inpatient hospital in Upstate New York, encountered the challenges of a national lorazepam injection shortage in July 2022. The pharmacists who were consulted to address the shortage were at a disadvantage because they did not have a pre-existing, facility-wide emergency management plan or a leadership consensus on how to prioritize the remaining supply of lorazepam injections, despite several patients who were court-ordered to receive lorazepam injection as a treatment over objection (TOO). After pharmacists communicated the shortage, prescribers discontinued some orders for lorazepam IM without providing orders for alternative medications, while other prescribers continued the PRN lorazepam orders without clear justification. Our research will describe the manner in which the lorazepam shortage was managed at the facility of record, which was just one of the many hospitals affected by the shortage. Additionally, we will explore the potential clinical impact on the psychiatric inpatients affected as a consequence of both the shortage and prescribing decisions made to address the shortage. We also underscore the need for a specific policy to deal with future medication shortages that advises strategic prescribing when needed, as the shortage crisis continues to evolve.
Methods
This study was a retrospective chart review that identified a subset of patients who were prescribed lorazepam injection to be administered intramuscularly between July and October 2022 and would be affected by the shortage. This screening process resulted in 13 patients who had an order for lorazepam injection and met the additional inclusion criteria of being inpatients during the specified dates of July 2, 2022, and October 4, 2022. Patient records were reviewed for information relevant to the use of lorazepam, order adjustments, and apparent changes in patient behavior. Patients with criminal procedure law (CPL) status were excluded, and the research was approved by the facility Institutional Review Board (IRB).
The records of lorazepam IM administration and order modification, if any, were compiled into an Excel database for later analysis. The following data was collected from this report for the specified time frame: patient name, patient sex, patient age, allergies, prescriber, PRN lorazepam dose and indication, lorazepam STAT administrations, lorazepam PRN administrations, whether PRN lorazepam order was continued or discontinued, last dose of PRN lorazepam administered, administrations of additional PRN medications, and any other medications on record. Additionally, the date and times (if multiple in one day) of code green (CG) and restraint or seclusion (RS) events were recorded. These events were used as a proxy for patient behavior. CG refers to an emergency alert signaling other hospital employees to arrive at an area where a patient is exhibiting behaviors that might escalate and require awareness and supportive interventions. Restraint refers to patients needing to be physically restrained, either by staff members or physical restraints, and seclusion refers to patients being placed in a separate area or room by themselves. These are implemented to allow patients to calm down and reduce risk of harm to themselves or to others. In this study, such occasions were utilized as references to negative behavior changes.
The CG and RS information was gathered by reviewing morning reports (i.e., documents describing patient events that occur when day shift staff are not present, namely interactions occurring at nighttime or weekends). The morning reports were distributed via email to appropriate hospital employees and were not saved in one location for archival purposes; thus, these documents were gathered for data collection by saving documents from email attachments sent by staff who had access to them. While this process resulted in the compilation of many reports, some were unavailable and unable to be reviewed. These reports were then read for patient behavior notes, which were listed in an Excel spreadsheet. This information was transferred to the data collection table for the relevant lorazepam injection patients.
Results
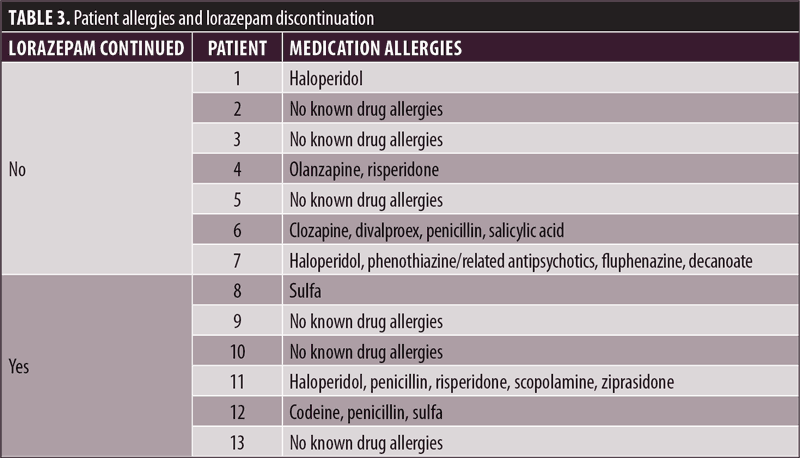
This study reviewed the clinical and hospital leadership management of the lorazepam injection shortage. When reviewing the available data, information was compared between patients who continued lorazepam injection therapy to patients whose medication was discontinued. As seen in Table 1, a total of seven patients had their lorazepam injection discontinued, and six patients had their lorazepam PRN orders continued. In terms of indication, Table 2 demonstrates the varied reasons for the use of lorazepam, with different types of agitation being the most common indication. Comparing medication allergies, Table 3 shows three patients in each group with no known drug allergies. Two patients who had lorazepam discontinued and one patient in the continued group were allergic to haloperidol. One patient in the discontinued group was allergic to penicillin, compared to two from the continued group. For other medication allergies, each group had one person with an allergy that had no matching allergy in the other group.

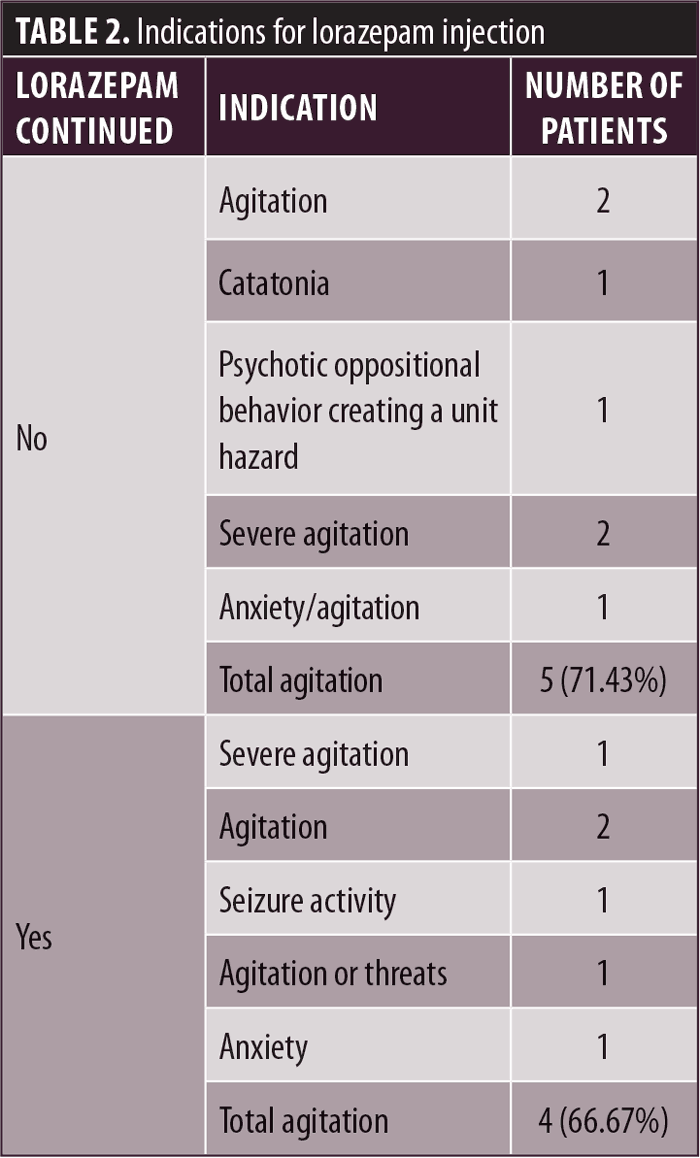
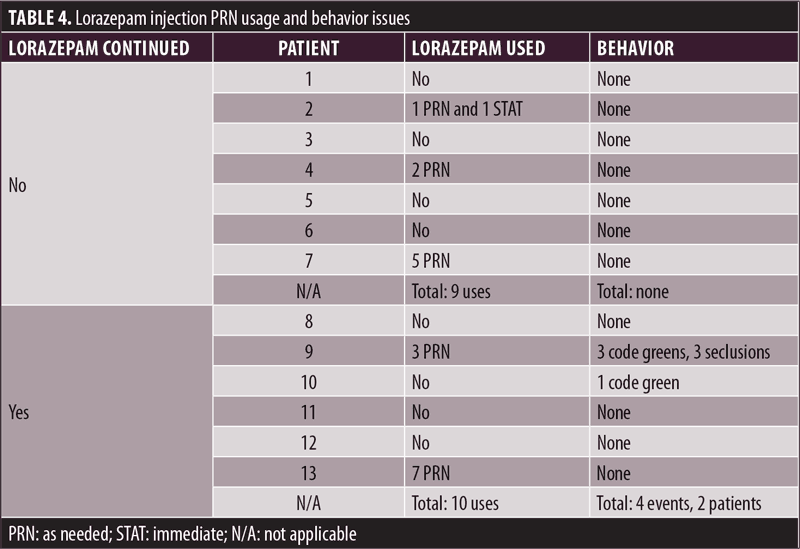
Table 4 describes medication usage following the lorazepam shortage and discontinuation, as well as noted behavior changes in the patients. No alternative medications were prescribed to any patient as a replacement for PRN lorazepam injection. Though there was a shortage of the medication, a limited supply remained available throughout the hospital. Thus, in the discontinuation group, lorazepam injection was used a total of nine times in three different patients (42.86%), compared to a total of 10 times in two patients (33.33%) in the continuation group. Of note, the discontinuation group did not have any reported behavior changes, while the continuation group had a total of four reported events for two patients (33.33% of the group). Interestingly, of these two patients, one was involved in a CG and had not received PRN lorazepam. The other patient received PRN lorazepam three times and was involved in behavioral issues three times, though only one of these events occurred on the same day a PRN lorazepam dose was administered. Furthermore, two patients provided us with interesting insight into medication use and behavior. Patient 7 from the discontinuation group received a PRN lorazepam injection five times and did not display behavioral changes. Similarly, Patient 13 from the continuation group received seven lorazepam injections and did not display behavioral changes.
In the search for potential alternative medications, the currently prescribed PRN medications for both groups of patients were reviewed and are reported in Table 5. In total, both groups had three patients on oral lorazepam PRN. The discontinuation group had three patients prescribed PRN olanzapine, compared to only one patient in the continuation group, with the same respective number of orders per group for PRN chlorpromazine. Additionally, PRN haloperidol was a current order for one patient in the discontinuation group, compared to two patients in the continuation group.

It is important to note that lorazepam injection is often used for patients who refuse or are otherwise unable to take oral medication. Therefore, perhaps a more essential comparison in these PRN medications are the ones that can be injected. For the discontinuation group, seven medications out of a total of 13 orders (53.85%) constituted PRN injectable medications, whereas the continuation group included four injectable medications out of a total of eight orders (50%). Furthermore, the discontinuation group included five patients out of seven with injectable PRN medications, whereas the continuation group included four patients out of six.
Discussion
Although the results show that no plan was made to address the shortage of lorazepam injection, a limited supply of the medication remained at the hospital and was utilized for certain patients. This demonstrates that although a shortage existed, the medication was never entirely out of stock and unavailable at the hospital. Nevertheless, this outcome was not a result of implementing a plan or proactive strategy to ensure availability for the continued use of lorazepam injection in the patients with the greatest needs. When examining allergies, for example, it is rational for patients who are allergic to alternative agents to be considered for prioritization of the drug in short supply when developing proactive emergency procedures. Additionally, patients subject to court-ordered treatment over objection (TOO) should also be afforded priority access to the medication in question.
There was no clear reason for the discontinuation of the medication for some patients but not others. No patients received alternative medication orders to replace the lorazepam injection when the shortage was announced. Additionally, none of the patients who had their lorazepam injection orders discontinued experienced psychiatric or behavioral exacerbation requiring CG, RS, or behavior notes entered on the morning report. There was no apparent pattern connecting the discontinuation or continuation of lorazepam with behavioral issues. It stands to reason that those who have their medication discontinued with no alternative would exhibit worsening symptoms that manifest as behavioral problems or exacerbation of psychiatric symptomatology. However, no patient in the discontinuation group demonstrated any behavioral problems or notable worsening psychiatric symptoms. The opposite was seen in two patients who continued their lorazepam and still presented concerning behaviors. This pattern poses the question, was there ever a need for lorazepam injection to be prescribed PRN? It is possible that the patients who had pre-existing behavioral issues would most likely experience symptoms of exacerbation regardless of the medication administered. While it is possible that the patients who continued the medication may have needed it more due to worse symptoms and behavior, this information was not available and could not be determined with the limited sample size. Therefore, this may be a case for deprescribing. If the discontinuation of lorazepam injection did not make a difference in behavior or symptomatology, they may have never needed this medication in the first place.
Drug shortages affect not only patient therapy, but also downstream factors in the patient’s care that have the capacity to contribute to patient harm. These factors include relapses in otherwise well-controlled disease states, hospitalization and/or increased length of stay, exposure to adverse drug events they would otherwise not have encountered, and increased mortality.14–16 The Institute for Safe Medication Practices (ISMP) notes that medication errors arise in times of shortages as well, with the potential need for the use of less common regimens, changes in existing complex regimens, and burden on dispensing roles in pharmacies.15,17 Cost to the institution, pharmacy, and patient can cause financial strain for all functioning recipients of the medication, such as increased procurement costs for alternative, more expensive drug forms or agents or formulary availability for the patient.17,18 Current guidance around drug shortages is scarce even in official capacities, including from pharmacy-led organizations. The American Society of Health-System Pharmacists (ASHP) manages a drug shortages list, which may contrast with the US Food and Drug Administration (FDA) drug shortages list, as well as a guideline for managing drug product shortages. The guideline, which details contributing factors, safety considerations, and planning steps, in addition to response recommendations to active shortages, outlines the best practices that are currently available.19
The ASHP recommendations include guidance on the planning of a response to a drug shortage for any of the reasons it might be occurring. The need for proper infrastructure is of note, with emphasis placed on the proper outlining of the process an institution would need to follow to therapeutically, ethically, and promptly respond to a shortage.19 The use of interprofessional teams and committees that can respond to shortages with identified member roles in drug information, purchasing and distribution, communication, information technologies, allocation, ethics, and therapeutic decision-making are crucial to not delay care or cause undue harm to patient populations, regardless of treatment setting.12,20–22 Responses to a drug shortage are detailed by the ASHP in a series of analyses, including operational, therapeutic, and shortage impact analyses. Analyses should be performed by appropriate committees prior to any implementation. It is here that the ASHP bolsters the importance of a pharmacy department’s involvement in decision-making within an interdisciplinary team, as their algorithm involves pharmacists at each analysis.19 Operational assessments serve to analyze the validity of shortages, address current supplies at each level of the supply chain, determine institutional needs based on purchase history, identify the availability of alternative agents, and estimate impact time. Therapeutic assessments should be utilized to identify the patient populations most affected by the shortage, be it by disease state, critical necessity of a specific agent, or ethical considerations, as well as to identify alternative medications. These two assessments should be done in tandem to inform the shortage impact analysis.19 Once the analysis considers what has been identified in the assessment stages, the impact on patient care can be determined. This includes determining the potential effects of changes in therapy, prescribing, distribution, administration, and any cost to both patient and institution.19 The utilization of electronic health records (EHRs) could address some potential medication errors that may arise from rapid changes brought about by shortages. However, EHRs could be a burden if the system cannot be changed on short notice or the financial strain or scope of the shortage cannot justify the change.20 This should culminate in the preparation of a final plan that includes communication of all the assessments’ findings and identified impacts of the shortage, as well as what is being implemented as a result. Stockpiling is also addressed and discouraged, citing the undue cost and stress it may cause.23
Limitations. There are some limitations to the methods of this retrospective review. The occurrences of CG or RS were not methodically recorded; rather, they were collected from the available reports that were sent via email. From the dates of July 2, 2022, to October 4, 2022, there were 17 days with no available reports. These days might have included descriptions of behavioral changes that were not included in the study. It is important to note that 10 of those dates were in July, when the shortage was first announced. While it is possible these days would have been the major area of impact from the shortage, the existence of additional doses means that the shortage’s effects might have been postponed or never truly affected the hospital. In addition, this review had a limited total sample size of 13 patients, with a comparison between one group of six patients and one group of seven patients. Therefore, generalizability is limited due to the small sample size.
Conclusion
It is important to create a strategy or algorithm to manage a future shortage. With the previous information presented regarding various medication shortages, it is reasonable to assume that additional shortages will continue to occur. Abruptly discontinuing lorazepam due to poor planning in the time of medication shortages could lead to withdrawal symptoms and negative impacts on the patient and their behavior. Having an algorithm in place to decide how to allocate limited medication supplies could provide better patient care by ensuring that the patients who need the medication most receive their doses. This could minimize the number of patients experiencing negative symptoms or exhibiting behavioral issues due to missing medications. Additionally, having a set plan in place would reduce confusion when a shortage is announced. In our analysis, the hospital’s staff did not have a protocol to follow when the lorazepam shortage began, which resulted in a disorganized method of deciding who would continue to receive the medication, which could have resulted in patients without alternatives having no medication intervention available. Furthermore, some of the patients who had the medication discontinued still received PRN and STAT doses (a total of 9 uses for 3 patients in a group of 7), which indicated that these patients did not fully stop this medication. Forming a plan to address these issues may lead to a clearer pattern of medication use, conservation of remaining doses, and better patient care.
No clear negative impact was seen in the behavior of patients who had their lorazepam injections discontinued during the medication shortage. While there were no obvious impacts on these patients, there was no official plan in place to manage the medication supply. In the future, it might be best to create an algorithm that will allow for the proper allocation of medication reserves to reduce errors, limit confusion among the hospital staff, provide medication to patients who need it most, and optimize patient care.
References
- American Society of Health-System Pharmacists. Drug shortages list. 2023. https://www.ashp.org/drug-shortages/current-shortages/drug-shortages-list?page=CurrentShortages. Accessed 28 Jun 2023.
- Goodman B. Empty pharmacy shelves shine a light on vulnerabilities in US drug supplies. CNN. 14 Dec 2022. https://www.cnn.com/2022/12/12/health/drug-shortages-explainer/index.html. Accessed 27 Dec 2022.
- Wheeler M, Chandramouli J. Drug shortage detail: lorazepam injection. American Society of Health-System Pharmacists. 23 Jun 2015. Updated 25 Aug 2023. https://www.ashp.org/drug-shortages/current-shortages/drug-shortage-detail.aspx?id=87. Accessed 29 Jun 2023.
- Tassey T. Out of Ativan?! The drug shortage crisis in the US. EMResident. 29 Jan 2016. https://www.emra.org/emresident/article/out-of-ativan-the-drug-shortage-crisis-in-the-u.s/. Accessed 27 Dec 2022.
- Shukar S, Zahoor F, Hayat K, et al. Drug shortage: causes, impact, and mitigation strategies. Front Pharmacol. 2021;12:693426.
- van Langenberg DR, Cheng RK, Garg M. Outcomes of a drug shortage requiring switching in patients with ulcerative colitis. World J Gastrointest Pathophysiol. 2020;11(2):32–42.
- Meloni ST, Chaplin B, Idoko J, et al. Drug resistance patterns following pharmacy stock shortage in Nigerian Antiretroviral Treatment Program. AIDS Res Ther. 2017;14(1):58.
- Bourneau-Martin D, Babin M, Grandvuillemin A, et al. Adverse drug reaction related to drug shortage: a retrospective study on the French National Pharmacovigilance Database. Br J Clin Pharmacol. 2023;89(3):1080–1088.
- LABEL: ATIVAN- lorazepam injection. US National Library of Medicine. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/18140s046lbl.pdf. Accessed 29 Nov 2023.
- Amore M, D’Andrea M, Fagiolini A. Treatment of agitation with lorazepam in clinical practice: a systematic review. Front Psychiatry. 2021;12:628965.
- Zaman H, Sampson SJ, Beck AL, et al. Benzodiazepines for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2017;12(12):CD003079.
- Flynn J, Chubbs K, Gladney-Martin S, et al. Triage in times of drug shortage. Healthc Manage Forum. 2015;28(5):202–205.
- Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26–34.
- Institute for Safe Medication Practices. A shortage of everything except errors: harm associated with drug shortages. 19 Apr 2012. https://www.ismp.org/resources/shortage-everything-except-errors-harm-associated-drug-shortages. Accessed 5 Jun 2023.
- Institute for Safe Medication Practices. Drug shortages threaten patient safety. 29 Jul 2010. https://www.ismp.org/resources/drug-shortages-threaten-patient-safety. Accessed 21 May 2023.
- McLaughlin M, Kotis D, Thomson K, Harrison M, et al. Effects on patient care caused by drug shortages: a survey. J Manag Care Pharm. 2013;19(9):783–788.
- Institute for Safe Medication Practices. Drug shortages continue to compromise patient care. 11 Jan 2018. https://www.ismp.org/resources/drug-shortages-continue-compromise-patient-care. Accessed 21 May 2023.
- Kaakeh R, Sweet BV, Reilly C, et al. Impact of drug shortages on US health systems. Am J Health Syst Pharm. 2011;68(19):1811–1819.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75(21):1742–1750.
- Pick AM, Massoomi F, Neff WJ, et al. A safety assessment tool for formulary candidates. Am J Health Syst Pharm. 2006;63(13):1269–1272.
- Rosoff PM. Unpredictable drug shortages: an ethical framework for short-term rationing in hospitals. Am J Bioeth. 2012;12(1):1–9.
- Rosoff PM, Patel KR, Scates A, et al. Coping with critical drug shortages: an ethical approach for allocating scarce resources in hospitals. Arch Intern Med. 2012;172(19):1494–1499.
- Myers CE. Artificial shortages of drug supplies. Am J Health Syst Pharm. 1999;56(8):727.


