
by Aline Hajj, PharmD, PhD; Souheil Hallit, PharmD, MSc, MPH, PhD; Rouba El-Khatib, PharmD; Sandra Abi Haidar, PharmD; Fabienne Hajj Moussa Lteif, MSc; Layal Hajj, MSc; Maguy Moudawar, MSc; and Lydia Rabbaa Khabbaz, PharmD, PhD
Drs. A. Hajj, El-Khatib, Abi Haidar, and Rabbaa Khabbaz are with the Faculty of Pharmacy, Saint-Joseph University in Beirut, Lebanon. Drs. A. Hajj and Rabbaa Khabbaz and Ms. Hajj Moussa Lteif are with Laboratoire de Pharmacologie, Pharmacie Clinique et Contrôle de Qualité des Médicaments, Saint-Joseph University in Beirut, Lebanon. Dr. Hallit is with the Faculty of Medicine and Medical Sciences, Holy Spirit University of Kaslik (USEK) in Jounieh, Lebanon; Research Department, Psychiatric Hospital of the Cross in Jal Eddib, Lebanon; and Psychology Department, College of Humanities, Effat University in Jeddah, Saudi Arabia. Ms. L. Hajj and Ms. Moudawar are with SESOBEL in Ain El Rihani, Lebanon.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(7–9):22–27.
Abstract
Objective: The prevalence of autism spectrum disorder (ASD) in Lebanon is higher than what is reported by the World Health Organization (WHO), leading to the thought that the Lebanese population has some specific risk factors for ASD. Therefore, it is important to conduct more robust studies on this population. We conducted this study to identify pre-, peri-, and neonatal risk factors for ASD. Our ultimate goal was to detect and change some modifiable risk factors, thus reducing the incidence of ASD.
Design: A case-control study was conducted using a random proportional sample of Lebanese children with ASD to explore whether risk factors, such as family history, pregnancy (including all medication and substances taken during pregnancy and infection history), gestational age, delivery, birth milestones, and the neonate’s condition at birth could be associated with a higher prevalence of ASD. The local ethical committee approved the study (USJ-2016-91), and all parents gave their written consent.
Results: A total of 66 children with ASD and 66 controls were included. The results of the multivariable analysis showed that a higher gestational weight gain (adjusted odds ratio [ORa]: 1.11) was significantly associated with higher odds of autism, whereas female sex (ORa: 0.13) and higher number of weeks of gestation (ORa: 0.76) were significantly associated with lower odds of autism.
Conclusion: Such results are of great relevance, since many of the identified factors herein could be avoidable or modifiable, suggesting the need for implementing timely and appropriate public health strategies for disease prevention in pregnant women that could reduce ASD.
Keywords: Autism spectrum disorder, case-control study, gestational weight gain, pregnancy, risk factors
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired communication and social interaction, as well as restricted repetitive patterns of behavior, interests, and activities.1 To date, the etiology of ASD remains largely unknown, but it is thought to be a multifactorial disorder, with a complex interplay between genetic and maternal/environmental factors.2–4 In a recent editorial, Jutla et al5 highlighted the importance of population-based studies to better understand the relative influence of predisposing factors to ASD. This editorial discussed, in particular, the results of the most comprehensive multinational, family-based study to date, aimed at estimating the additive genetic, maternal, and environmental effect in ASD.6 The authors concluded that heritability could be accounted for approximatively 80 percent of this effect, leaving a nonnegligible contribution of environmental factors. Other studies also estimated that the contribution of shared environment could range from 7 to 35 percent.2 Even with this small contribution to ASD risk, researchers still emphasize the importance of understanding environmental risk patterns that remain relatively poorly studied.5,7
In that context, factors related to the mother during the prenatal period have been suggested as risk factors for ASD, including sociodemographic, clinical, and nutritional factors, as well as medication/substance intake during pregnancy. These factors include advanced conception age, assisted reproductive technologies, pre-pregnancy and pregnancy obesity, excessive gestational weight gain, bacterial and viral infections, gestational diabetes, migraine, vaccination, medications, supplement intake, smoking, and alcohol consumption.8–12
Current evidence points to some perinatal factors as well, such as caesarian section delivery, obstetric/birth complications associated with fetal distress (trauma or ischemia and hypoxia),13–15 low birthweight, and preterm birth.16,17
The exact mechanisms explaining predisposition to ASD remain largely debated, but several hypotheses have been suggested, including inflammation/elevation of cytokine levels, endocrine disruption, alterations in signaling pathways and neurotransmitters, and oxidative stress, that might all lead to epigenetic changes in the fetal brain, thus leading to deleterious long-term developmental consequences, including vulnerability to develop ASD.7,18–20
The prevalence of ASD in Lebanon is higher than what is reported by the World Health Organization (WHO) (1 per 66 children in Lebanon21 and 1.48 percent22 vs. 1 per 160 children in the world23); therefore, it is possible that the Lebanese population has some specific risk factors, highlighting the importance of conducting national and regional studies. Several Lebanese studies have explored the environmental risk factors for ASD, yielding conflicting results.22,24,25 There were limitations to the design of each study, and the authors concluded that more robust studies exploring a full panel of risk factors associated with ASD in the region were necessary to determine the cultural and environmental characteristics.
Based on the aforementioned reasons, we aimed to replicate these findings and check if the previously described factors held among a representative sample of Lebanese children. Therefore, the primary purpose of our study was to determine the pre-, peri-, and neonatal factors associated with ASD among Lebanese children. Our ultimate goal would be to detect and change some modifiable risk factors that could reduce the incidence of this disorder.
Methods
General design of the study. A case-control study was conducted from February to May 2018, using a random proportional sample of Lebanese children with ASD from all districts (mouhafazat) of Lebanon (Beirut, Mount Lebanon, North, South, and Bekaa). A list of children was provided by the different centers (American Academy of Beirut and the Lebanese Autism Society–Beirut; Sesobel–Mount Lebanon, North Autism Center–North, Sidon Orphan Welfare Society–South and Rayon d’espoir–Zahle, Bekaa). A group of controls matched for age and mouhafaza was also included in this study.
Ethics disclosure. The study protocol was approved by the Ethics Committee of Saint Joseph University in Beirut (reference number: USJ-2016-91). Written informed consent was obtained from the parent answering the questions the day of the recruitment. All patients or their legal representatives gave their written informed consent prior to inclusion.
Questionnaire and data collection. The questionnaire (Appendix 1; https://innovationscns.com/wp-content/uploads/Hajj_Appendix-1.pdf) was completed via a face-to-face interview with the child’s parent. On average, the questionnaire was completed in about 15 to 20 minutes. The parent had the choice of accepting or refusing to complete the questionnaire. At the end of the process, the completed questionnaires were collected and sent for data entry. The anonymous questionnaire was in French. It consisted of different sections: sociodemographic characteristics (e.g., age, sex, mouhafaza, family history of ASD, age of diagnosis with ASD), data on care services, and questions related to the maternal risk factors (e.g., details related to the previous pregnancies and details related to the pregnancy of the child with ASD, including, among others, gestational weight gain, administration of vaccines and infections during pregnancy, supplementation and medication during pregnancy, etc.). Respondents were also asked about the childbirth, the perinatal period, and comorbidities and treatment of children with ASD.
Statistical analysis. The SPSS statistical program version 23 was used for all statistical analyses. Descriptive statistics were calculated for all variables in the study. This includes the mean and standard deviation for measurements, counts, and continuous percentages for categorical variables. The Chi-squared test was used to compare between dichotomous and categorical variables and the presence versus absence of ASD, whereas the student’s t-test was used to compare between two means of the two groups. Finally, a forward logistic regression was conducted, taking the presence versus absence of autism as the dependent variable and taking all variables that showed a value of p less than 0.1 in the bivariate analysis as independent variables to minimize confounding factors as much as possible. In all cases, a value of p less than 0.05 was considered significant.
Results
A total of 66 children with ASD and 66 controls were included in this study. For children with ASD, the mean age at diagnosis was 2.9±1.57 years, and only three (4.5%) had a family history of ASD. Several comorbidities were noted among these children, mainly hyperactivity (n=36; 54.5%), sleep disorders (n=18; 27.3%), aggressiveness (n=13; 19.7%), auto-aggressiveness (n=11; 16.7%), and behavioral/anger crisis (n=11; 16.7%); others included epilepsy, irritability, impulsivity, anxiety, auto-mutilation, and mental retardation. The majority of children with ASD had received pharmacological (n=16; 24.2%) and interventional treatments. The most reported pharmacological treatments consisted of risperidone (n=33; 50%), valproic acid (n=6; 9.1%), and atomoxetine (n=5; 7.6%) but aripiprazole, fluoxetine, sertraline, citalopram, topiramate, and methylphenidate (n=1; 1.5% for each) were also reported. The most common interventions consisted of psychomotor therapy (n=47; 71.2%), speech therapy (n=47; 71.2%), psychoeducation (n=10; 15.2%), and other therapies, including ergotherapy, art therapy, and music therapy.
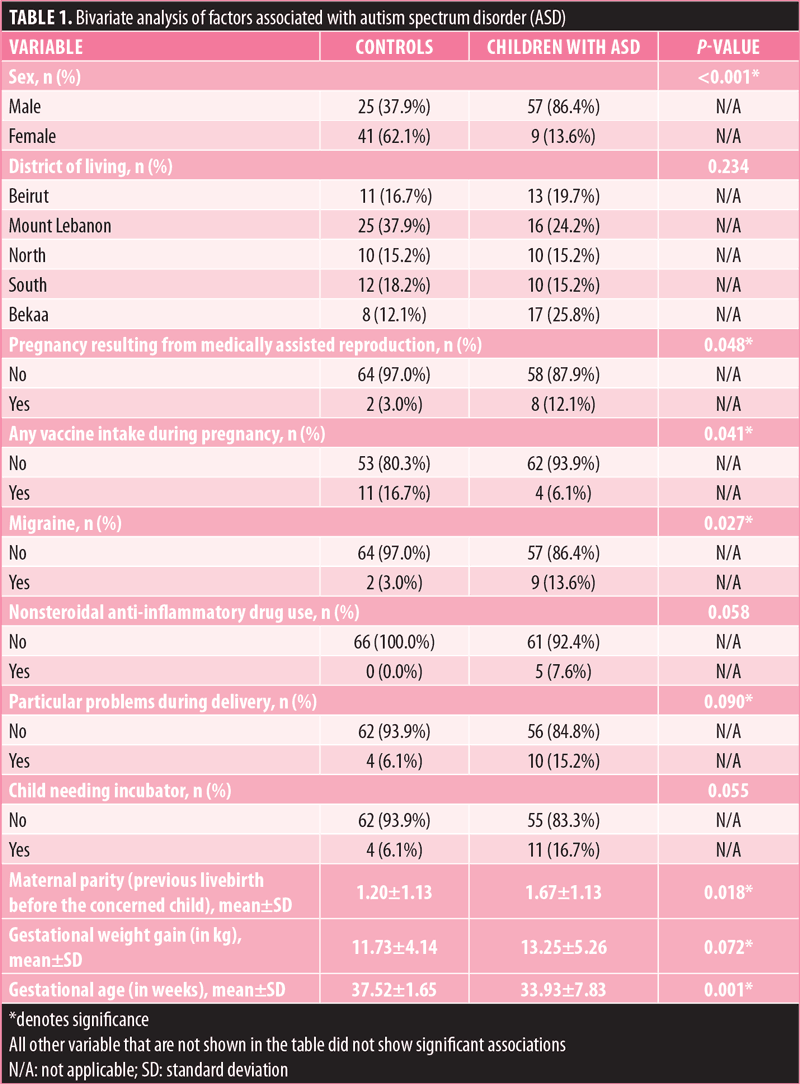
Bivariate analysis of the sociodemographic and other characteristics. The results of the bivariate analysis of factors associated with the presence/absence of autism are summarized in Table 1. Male sex (86.4% vs. 37.9%), pregnancy as a result of a medically assisted reproduction (12.1% vs. 3.0%), and migraine (13.6% vs. 3.0%) were significantly associated with higher odds of ASD, whereas taking any vaccine during pregnancy (16.7% vs. 6.1%) was significantly associated with lower odds of ASD. Finally, higher mean number of pregnancies before the concerned child (1.67 vs. 1.20) and higher gestational weight gain (13.25 vs. 11.73) were associated with higher odds of ASD, whereas higher gestational age (37.52 vs. 33.93) was significantly associated with lower odds of ASD in children.
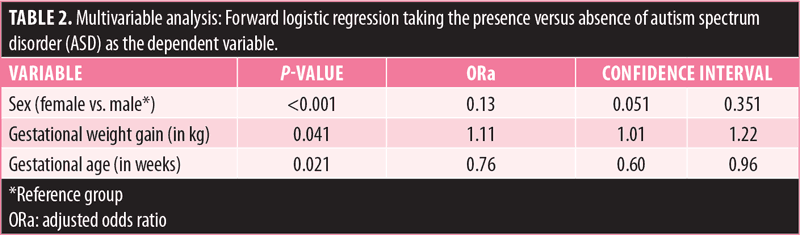
Multivariable analysis. The results of forward logistic regression, taking the presence versus absence of autism as the dependent variable, showed that higher gestational weight gain (adjusted odds ratio [ORa]: 1.11) was significantly associated with higher odds of autism, whereas female sex (ORa: 0.13) and higher number of weeks of gestation (ORa: 0.76) were significantly associated with lower odds of autism in the sample (Table 2).
Discussion
This study is one of a few in Lebanon that evaluated the association of pre-, peri-, and neonatal factors associated with autism among a sample of Lebanese children.24,25
The results of our study showed a predominance of male children (86.4%) among those with ASD, compared to controls. This is not surprising, since a striking and consistent feature of ASD is that it is more commonly diagnosed in male individuals than in female individuals.26,27 We therefore replicated what was previously observed in the Lebanese studies,22,24,25 and consolidated the findings of a recent meta-analysis, including 53,972 children who met the criteria for ASD, that showed a male-to-female ratio estimated to be 3:1.28 Several studies have reported the heritability of ASD,29,30 but we only identified a family history of ASD in 4.5 percent of children, suggesting a lower heritability rate. Although it is hard to draw conclusions with a small sample, this result is similar to previous findings reported in a Lebanese study.24 They are also in line with the findings of Hallmayer et al,31 highlighting a moderate genetic heritability and suggesting that other shared environmental factors (e.g., exposure to pollutants/toxicants, smoking environment, environmental or social stressors7,24) more prevalent in Lebanon might underlie susceptibility to ASD.
Aside from the environmental risk factors, our study highlighted an important modifiable risk factor for ASD that was significantly associated with higher odds of autism: gestational weight gain. This factor remained significant even in the multivariable analyses. These results are consistent with the ones observed in a large Chinese population, where the authors demonstrated that excessive gestational weight gain was associated with autism risk (OR: 1.327) after adjusting for children’s sex, parental age, and family annual income.32 The authors stipulated that the evidence was stronger for a link between gestational weight gain and ASD then between pre-pregnancy body mass index (BMI) and ASD, suggesting the role of the metabolic process and nutritional status during pregnancy.32,33 One hypothesis put forward to explain these results is related to glucose abnormalities. Hence, recent hypotheses have been suggested between intrauterine hyperglycemia/hyperinsulinemia and neurological disorders, such as ASD.34 In fact, authors have speculated that the disrupted neural connectivity, disordered neurological migration, and mitochondrial dysfunction observed in children with ASD could be due to prolonged fetal/neonatal dysglycemia (hypo/hyperglycemia), among other factors.34 Some authors have demonstrated that even a transient hyperglycemia might cause epigenetic changes.34 Controlled studies are needed to clarify all these factors and evaluate whether specific interventions, aimed at controlling gestational weight gain, would help reduce the risk of having children with ASD.11,34 It is noteworthy to add that such results are alarming if we look at recently published national data from 1,000 full-term deliveries of women enrolled through the National Collaborative Perinatal Neonatal Network (Lebanon). Authors have shown that while 16 percent of women with normal BMI exceeded the recommended gestational weight gain, more than half of the participants in the overweight/obesity group went above the limits set by the Institute of Medicine.35
Moreover, a decreased gestational age was significantly associated with ASD. Although not classified as a disease or a syndrome,36 gestational age has been identified as another risk factor associated with ASD.37–39 Many theories have been suggested to explain this association; inadequate maternal micronutrient supplementation might compromise both birth weight and gestational age.40,41 Premature children (born before 28 weeks of gestation) bear the risk of neurodevelopmental and physiological impairments, including autism.39 Conversely, premature delivery might compromise normal brain development, increasing the risk of ASD.42,43
Another factor that could be indirectly related to the maternal nutritional status during pregnancy is parity, which was shown to be associated with an increased risk of ASD in the bivariate analyses. Some studies have suggested that parity could be considered a risk factor for ASD, particularly being firstborn (vs. being third-born or later).44 In our study, however, the number of previous pregnancies was significantly associated with a higher risk of ASD, as reported by the previous Lebanese study.22 One possible explanation could be related to the physical health status of mothers in between subsequent pregnancies, where nutrient deficiency and higher maternal weight could be implicated in worsening risks.
Due to the previously mentioned factors and as recommended by the WHO, nutritional education and counseling for pregnant women would allow them to maintain a balanced nutritional status provided by a healthy diet during this critical developmental phase. Such strategies, including both diet and exercise, along with closer follow-up by the medical team, would promote optimal weight gain during pregnancy, improve birth outcomes, and reduce prematurity and delivery complications.45–47
Other than the previously mentioned factors, we have shown that women suffering from migraine might be at a higher risk of having a child with ASD. The effects of maternal migraine on pregnancy outcomes are poorly investigated, and studies have yielded inconclusive and conflicting results.17 Hence, some authors failed to identify any difference in rates of major congenital malformations or other outcomes, such as pregnancy duration or neonatal birth, compared to controls.48 Nevertheless, others have pointed out an association between migraines and increased rates of major congenital malformations, preterm delivery, preeclampsia, and low birthweight.49,50 A recent nationwide, population-based study including 22,841 pregnancies among women with migraine and 228,324 matched pregnancies without migraine examined the association between migraine in pregnancy and postnatal neurological complications in children.51 The authors identified greater risks of several adverse outcomes in women with migraine (e.g., respiratory distress syndrome, febrile seizures, etc.). However, nothing was reported about neurodevelopmental disorders, such as ASD, because the study evaluated neonatal outcomes within the first year of life, and ASD cannot be identified within this period.51 Pregnant women with migraine should be closely monitored to avoid having crises during pregnancy. Even if a meta-analysis suggested that the use of triptans during pregnancy does not seem to increase the risk of prematurity of malformations, authors have declared that the routine use of triptans in pregnancy should be carefully evaluated, and further research is required to ensure the safety of their prescription in pregnant women.17
Finally, concerning the link between vaccination and ASD, we have shown that maternal vaccination during pregnancy was significantly associated with lower odds of autism in children. In our study, the only reported vaccine to be administered to the pregnant women was the influenza vaccine. That could mean that this vaccine could have a protective effect against the development of ASD. One study has investigated the link between maternal influenza vaccination during pregnancy and ASD risk in a cohort of 196,929 children.52 The authors did not find any association between influenza infection, vaccination during pregnancy, and risk of ASD. Other studies have shown that mothers of children with ASD were more likely to have had or been exposed to influenza during gestation or fever secondary to influenza.52–54 Therefore, the vaccine, by preventing fever due to influenza, could have protected the children from developing symptoms of ASD.
Limitations and Strengths
A limitation of this study included its retrospective design. Hence, it relied on information based on the recall of parents of some details that could date from several years prior. Moreover, our sample was matched for age and district of living, but not for sex. It was composed of more female than male children, predisposing us to a selection bias. In addition, the sample size could be considered small, but it remains large enough to provide a sufficient statistical power for our analyses, and we performed a multivariable analysis taking the presence versus the absence of ASD as a dependent variable to avoid any confounding factor. Also, we did not report any detailed information related to pollution, especially with the waste burning consequent to the waste management crisis between 2015 and 2017.55
However, we included a representative sample of the Lebanese population with ASD with several factors that were evaluated comparatively with a random sample of controls matched for age and district. All children with ASD were diagnosed by a psychiatrist, and face-to-face interviews were conducted with the parents who had the medical health record at the moment of the interview, which reduced the risk of information bias.
Conclusion
Our results demonstrated the importance of conducting regional studies to elucidate the particularities of the environmental factors that might contribute to ASD. Such results are of great relevance, since some of the identified factors herein could be avoidable or modifiable, suggesting the need for implementing timely and appropriate evidence-based practices and public health strategies, which emphasize health promotion culture, screenings, and diagnosis, as well as disease prevention in pregnant women that could reduce ASD, among other disorders, especially in highly prevalent countries, such as Lebanon. More studies are also crucial to better identify the combination of factors and gene-environment interactions that could be considered as potential risk factors for ASD, thereby improving earlier detection and better management of ASD. Future studies analyzing biological samples for genetic, epigenetic, and inflammatory markers are necessary to elucidate the underlying mechanisms and start a new era for research studies based on modifiable risk factors for developmental disorders.
Author Contributions
Aline Hajj and Souheil Hallit designed the study and wrote the protocol. Lydia Khabbaz contributed to the design. Aline Hajj, Souheil Hallit, and Layal Hajj managed the literature searches and analyses. Aline Hajj, Rouba El-Khatib, Sandra Abi Haidar, Fabienne Hajj Moussa, Layal Hajj, and Maguy Moudawar included the patients and performed the survey. Souheil Hallit undertook the statistical analysis. Aline Hajj and Souheil Hallit wrote the first draft of the manuscript. Lydia Rabbaa Khabbaz supervised the whole process and critically reviewed the article. All authors contributed to and have approved the final manuscript.
References
- World Health Organization. Meeting report: autism spectrum disorders and other developmental disorders: from raising awareness to building capacity: World Health Organization, Geneva, Switzerland 16–18 September 2013. World Health Organization; 2013.
- Tick B, Bolton P, Happe F, et al. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585–595.
- Plomin R. Commentary: why are children in the same family so different? Non-shared environment three decades later. Int J Epidemiol. 2011;40(3):582–592.
- Muhle RA, Reed HE, Stratigos KA, Veenstra-VanderWeele J. The emerging clinical neuroscience of autism spectrum disorder: a review. JAMA Psychiatry. 2018;75(5):514–523.
- Jutla A, Reed H, Veenstra-VanderWeele J. The architecture of autism spectrum disorder risk: what do we know, and where do we go from here? JAMA Psychiatry. 2019;76(10):1005–1006.
- Bai D, Yip BHK, Windham GC, et al. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry. 2019;76(10):1035–1043.
- Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13.
- Lee BK, Magnusson C, Gardner RM, Blomstrom A, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–105.
- Cordero C, Windham GC, Schieve LA, et al. Maternal diabetes and hypertensive disorders in association with autism spectrum disorder. Autism Res. 2019;12(6):967–975.
- Hamra GB, Lyall K, Windham GC, et al. Prenatal exposure to endocrine-disrupting chemicals in relation to autism spectrum disorder and intellectual disability. Epidemiology. 2019;30(3):418–426.
- Windham GC, Anderson M, Lyall K, et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res. 2019;12(2):316–327.
- Kim JY, Son MJ, Son CY, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600.
- Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123(5):1293–1300.
- Johnson S, Hollis C, Kochhar P, et al. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156(4):525–531.e2.
- Kroger A, Hanig S, Seitz C, et al. Risk factors of autistic symptoms in children with ADHD. Eur Child Adolesc Psychiatry. 2011;20(11–12):561–570.
- Brašić JR, Holland JA. A qualitative and quantitative review of obstetric complications and autistic disorder. J Dev Phys Disabil. 2007;19(4):337–364.
- Marchenko A, Etwel F, Olutunfese O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015;55(4):490–501.
- Ponzio NM, Servatius R, Beck K, et al. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci. 2007;1107:118–128.
- Atladottir HO, Thorsen P, Ostergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430.
- Yirmiya N, Charman T. The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics. J Child Psychol Psychiatry. 2010;51(4):432–458.
- Chaaya M, Saab D, Maalouf FT, Boustany RM. Prevalence of autism spectrum disorder in nurseries in Lebanon: a cross sectional study. J Autism Dev Disord. 2016;46(2):514–522.
- Saab D, Chaaya M, Boustany R. National prevalence and correlates of autism: a Lebanese cross-sectional study. Autism Open Access. 2018;8(1).
- World Health Organization. Meeting report: autism spectrum disorders and other developmental disorders: from raising awareness to building capacity: World Health Organization, Geneva, Switzerland 16-18 September 2013. World Health Organization; 2013.
- Hamade A, Salameh P, Medlej-Hashim M, et al. Autism in children and correlates in Lebanon: a pilot case-control study. J Res Health Sci. 2013;13(2):119–124.
- Guisso DR, Saadeh FS, Saab D, et al. Association of autism with maternal infections, perinatal and other risk factors: a case-control study. J Autism Dev Disord. 2018;48(6):2010–2021.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Association; 2013.
- Messinger DS, Young GS, Webb SJ, et al. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol Autism. 2015;6:32.
- Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466–474.
- Waye MMY, Cheng HY. Genetics and epigenetics of autism: a review. Psychiatry Clin Neurosci. 2018;72(4):228–244.
- Genovese A, Butler MG. Clinical assessment, genetics, and treatment approaches in autism spectrum disorder (ASD). Int J Mol Sci. 2020;21(13):4726.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102.
- Shen Y, Dong H, Lu X, et al. Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC Psychiatry. 2018;18(1):11.
- Gardner RM, Lee BK, Magnusson C, et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: results from a Swedish total population and discordant sibling study. Int J Epidemiol. 2015;44(3):870–883.
- Hoirisch-Clapauch S, Nardi AE. Autism spectrum disorders: let’s talk about glucose? Transl Psychiatry. 2019;9(1):51.
- Papazian T, Abi Tayeh G, Sibai D, et al. Impact of maternal body mass index and gestational weight gain on neonatal outcomes among healthy Middle-Eastern females. PLoS One. 2017;12(7):e0181255.
- Butler AS, Behrman RE. Preterm birth: causes, consequences, and prevention. National Academies Press; 2007.
- Leavey A, Zwaigenbaum L, Heavner K, Burstyn I. Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. J Pediatr. 2013;162(2):361–368.
- Atladottir HO, Schendel DE, Henriksen TB, et al. Gestational age and autism spectrum disorder: trends in risk over time. Autism Res. 2016;9(2):224–231.
- Limperopoulos C. Autism spectrum disorders in survivors of extreme prematurity. Clin Perinatol. 2009;36(4):791–805, vi.
- Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42(5):1340–1355.
- Gernand AD, Schulze KJ, Stewart CP, et al. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–289.
- Atladottir HO, Schendel DE, Parner ET, Henriksen TB. A descriptive study on the neonatal morbidity profile of autism spectrum disorders, including a comparison with other neurodevelopmental disorders. J Autism Dev Disord. 2015;45(8):2429–2442.
- Buchmayer S, Johansson S, Johansson A, et al. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124(5):e817–e825.
- Guinchat V, Thorsen P, Laurent C, et al. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand. 2012;91(3):287–300.
- World Health Organization Regional Office for Europe. Healthy eating during pregnancy and breastfeeding: booklet for mothers. World Health Organization; 2001.
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization; 2016.
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015(6):CD007145.
- Banhidy F, Acs N, Horvath-Puho E, Czeizel AE. Pregnancy complications and delivery outcomes in pregnant women with severe migraine. Eur J Obstet Gynecol Reprod Biol. 2007;134(2):157–163.
- Banhidy F, Acs N, Horvath-Puho E, Czeizel AE. Maternal severe migraine and risk of congenital limb deficiencies. Birth Defects Res A Clin Mol Teratol. 2006;76(8):592–601.
- Grossman TB, Robbins MS, Govindappagari S, Dayal AK. Delivery outcomes of patients with acute migraine in pregnancy: a retrospective study. Headache. 2017;57(4):605–611.
- Skajaa N, Szepligeti SK, Xue F, et al. Pregnancy, birth, neonatal, and postnatal neurological outcomes after pregnancy with migraine. Headache. 2019;59(6):869–879.
- Zerbo O, Qian Y, Yoshida C, et al. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr. 2017;171(1):e163609.
- Deykin EY, MacMahon B. Viral exposure and autism. Am J Epidemiol. 1979;109(6):628–638.
- Zerbo O, Iosif AM, Walker C, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33.
- Morsi RZ, Safa R, Baroud SF, et al. The protracted waste crisis and physical health of workers in Beirut: a comparative cross-sectional study. Environ Health. 2017;16(1):39.


